1. Introduction
Polyethylene glycols (PEGs) are neutral polymers composed of repeated ethylene glycol units with different chain lengths [
1]. PEGs are one of the best-investigated polymers with a large number of applications in different fields. The water-solubility and hydrophilic properties of PEGs combined with a low intrinsic toxicity make them ideally suited for biological applications. Indeed, their use is approved by the Food and Drugs Administration (FDA) and European Medicines Agency (EMA) in several fields such as the pharmaceutical, cosmetic, packing, food, and clinical fields [
2].
Evidence from different experimental studies has suggested beneficial effects of PEGs in different pathological situations. PEGs have demonstrated membrane-protective effects in a variety of cells or organs against various insults. In this sense, PEGs reduce oxidative stress mainly through preservation of cell membrane integrity and protection against reactive oxygen species production during ischemia and reperfusion injury [
3]. PEG ameliorated the early and late effects of radiation in mice intestine through the stabilization and preservation of lipid-raft signaling, leading to preservation of membrane integrity [
4]. PEG has also been shown to reinforce epithelial barriers and to reduce inflammation of the colon in experimental colitis [
5]. The intravenous administration of PEG is effective in minimizing myocardial ischemia-reperfusion injury and in preserving ventricular function through the inhibition of apoptotic signaling and upregulation of cell survival signaling [
6]. Finally, the potential role of PEG in organ preservation has been demonstrated in experimental kidney [
3], liver [
7], and pancreatic [
8] transplantation. The presence of PEG in the preservation solution ameliorated the deleterious consequences of ischemia-reperfusion by triggering protective cell signaling pathways.
However, there is much less information regarding the precise mechanisms involved in the described beneficial effects of PEGs. In particular, little is known about interactions between PEGs and the different biological processes mediated by extracellular vesicles and, more specifically, exosomes. Exosomes are the smallest subtype of extracellular vesicles secreted constitutively by fundamentally all cells in physiological conditions into the extracellular space [
9]. They have received increasing attention since they were discovered to enclose functional proteins, lipids, DNA, mRNA, microRNA, and a large variety of other small noncoding RNA species [
10,
11]. Such exosomal cargo can be delivered into neighboring and distal cell subpopulations to either confer pathogenic or therapeutic effects through modulation of immune responses. Moreover, exosomes can be localized in many biological body fluids including blood, saliva, ascitic fluid, cerebrospinal fluid, and urine, thereby facilitating intercellular communication [
12].
Over the past years, a number of studies have provided evidence of exosomes’ implication in physiological and pathological cellular events [
13]. It has been reported that exosomes play relevant roles in processes as cancer, metastasis, neurological disorders, or inflammation. Previous studies of our group have determined the involvement of exosomes in lung damage associated with experimental acute pancreatitis (AP). Circulating exosomes from AP-induced rats reached the alveolar compartment and polarized macrophages to a pro-inflammatory phenotype [
14]. Hence, exosomes are now recognized as promising targets in a large number of pathologies and any agent with capability to modulate their function can be considered a potential therapeutic tool.
Interestingly, we have recently reported an anti-inflammatory role of 35-kDa molecular weight PEG (PEG35) in experimental AP and associated lung injury [
15]. Intravenous administration of PEG35 was able to reduce the severity of the inflammatory damage and to improve outcomes when administered following the initiation of AP-associated systemic effects. These effects may be attributed to the direct anti-inflammatory activity of PEG35, but a potential effect of this polymer on circulating exosomes could also be hypothesized.
In the present paper, we aimed to explore the interaction between exosomes and PEG35 in differentiated inflammatory cells in order to determine the potential role of PEG35 on the regulation of exosome uptake and function.
2. Materials and Methods
2.1. Cell Culture
The human pro-monocytic THP-1 cell line was purchased from Sigma-Aldrich (St. Louis, MO, USA). The cells were grown at 37 °C in suspension in RPMI 1640 medium GlutaMAX™ (Fisher Scientific; Madrid, Spain) supplemented with 10% fetal bovine serum (FBS), 100 U/mL penicillin, and 100 μg/mL streptomycin. THP-1 cells were plated at a density of 3 × 105 in 24-well culture plates and differentiated to macrophages through a first incubation with 100 nM phorbol 12-myristate13-acetate (PMA) (Sigma-Aldrich, St. Louis, MO, USA) for 24 h. The PMA-containing media was discarded and replaced with fresh media without PMA for a further 24 h.
The human epithelial cell line BICR-18 (from larynx squamous cell carcinoma) and CAPAN-2 (from pancreatic ductal adenocarcinoma) were obtained from American Type Cell Collection (ATCC, Manassas, VA, USA) and maintained at 37 °C in DMEM GlutaMAX™ (Fisher Scientific; Madrid, Spain) supplemented with 10% FBS, 100 U/mL penicillin, and 100 μg/mL streptomycin. Cultures were split every 3 days by trypsinization with 0.1% trypsin in Ca2+/Mg2+-free phosphate-buffered saline (PBS) containing 0.9 mM ethylenediaminetetraacetic acid (EDTA) (Sigma-Aldrich; St. Louis, MO, USA).
All cells were cultured at 37 °C in humidified atmosphere of 95% air and 5% CO2.
2.2. Experimental Animals
Male Wistar rats weighing 200–250 g (7–9 weeks old) were housed in a controlled environment with free access to standard laboratory pelleted formula (A04; Panlab, Barcelona, Spain) and tap water. A period of one week was allowed for animals to acclimatize before any experimentation. All procedures were conducted in accordance with European Union regulatory standards for animal experimentation (Directive 2010/63/EU on the protection of animals used for scientific purposes). The Ethical Committee for Animal Experimentation (CEEA, Directive 396/12, University of Barcelona) approved the animal experiments (ethic approval number: 211/18, University of Barcelona, 11/04/2018).
2.3. Exosome Isolation
In order to generate exosome-free media, exosomes present in FBS were removed by overnight centrifugation at 100,000×
g followed by filtration through 0.2-μm syringe-fitted filters (Millipore, Burlington, MA, USA). This exosome-depleted FBS was used for cell culture (corresponding culture media supplemented with 10% exosome-free FBS). For exosome isolation, BICR-18 cell supernatants and plasma samples from AP-induced rats were collected and centrifuged at 2000×
g and 10,000×
g for 10 and 30 min, respectively, at 4 °C. The last supernatant was filtered through a 0.22-µm syringe filter (Millipore, Burlington, MA, USA) and ultra-centrifuged at 120,000×
g for 70 min. After that, the pelleted vesicles were washed with PBS and centrifuged again at 120,000×
g [
16].
The quality of exosome preparation was verified by nanoparticle tracking analysis and by determining the presence of the exosomal marker TSG101 and the absence of calnexin by Western blot. The number of exosomes obtained was also quantified by measuring their protein content using a Bradford assay (Bio-Rad Laboratories, Hercules, CA, USA).
2.4. Exosomes and Cell Staining
For internalization assays, exosomes were isolated from BICR-18 cells (ExoB) or from AP-induced rats (ExoAP) and labelled with the PKH26 red fluorescent cell linker dye (Sigma-Aldrich, St. Louis, MO, USA) for 5 min. The staining reaction was stopped with 3% bovine serum albumin (BSA) for 1 min. In order to remove the unbound dye, exosomes were washed three times with PBS using 300 kDa Nanosep centrifugal devices (Pall Corporation, New York, NY, USA). Fixed cells were also stained with the DNA-specific blue, fluorescent stain 4′, 6-diamidino-2-phenylindole (DAPI) (Sigma-Aldrich, St. Louis, MO, USA) for 1–5 min at room temperature. In some experiments, THP-1 macrophages were stained with the PKH67 green, fluorescent cell linker dye for general cell membrane.
2.5. Cell Treatments
To analyze exosome uptake in the presence of PEG35, differentiated THP-1 cells were incubated with 4% PEG35 diluted in PBS for 30 min prior to treatment with the appropriate stimuli: 10 µg/mL PKH26-labelled ExoB or 10 µg/mL PKH26-labelled ExoAP for 1.5 h. As well, epithelial BICR-18 and CAPAN-2 cells were incubated with 4% PEG35 for 30 min prior to treatment with PKH26-labelled ExoB for 1.5 h. The concentration of exosomes was selected according to previous in vitro studies [
17]. Exosome internalization was analyzed by confocal microscopy imaging or by flow cytometry.
To analyze the modulation of the inflammatory response induced by PEG35, differentiated THP-1 cells were incubated with increasing concentrations of PEG35 (1, 2, 4, and 6%) for 30 min prior to treatment with the appropriate stimuli: 10 µg/mL ExoAP or 0.1 µg/mL lipopolysaccharide (LPS) (Sigma-Aldrich, St. Louis, MO, USA) for another 1.5 h. In another set of experiments, to determine the nuclear shift of p65 subunit of Nuclear Factor Kappa B (NFκB), differentiated THP-1 cells were incubated with 4% PEG35 for 30 min prior to treatment with 10 µg/mL ExoAP or 0.1 µg/mL LPS for another 30 min.
2.6. Nanoparticle Tracking Analysis
The size distribution and concentration of exosomes were measured using a NanoSight LM10 machine (NanoSight, Salisbury, UK). All parameters of the analysis were set at the same values for all samples, and three one-min-long videos were recorded in all cases. The background was measured by testing filtered PBS, which revealed no signal.
2.7. Animal Model of AP
Rats (n = 5) were anesthetized with an intraperitoneal injection of pentobarbital at a dose of 50 mg/kg. After midline laparotomy, a polyethylene catheter connected to an infusion pump was inserted through the duodenum via the Ampulla of Vater, 3–4 mm into the biliopancreatic duct. A bulldog clamp was applied to the proximal biliopancreatic duct (near the liver) to prevent infusion into the liver. The experimental model of AP was induced in the rats by retrograde injection of 5% sodium taurocholate in saline solution at 1 mL/kg for 1 min using an infusion pump (Harvard Instruments, Edenbridge, UK). Buprenorphine (0.05 mg/kg) was intravenously administered as an analgesic immediately before surgery. Three hours after AP induction, the animals were euthanized and blood was collected in heparinized syringes from the vena cava for exosome isolation.
2.8. Confocal Microscopy
Cells were imaged using an inverted Nikon Eclipse Ti2-E microscope (Nikon Instruments, Melville, NY, USA) attached to the spinning disk unit Andor Dragonfly. For all experiments, an oil-immersion objective (Plan Fluor 20×, numerical aperture (NA) 0.75, oil) was used. Samples were excited with 405 nM, 488 nM, and 561 nM laser diodes. The beam was coupled into a multimode fiber going through the Andor Borealis unit reshaping the beam from a Gaussian profile to a homogenous flat top. From there, it was passed through the 40-µm pinhole disk. Cells were imaged on a high-resolution scientific complementary metal oxide semiconductor (sCMOS) camera (Zyla 4.2, 2.0 Andor, Oxford Instruments Company, Concord, MA, USA). Fusion software from Oxford Instruments Company was used for acquisition of images. Image deconvolution was performed after acquisition. Image processing and analysis were performed with Image J/Fiji open source software using Image J Macro Language.
2.9. SDS-PAGE and Western Blot
For characterization of the exosomes, exosomal proteins were extracted in ice-cold radioimmunoprecipitation assay (RIPA) buffer in the presence of protease inhibitors. Extracts were then centrifuged at 15,000× g for 20 min at 4 °C, and the supernatants were collected. Cell lysates, used as a negative control, were obtained from differentiated THP-1 cells. Protein concentrations of the supernatants were determined by the Bradford protein assay (Bio-Rad Laboratories, Hercules, CA, USA). SDS-PAGE was performed on a 10% gel, and proteins were transferred to a polyvinylidene difluoride (PVDF) membrane for blotting (Bio Rad, Hercules, CA, USA). Membranes were blocked for 1 h in 5% non-fat milk in PBS, followed by overnight incubation at 4 °C with the following antibodies from Proteintech (Manchester, UK): anti-rabbit TSG101 (1:1000 dilution, reference 14497-1-AP) and anti-rabbit calnexin as a negative control (1:1000 dilution, reference 10427-2-AP).
To determine NFκB activation, the nuclear extract from treated THP-1 cells was prepared according to the protocol described in the nuclear extraction kit from Active Motif (Carlsbad, CA, USA). Nuclear protein concentration was determined, and SDS-PAGE was performed on a 10% gel. Then, proteins were transferred to a PVDF membrane for blotting. Membranes were blocked for 1 h in 5% non-fat milk in PBS, followed by overnight incubation at 4 °C with anti-rat p65 antibody (1:400 dilution; reference sc-372; Santa Cruz Biotechnology, Santa Cruz, CA, USA), or were blocked for 1 h in 5% BSA in PBS for subsequent overnight incubation with anti-mouse TATA binding protein (TBP) antibody (1:500 dilution; reference ab818; Abcam, Cambridge, UK).
All blots were washed and incubated with the corresponding horseradish peroxidase (HRP)-conjugated secondary antibody. Bound antibodies were detected using enhanced chemiluminescence (ECL) (Bio-Rad Laboratories, Hercules, CA, USA), and were analyzed using ChemiDoc™ Touch Imaging System (Bio-Rad Laboratories, Hercules, CA, USA).
2.10. Immunofluorescence
To determine NFκB or Signal transducer and activator of transcription 3 (STAT3) nuclear translocation, THP-1 macrophages were incubated in coverslips overnight at 37 °C under 5% CO2 in air. Treated cells were fixed with 3.5% formaldehyde for 5 min at room temperature and permeabilized with Triton X-100. The cells were stained with the following antibodies from Santa Cruz (Santa Cruz Biotechnology, Santa Cruz, CA, USA): anti-NFκB p65 (1:400 dilution, reference sc-372) and anti-STAT3 antibody (1:400 dilution, reference sc-483). Alexa Fluor 488-conjugated anti-goat antibody (Molecular Probes, Eugene, OR, USA) was used as a secondary antibody. Localization of NFκB and STAT3 was examined by confocal microscopy imaging.
2.11. Real-Time qRT-PCR
The total RNA from the cells was extracted with Nucleozol reagent (Macherey-Nagel, Dueren, Germany) according to the manufacturer’s protocol. RNA concentration and quality were measured with the optical density (OD) A260/A280 ratio and OD A260/A230 ratio respectively, and the integrity of the 18S and 28S ribosomal bands for all RNA preparations was verified by running a 1% agarose gel electrophoresis. Reverse transcription was conducted on a 1-µg RNA sample using the iScript cDNA Synthesis Kit (Bio-Rad Laboratories, Hercules, CA, USA). Subsequent PCR amplification was conducted using SsoAdvanced™ Universal SYBR
® Green Supermix (Bio-Rad Laboratories, Hercules, CA, USA) on a CFX Real-Time PCR Detection System (Bio-Rad Laboratories, Hercules, CA, USA) using 10 µL of amplification mixtures containing 50 ng of reverse-transcribed RNA and 250 nM of the corresponding forward and reverse primers. PCR primers for the detection of Interleukin 1β (IL1β) and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were designed with Primer3.0 plus [
18]. The sequences were as follows: IL1β forward: 5′-GGACAAGCTGAGGAAGATGC-3′ and reverse: 5′-TCGTTATCCCATGTGTCGAA-3′, and GAPDH forward: 5′-GATCATGAGCAATGCCTCCT-3′ and reverse: 5′-TGTGGTCATGAGTCGTTCCA-3′. The specificity of the amplicons was determined by melting curve analysis. Reactions were carried out in duplicate, and threshold cycle values were normalized to GAPDH gene expression. The ratio of the relative expressions of target genes to GAPDH was calculated by the ΔCt formula.
2.12. Flow Cytometry
For flow cytometry analysis, differentiated THP-1 cells were pretreated with increasing concentrations of PEG35 and then co-incubated with PKH26-labeled ExoAP. Subsequently, cells were washed with PBS and trypsinized. Pelleted cells were fixated in 4% paraformaldehyde and incubated for 15 min at 4 °C. After incubation, cells were washed again with PBS and resuspended in 0.3 mL of PBS buffer for flow cytometry analysis. The PKH26 fluorescence was measured on a BD LSR Fortessa SORP cytometer using the FacsDiva software (BD Biosciences, San Jose, CA, USA). Data analyses were performed with FlowJo software (version 4.4.1; FlowJo LLC, Ashland, OR, USA).
2.13. IL1β Immunoassay
Interleukin-1 β (IL1β) was measured in the supernatant of treated THP-1 macrophages using a commercially available ELISA Kit (R&D Systems, Minneapolis, MN, USA) in accordance with the manufacturer’s instructions. Briefly, standards and samples reacted with a specific antibody against IL1β immobilized in a microplate. Another antibody specific for human IL1β conjugated to HRP was then added to the wells. After washing, a substrate solution was added, yielding a yellow product. The intensity of the color measured is in proportion to the amount of IL1β. The optical density was measured at 450 nM using an automated microplate reader (iEMS Reader MF; Labsystems, Helsinki, Finland). IL1β levels were obtained in pg/mL. All samples were run in duplicate.
2.14. Statistical Analysis
All data were exported into Graph Pad Prism 4 (GraphPad Software, Inc.) and presented as means ± SEM. Statistical analyses were carried out by one-way analysis of variance (ANOVA), followed by Tukey’s multiple comparison test to determine the significance between pairs. The minimal level of statistical significance was <0.05.
4. Discussion
Over the last decade, considerable interest has grown in the study of PEG properties and their use has extended to different fields. Recently, our group has demonstrated the anti-inflammatory effect of prophylactic administration of PEG35 in an experimental model of acute necrotizing pancreatitis, and besides, we have shown that therapeutic treatment with this polymer exerted significant protection against acute lung inflammation associated with this disease [
15].
In the current study, we explored whether this protective action of PEG35 is related to effects on the cellular uptake of exosomes. It is well known that these nanovesicles are able to trigger the inflammatory response in a broad spectrum of pathologies. In relation to the pancreas, there is scarce bibliographical documentation on the role of exosomes in pancreatitis-associated inflammation. Our group has previously reported that circulating exosomes may act as pro-inflammatory mediators and may activate alveolar macrophages in experimental AP [
14]. More recently, another mechanism of exosome-induced inflammation in AP have been elucidated in which NLRP3 inflammasome activation and subsequent pyroptosis in alveolar macrophages are responsible for the lung injury secondary to AP [
21].
Since PEG has the capability to bind biological membranes [
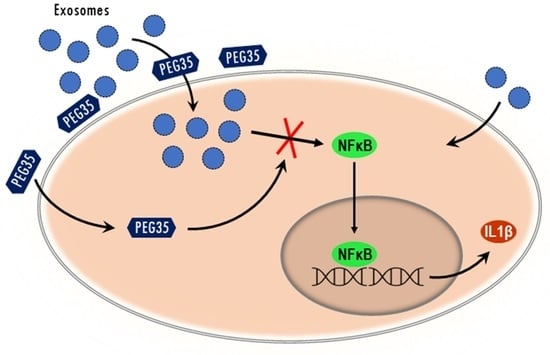
22], it could be hypothesized that its presence impairs the process of exosome uptake, thus reducing the associated inflammatory response triggered by these nanovesicles. Unexpectedly, our results indicate the opposite mechanism and exosome uptake increased in a dose-dependent manner in the presence of PEG35.
We then explored whether this mechanism was related to the high phagocytic capacity of macrophages and evaluated the effect of PEG35 on different epithelial cell lines. In all cases, the presence of 4% PEG35 increased exosome uptake, pointing to a nonspecific mechanism which probably involves the binding process between exosomal and cell membranes.
Our group first clarified that exosomes are involved in AP and that circulating exosomes transform alveolar macrophages into a pro-inflammatory phenotype. This change seems to be related to the content of exosomes, which has been found to be enriched in immune response-associated proteins such as S100-A8, S100-A9, Ficolin-2, or LPS-binding protein [
14]. In addition, exosomes released from taurolithocholate-treated pancreatic acinar cells contain differentially expressed miRNAs, which promote inflammation in macrophages through NF𝜅B activation [
23]. Therefore, it seems clear that the exosomes from AP carry compounds with relevant pro-inflammatory activity that significantly contributes to disease progression.
As expected, our results confirm that exosomes from AP increased the expression of pro-inflammatory IL1β on THP-1 macrophages, revealing their activation. Interestingly, PEG35 treatment succeeded in the inhibition of these inflammatory cytokine’s expressions. This fact suggests that, despite the increase in internalization of exosomes induced by PEG35, its anti-inflammatory effect is able to prevent the activation induced by these nanovesicles.
Next, we determined the direct anti-inflammatory effect of PEG35 on the activation of macrophages and confirmed that this polymer markedly prevented IL1β mRNA and protein expression induced by LPS treatment in a concentration-dependent manner. This is consistent with the results obtained in a previous study in which we demonstrated a direct anti-inflammatory effect of PEG35 in pancreatic acinar AR42J cells [
24]. In this sense, we found that administration of this polymer attenuated the expression of pro-inflammatory cytokines and associated cell death markers following TNFα or cerulein treatment.
Given that activation of NFκB and STAT3 plays a crucial role in inflammatory processes, it was conceivable that these signaling pathways could be involved in PEG35 inhibition of inflammation. Although triggered by different pathways, activated STATs and NFκB translocate into the nucleus and function either individually or collaboratively in regulating inflammatory gene expression. Our data indicated that macrophage treatment with PEG35 caused a shift in the p65 nuclear localization observed under LPS or ExoAP stimulation. However, STAT3 was not found to be a target for PEG35-mediated attenuation of inflammation in LPS-treated macrophages. The direct inhibitory effect of PEG35 on NFκB allows for understanding the fact that, although more exosomes are uptaken, thus implying a greater pro-inflammatory stimulus, PEG35 inhibits its activation at a subsequent stage and the outcome is a lower expression of inflammatory mediators.