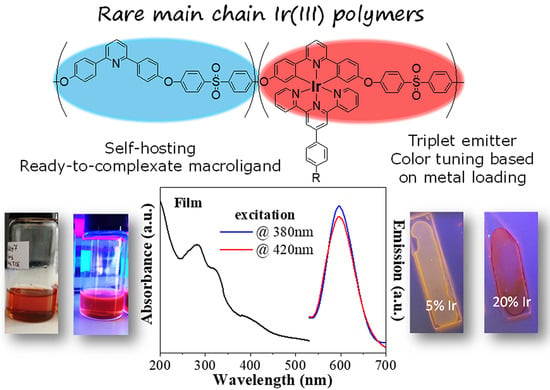
Bis-Tridendate Ir(III) Polymer-Metallocomplexes: Hybrid, Main-Chain Polymer Phosphors for Orange–Red Light Emission
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Instrumentation
2.3. Synthesis of Compounds
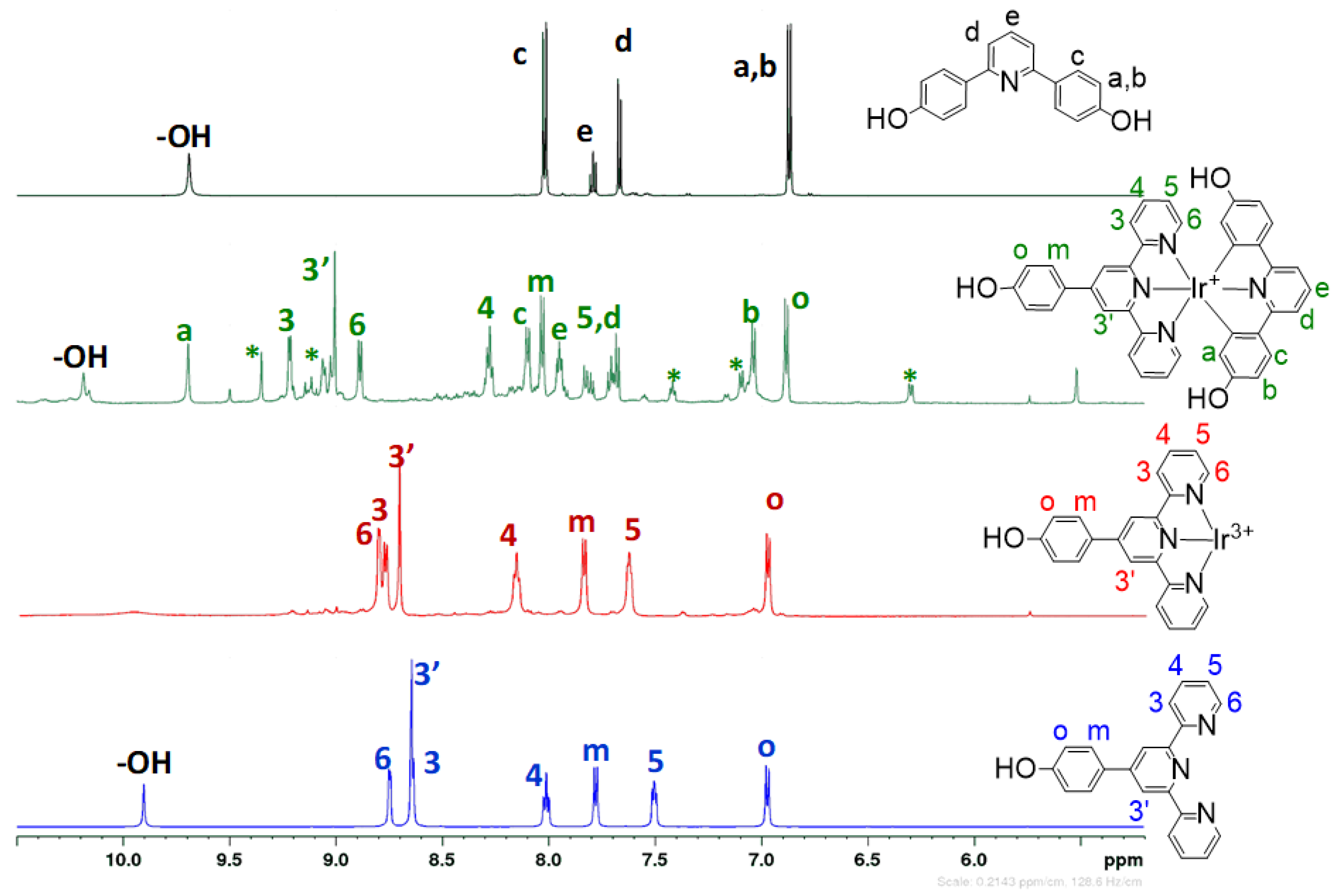
2.3.1. Monocomplexes R-tpy-IrCl3
2.3.2. Dicomplexes R-tpy-Ir-HOpy
2.3.3. Copolymer Metallocomplexes CPOL-Rtpy-Ir
3. Results
3.1. Synthesis of Compounds
3.2. Structural Characterization
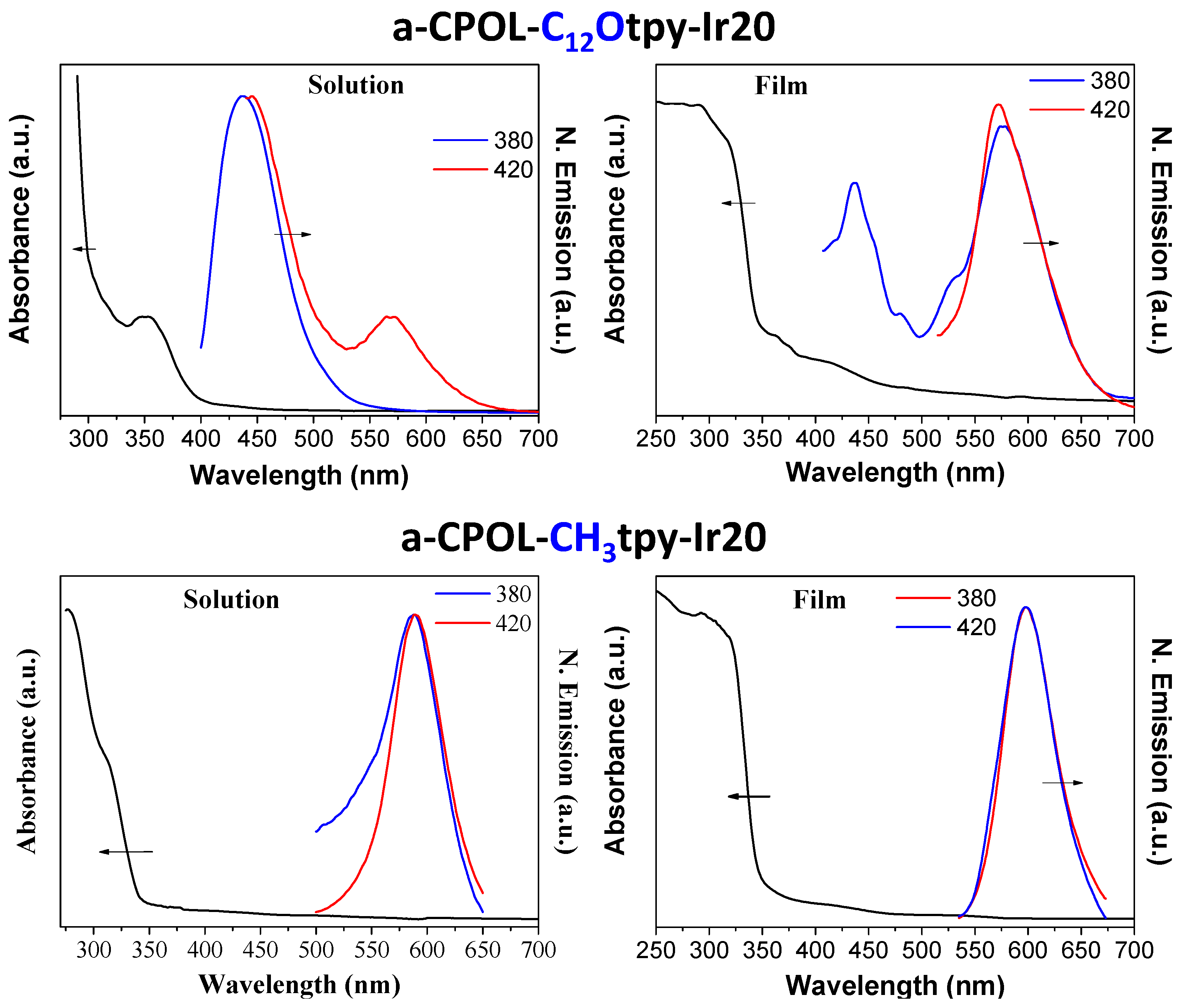
3.3. Photophysical Properties
3.4. Electrochemical Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Baldo, M.A.; You, Y.; O’Brien, D.F.; Shoustikov, A.; Sibley, S.; Thompson, M.E.; Forrest, S.R. Highly efficient phosphorescent emission from organic electroluminescent devices. Nature 1998, 395, 151. [Google Scholar] [CrossRef]
- Ma, D.; Tsuboi, T.; Qiu, Y.; Duan, L. Recent progress in ionic iridium(III) complexes for organic electronic devices. Adv. Mater. 2017, 29. [Google Scholar] [CrossRef] [PubMed]
- Mao, H.T.; Li, G.F.; Shan, G.G.; Wang, X.L.; Su, Z.M. Recent progress in phosphorescent Ir(III) complexes for nondoped organic light-emitting diodes. Coord. Chem. Rev. 2020, 413, 213283. [Google Scholar] [CrossRef]
- Henwood, A.F.; Zysman-Colman, E. Luminescent iridium complexes used in light-emitting electrochemical cells (LEECs). Top. Curr. Chem. 2016, 374, 1–41. [Google Scholar] [CrossRef] [Green Version]
- Namanga, J.E.; Pei, H.; Bousrez, G.; Mallick, B.; Smetana, V.; Gerlitzki, N.; Mudring, A.V. Efficient and long lived green light-emitting electrochemical cells. Adv. Funct. Mater. 2020, 30. [Google Scholar] [CrossRef]
- Caporale, C.; Massi, M. Cyclometalated iridium(III) complexes for life science. Coord. Chem. Rev. 2018, 363, 71–91. [Google Scholar] [CrossRef] [Green Version]
- Glaser, F.; Wenger, O.S. Recent progress in the development of transition-metal based photoredox catalysts. Coord. Chem. Rev. 2020, 405, 213129. [Google Scholar] [CrossRef]
- Wang, T.; Sun, R.; Shi, M.; Pan, F.; Hu, Z.; Huang, F.; Li, Y.; Min, J. Solution-processed polymer solar cells with over 17% efficiency enabled by an iridium complexation approach. Adv. Energy Mater. 2020, 10, 1–9. [Google Scholar] [CrossRef]
- Housecroft, C.E. Iridium: Inorganic & coordination chemistry. In Encyclopedia of Inorganic Chemistry; King, R.B., Crabtree, R.H., Lukehart, C.M., Atwood, D.A., Scott, R.A., Eds.; John Wiley & Sons, Ltd: Hoboken, NJ, USA, 2006; Volume 6, pp. 1–18. [Google Scholar]
- Yang, H.; Meng, G.; Zhou, Y.; Tang, H.; Zhao, J.; Wang, Z. The photoluminescent properties of new cationic iridium(III) complexes using different anions and their applications in white light-emitting diodes. Materials 2015, 8, 6105–6116. [Google Scholar] [CrossRef] [Green Version]
- Tang, H.; Meng, G.; Chen, Z.; Wang, K.; Zhou, Q.; Wang, Z. Warm white light-emitting diodes based on a novel orange cationic iridium(III) complex. Materials 2017, 10, 657. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Zhang, H.; Zhang, B.; Xie, Z.; Wong, W.Y. Towards high-power-efficiency solution-processed OLEDs: Material and device perspectives. Mater. Sci. Eng. R Rep. 2020, 140, 100547. [Google Scholar] [CrossRef]
- Adeloye, A.O. Exploration of the structural and photophysical characteristics of mono- and binuclear Ir (III) cyclometalated complexes for optoelectronic applications. Materials 2019, 12, 2734. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baranoff, E.; Curchod, B.F.E. FIrpic: Archetypal blue phosphorescent emitter for electroluminescence. Dalt. Trans. 2015, 44, 8318–8329. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Li, S.; Sun, Q.; Huang, C.; Fung, M.K. Highly efficient white organic light-emitting diodes with ultrathin emissive layers and a spacer-free structure. Sci. Rep. 2016, 6, 4–11. [Google Scholar] [CrossRef]
- Collin, J.P.; Dixon, I.M.; Sauvage, J.P.; Williams, J.A.G.; Barigelletti, F.; Flamigni, L. Synthesis and photophysical properties of iridium(III) bisterpyridine and its homologues: A family of complexes with a long-lived excited state. J. Am. Chem. Soc. 1999, 121, 5009–5016. [Google Scholar] [CrossRef]
- Polson, M.; Fracasso, S.; Bertolasi, V.; Ravaglia, M.; Scandola, F. Iridium cyclometalated complexes with axial symmetry. Synthesis and photophysical properties of a trans-biscyclometalated complex containing the terdentate ligand 2,6-diphenylpyridine. Inorg. Chem. 2004, 43, 1950–1956. [Google Scholar] [CrossRef]
- Williams, J.A.G.; Wilkinson, A.J.; Whittle, V.L. Light-emitting iridium complexes with tridentate ligands. J. Chem. Soc. Dalt. Trans. 2008, 2081–2099. [Google Scholar] [CrossRef]
- Chi, Y.; Chang, T.K.; Ganesan, P.; Rajakannu, P. Emissive bis-tridentate Ir(III) metal complexes: Tactics, photophysics and applications. Coord. Chem. Rev. 2017, 346, 91–100. [Google Scholar] [CrossRef]
- Goodall, W.; Williams, J.A.G. Iridium(III) bis-terpyridine complexes incorporating pendent YV-methylpyridinium groups: Luminescent sensors for chloride ions. J. Chem. Soc. Dalt. Trans. 2000, 2893–2895. [Google Scholar] [CrossRef]
- Whittle, V.L.; Williams, J.A.G. A new class of iridium complexes suitable for stepwise incorporation into linear assemblies: Synthesis, electrochemistry, and luminescence. Inorg. Chem. 2008, 47, 6596–6607. [Google Scholar] [CrossRef]
- Agosti, A.; Kuna, E.; Bergamini, G. Divergent terpyridine-based coordination for the construction of photoactive supramolecular structures. Eur. J. Inorg. Chem. 2019, 2019, 577–584. [Google Scholar] [CrossRef] [Green Version]
- Liu, B.; Monro, S.; Li, Z.; Jabed, M.A.; Ramirez, D.; Cameron, C.G.; Colón, K.; Roque, J.; Kilina, S.; Tian, J.; et al. New class of homoleptic and heteroleptic bis(terpyridine) iridium(III) complexes with strong photodynamic therapy effects. ACS Appl. Bio Mater. 2019, 2, 2964–2977. [Google Scholar] [CrossRef] [PubMed]
- Darmawan, N.; Yang, C.H.; Mauro, M.; Raynal, M.; Heun, S.; Pan, J.; Buchholz, H.; Braunstein, P.; de Cola, L. Efficient near-UV emitters based on cationic bis-pincer iridium(III) carbene complexes. Inorg. Chem. 2013, 52, 10756–10765. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Chau, N.Y.; Liao, J.L.; Wong, W.Y.; Lu, C.Y.; Sie, Z.T.; Chang, C.H.; Fox, M.A.; Low, P.J.; Lee, G.H.; et al. Bis-tridentate iridium(III) phosphors bearing functional 2-phenyl-6-(imidazol-2-ylidene)pyridine and 2-(pyrazol-3-Yl)-6-phenylpyridine chelates for efficient OLEDs. Organometallics 2016, 35, 1813–1824. [Google Scholar] [CrossRef] [Green Version]
- Kuo, H.H.; Chen, Y.T.; Devereux, L.R.; Wu, C.C.; Fox, M.A.; Kuei, C.Y.; Chi, Y.; Lee, G.H. Bis-tridentate Ir(III) metal phosphors for efficient deep-blue organic light-emitting diodes. Adv. Mater. 2017, 29, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.K.; Kuo, H.H.; Luo, D.; Lai, Y.N.; Li, W.C.; Chang, C.H.; Escudero, D.; Jen, A.K.Y.; Hsu, L.Y.; Chi, Y. Phenyl-A Nd pyrazolyl-functionalized pyrimidine: Versatile chromophore of bis-tridentate Ir(III) phosphors for organic light-emitting diodes. Chem. Mater. 2019, 31, 6453–6464. [Google Scholar] [CrossRef]
- Wilkinson, A.J.; Goeta, A.E.; Foster, C.E.; Williams, J.A.G. Synthesis and luminescence of a charge-neutral, cyclometalated iridium(III) complex containing N/\C/\N- and C/\N/\C-coordinating terdentate ligands. Inorg. Chem. 2004, 43, 6513–6515. [Google Scholar] [CrossRef]
- Adamovich, V.; Boudreault, P.L.T.; Esteruelas, M.A.; Gómez-Bautista, D.; López, A.M.; Oñate, E.; Tsai, J.Y. Preparation via a NHC dimer complex, photophysical properties, and device performance of heteroleptic bis(tridentate) iridium(III) emitters. Organometallics 2019, 38, 2738–2747. [Google Scholar] [CrossRef]
- Hu, Y.Y.; Luo, W.; Wang, Y.; Tong, B.H.; Fung, M.K.; Zhang, Q.F. Efficient yellow OLEDs based on bis-tridentate iridium(III) complexes with two C∧N∧N-coordinating ligands. Inorg. Chim. Acta 2020, 499, 119168. [Google Scholar] [CrossRef]
- Kuo, H.H.; Zhu, Z.; Lee, C.S.; Chen, Y.K.; Liu, S.H.; Chou, P.T.; Jen, A.K.Y.; Chi, Y. Bis-tridentate iridium(III) phosphors with very high photostability and fabrication of blue-emitting OLEDs. Adv. Sci. 2018, 5, 1–7. [Google Scholar] [CrossRef]
- Kuei, C.Y.; Tsai, W.L.; Tong, B.; Jiao, M.; Lee, W.K.; Chi, Y.; Wu, C.C.; Liu, S.H.; Lee, G.H.; Chou, P.T. Bis-tridentate Ir(III) complexes with nearly unitary RGB phosphorescence and organic light-emitting diodes with external quantum efficiency exceeding 31%. Adv. Mater. 2016, 28, 2795–2800. [Google Scholar] [CrossRef] [PubMed]
- Andreopoulou, A.K.; Gioti, M.; Kallitsis, J.K. Organic light-emitting diodes based on solution-processable organic materials. In Solution-Processable Components for Organic Electronic Devices; Ulanski, J., Luszczynska, M., Matyjaszewski, K., Eds.; Wiley-VCH Verlag GmbH & Co.: Weinheim, Germany, 2019. [Google Scholar] [CrossRef]
- Chen, X.; Liao, J.L.; Liang, Y.; Ahmed, M.O.; Tseng, H.E.; Chen, S.A. High-efficiency red-light emission from polyfluorenes grafted with cyclometalated iridium complexes and charge transport moiety. J. Am. Chem. Soc. 2003, 125, 636–637. [Google Scholar] [CrossRef] [PubMed]
- Baschieri, A.; Monti, F.; Armaroli, N.; Mazzotti, G.; Giorgini, L.; Sambri, L.; Benelli, T. Luminescent methacrylic copolymers with side-chain cyclometalated iridium(III) complexes. Dye. Pigment. 2019, 160, 188–197. [Google Scholar] [CrossRef]
- Xu, F.; Kim, H.U.; Kim, J.H.; Jung, B.J.; Grimsdale, A.C.; Hwang, D.H. Progress and perspective of iridium-containing phosphorescent polymers for light-emitting diodes. In Progress in Polymer Science; Elsevier Ltd.: Amsterdam, The Netherlands, 2015; pp. 92–121. [Google Scholar] [CrossRef]
- Tang, H.; Dong, X.; Chen, M.; Chen, Q.; Ren, M.; Wang, K.; Zhou, Q.; Wang, Z. A novel polymethyl methacrylate derivative grafted with cationic iridium(III) complex units: Synthesis and application in white light-emitting diodes. Polymers 2019, 11, 499. [Google Scholar] [CrossRef] [Green Version]
- Page, Z.A.; Chiu, C.Y.; Narupai, B.; Laitar, D.S.; Mukhopadhyay, S.; Sokolov, A.; Hudson, Z.M.; Bou Zerdan, R.; McGrath, A.J.; Kramer, J.W.; et al. Highly photoluminescent nonconjugated polymers for single-layer light emitting diodes. ACS Photonics 2017, 4, 631–641. [Google Scholar] [CrossRef]
- Liu, B.; Dang, F.; Tian, Z.; Feng, Z.; Jin, D. High triplet energy-level achieved by tuning the arrangement of building blocks in phosphorescent polymer backbones for furnishing high electroluminescent performances in both blue and white organic light-emitting devices high triplet energy-level achieve. ACS Appl. Mater. Interfaces 2017, 9, 16360–16374. [Google Scholar] [CrossRef]
- Aamer, K.A.; Tew, G.N. Synthesis, dynamic light scattering, and luminescence properties of copolymers containing iridium(III) bisterpyridine in the side chain. J. Polym. Sci. Part A Polym. Chem. 2007, 45, 1109–1121. [Google Scholar] [CrossRef] [Green Version]
- Zhen, H.; Jiang, C.; Yang, W.; Jiang, J.; Huang, F.; Cao, Y. Synthesis and properties of electrophosphorescent chelating polymers with iridium complexes in the conjugated backbone. Chem. A Eur. J. 2005, 11, 5007–5016. [Google Scholar] [CrossRef]
- Schulz, G.L.; Chen, X.; Chen, S.A.; Holdcroft, S. Enhancement of phosphorescence of Ir complexes bound to conjugated polymers: Increasing the triplet level of the main chain. Macromolecules 2006, 39, 9157–9165. [Google Scholar] [CrossRef]
- Liu, J.; Pei, Q. Electrophosphorescent polymers for high-efficiency light-emitting diodes. Curr. Org. Chem. 2010, 14, 2133–2144. [Google Scholar] [CrossRef]
- Langecker, J.; Rehahn, M. Iridium-functionalized polyfluorenes: Advantages and limitations of Thes Suzuki and Yamamoto approaches. Macromol. Chem. Phys. 2008, 209, 258–271. [Google Scholar] [CrossRef]
- Huang, S.P.; Jen, T.H.; Chen, Y.C.; Hsiao, A.E.; Yin, S.H.; Chen, H.Y.; Chen, S.A. Effective shielding of triplet energy transfer to conjugated polymer by its dense side chains from phosphor dopant for highly efficient electrophosphorescence. J. Am. Chem. Soc. 2008, 130, 4699–4707. [Google Scholar] [CrossRef] [PubMed]
- Hammond, M.R.; Andreopoulou, A.K.; Pefkianakis, E.; Kallitsis, J.K.; Mezzenga, R. Metallosupramolecular side-chain polymers and polyelectrolyte- metallosupramolecular surfactant complexes. Chem. Mater. 2009, 21, 2169–2172. [Google Scholar] [CrossRef] [Green Version]
- Gourdoupi, N.; Andreopoulou, A.K.; Deimede, V.; Kallitsis, J.K. Novel proton-conducting polyelectrolyte composed of an aromatic polyether containing main-chain pyridine units for fuel cell applications. Chem. Mater. 2003, 15, 5044–5050. [Google Scholar] [CrossRef]
- Koromilas, N.D.; Anastasopoulos, C.; Oikonomou, E.K.; Kallitsis, J.K. Preparation of porous polymeric membranes based on a pyridine containing aromatic polyether sulfone. Polymers 2019, 11, 59. [Google Scholar] [CrossRef] [Green Version]
- Heller, M.; Schubert, U.S. Syntheses of functionalized 2,2′:6′,2″-terpyridines. Eur. J. Org. Chem. 2003, 947–961. [Google Scholar] [CrossRef]
- Constable, E.C.; Harverson, P.; Smith, D.R.; Whall, L. The coordination chemistry of 4′-(4-tert-butylphenyl)-2,2′:6′,2″-terpyridine—A solubilising oligopyridine. Polyhedron 1997, 16, 3615–3623. [Google Scholar] [CrossRef]
- Andreopoulou, A.K.; Kallitsis, J.K. An “Attachment through coordination” approach to side chain dendritic polymers. European J. Org. Chem. 2005, 4448–4458. [Google Scholar] [CrossRef]
- Kallitsis, J.K.; Andreopoulou, A.K.; Daletou, M.; Neophytides, S. Pyridine Containing Aromatic Polyether Membranes; Li, Q., Aili, D., Hjuler, H., Jensen, J., Eds.; Springer: Cham, Switzerland, 2016. [Google Scholar] [CrossRef] [Green Version]
- Sasabe, H.; Seino, Y.; Kimura, M.; Kido, J. A m-terphenyl-modifed sulfone derivative as a host material for high-efficiency blue and green phosphorescent OLEDs. Chem. Mater. 2012, 24, 1404–1406. [Google Scholar] [CrossRef]
- Dixon, I.M.; Collin, J.P.; Sauvage, J.P.; Flamigni, L.; Encinas Perea, S.; Barigelletti, F. A family of luminescent coordination compounds: Iridium(III) polyimine complexes. Chem. Soc. Rev. 2000, 29, 385–391. [Google Scholar] [CrossRef]

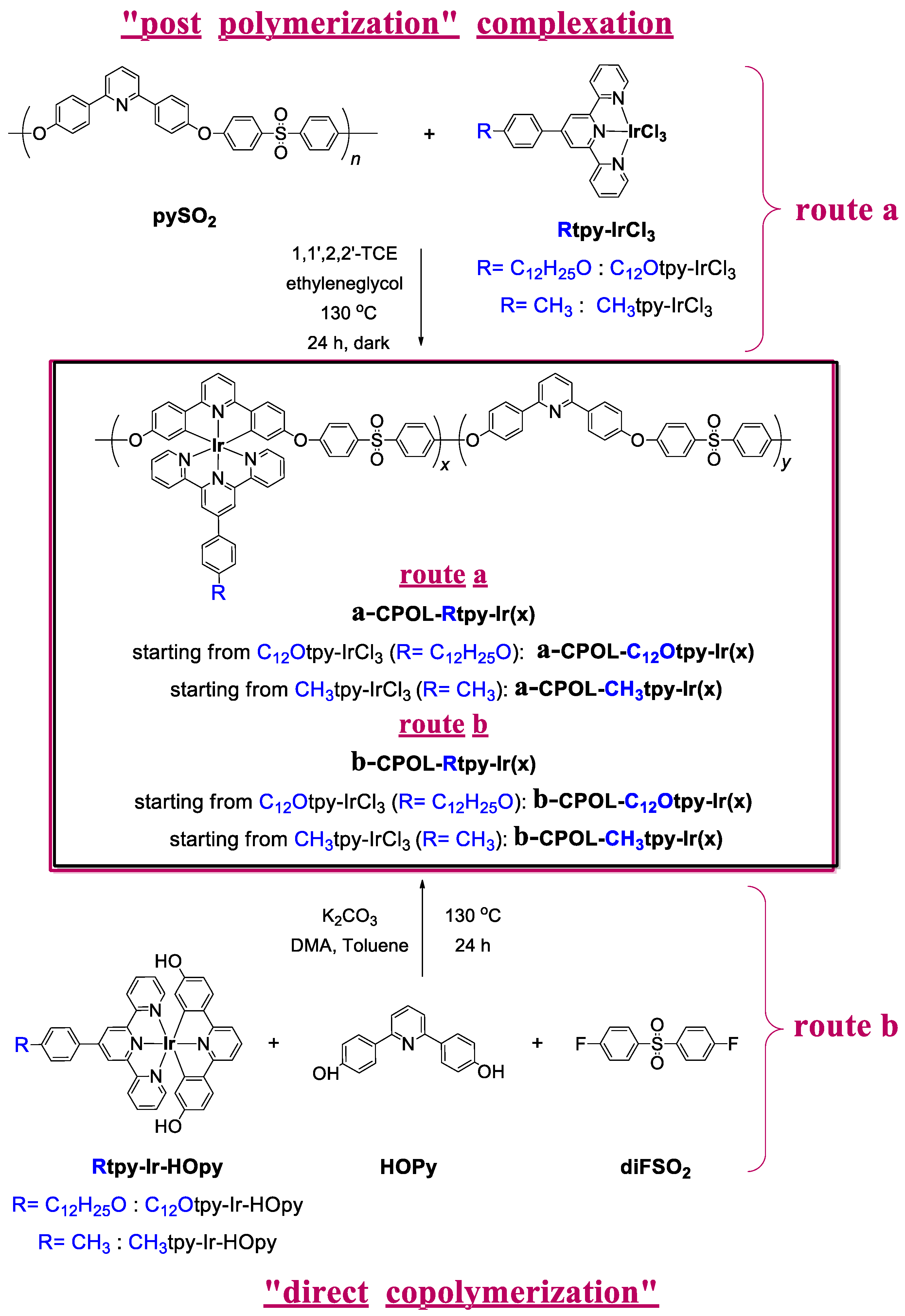






| Code | Ir (%) a | Synthetic Method | Solubility b,c | |
|---|---|---|---|---|
| “Post-Polymerization” Complexation a-CPOL-Rtpy-Ir(x) | ||||
| a-CPOL-C12Otpy-Ir5 | 5 | Post-complexation | TCE | ✓✓✓ |
| a-CPOL-C12Otpy-Ir20 | 20 | Post-complexation | TCE | ✓✓✓ |
| a-CPOL-C12Otpy-Ir50 | 50 | Post-complexation | CHCl3 | ✓ |
| DMSO, NMP | ✓✓ | |||
| DMF, DMA, TCE | ✓✓✓ | |||
| a-CPOL-CH3tpy-Ir5 | 5 | Post-complexation | TCE | |
| a-CPOL-CH3tpy-Ir20 | 20 | Post-complexation | CHCl3 | ✓ |
| DMSO, NMP | ✓✓ | |||
| DMF, DMA, TCE | ✓✓✓ | |||
| a-CPOL-CH3tpy-Ir50 | 50 | Post-complexation | CHCl3 | ✓ |
| DMSO, NMP | ✓✓ | |||
| DMF, DMA, TCE | ✓✓✓ | |||
| “Direct copolymerization” b-CPOL-Rtpy-Ir(x) | ||||
| b-CPOL-C12Otpy-Ir5 | 5 | Co-polymerization | TCE | ✓✓✓ |
| b-CPOL-C12Otpy-Ir25 | 25 | Co-polymerization | CHCl3 | ✓ |
| DMSO, NMP | ✓✓ | |||
| DMF, DMA, TCE | ✓✓✓ | |||
| b-CPOL-C12Otpy-Ir50 | 50 | Co-polymerization | CHCl3, | ✓ |
| NMP, DMSO | ✓✓ | |||
| TCE, DMF, DMA | ✓✓✓ | |||
| b-CPOL-CH3tpy-Ir5 | 5 | Co-polymerization | TCE | ✓✓✓ |
| b-CPOL-CH3tpy-Ir20 | 20 | Co-polymerization | CHCl3, | ✓ |
| NMP, DMSO | ✓✓ | |||
| TCE, DMF, DMA | ✓✓✓ | |||
| b-CPOL-CH3tpy-Ir50 | 50 | Co-polymerization | CHCl3, | ✓ |
| NMP, DMSO | ✓✓ | |||
| TCE, DMF, DMA | ✓✓✓ | |||
| Polymer | Mn b | Mw b | PD b |
|---|---|---|---|
| pySO2 | 16,400 | 27,500 | 1.7 |
| a-CPOL-C12Otpy-Ir5 | - | - | - |
| a-CPOL-C12Otpy-Ir20 | 15,640 | 30,470 | 1.9 |
| a-CPOL-C12Otpy-Ir50 | 4070 | 6660 | 1.6 |
| b-CPOL-C12Otpy-Ir5 | 7400 | 17,330 | 2.3 |
| b-CPOL-C12Otpy-Ir20 | 4360 | 6790 | 1.6 |
| b-CPOL-C12Otpy-Ir50 | 13,900 | 32,000 | 2.3 |
| a-CPOL-CH3tpy-Ir5 | 11,480 | 25,370 | 2.2 |
| a-CPOL-CH3tpy-Ir20 | 11,100 | 19,550 | 1.8 |
| a-CPOL-CH3tpy-Ir50 | 7200 | 9500 | 1.3 |
| b-CPOL-CH3tpy-Ir5 | 9230 | 17,100 | 1.8 |
| b-CPOL-CH3tpy-Ir20 | 7780 | 12,130 | 1.6 |
| b-CPOL-CH3tpy-Ir50 | 4600 | 6600 | 1.5 |
| Compound | Absorption (nm) | Emission (nm) | Medium |
|---|---|---|---|
| HOtpy-IrCl3 | 287, 327, 401, and 490 | 566 | DMF |
| HOtpy-Ir-HOpy | 288, 328, 415, and 523 | 590 | DMF |
| C12Otpy-IrCl3 | 287, 332, 410, 494, and 526 | 589 | DMF |
| C12Otpy-Ir-HOpy | 285, 312 (shoulder), 418, 457, and 525 | 594 | DMF |
| CH3tpy-IrCl3 | 282, 317 (shoulder), 418, and 520 | 592 | DMF |
| CH3tpy-Ir-HOpy | 285, 317, and 419 | 592 and 705 | DMF |
| pySO2 | |||
| a-CPOL-C12Otpy-Ir20 | 353 | 437 and 571 | TCE |
| a-CPOL-C12Otpy-Ir20 | 290 and 415 | 438 and 573 | Film |
| a-CPOL-CH3tpy-Ir20 | 278 and 311 | 590 | TCE |
| a-CPOL-CH3tpy-Ir20 | 237 and 293 | 600 | Film |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andrikopoulos, K.; Anastasopoulos, C.; Kallitsis, J.K.; Andreopoulou, A.K. Bis-Tridendate Ir(III) Polymer-Metallocomplexes: Hybrid, Main-Chain Polymer Phosphors for Orange–Red Light Emission. Polymers 2020, 12, 2976. https://doi.org/10.3390/polym12122976
Andrikopoulos K, Anastasopoulos C, Kallitsis JK, Andreopoulou AK. Bis-Tridendate Ir(III) Polymer-Metallocomplexes: Hybrid, Main-Chain Polymer Phosphors for Orange–Red Light Emission. Polymers. 2020; 12(12):2976. https://doi.org/10.3390/polym12122976
Chicago/Turabian StyleAndrikopoulos, Konstantinos, Charalampos Anastasopoulos, Joannis K. Kallitsis, and Aikaterini K. Andreopoulou. 2020. "Bis-Tridendate Ir(III) Polymer-Metallocomplexes: Hybrid, Main-Chain Polymer Phosphors for Orange–Red Light Emission" Polymers 12, no. 12: 2976. https://doi.org/10.3390/polym12122976