High Efficiency Gas Permeability Membranes from Ethyl Cellulose Grafted with Ionic Liquids
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
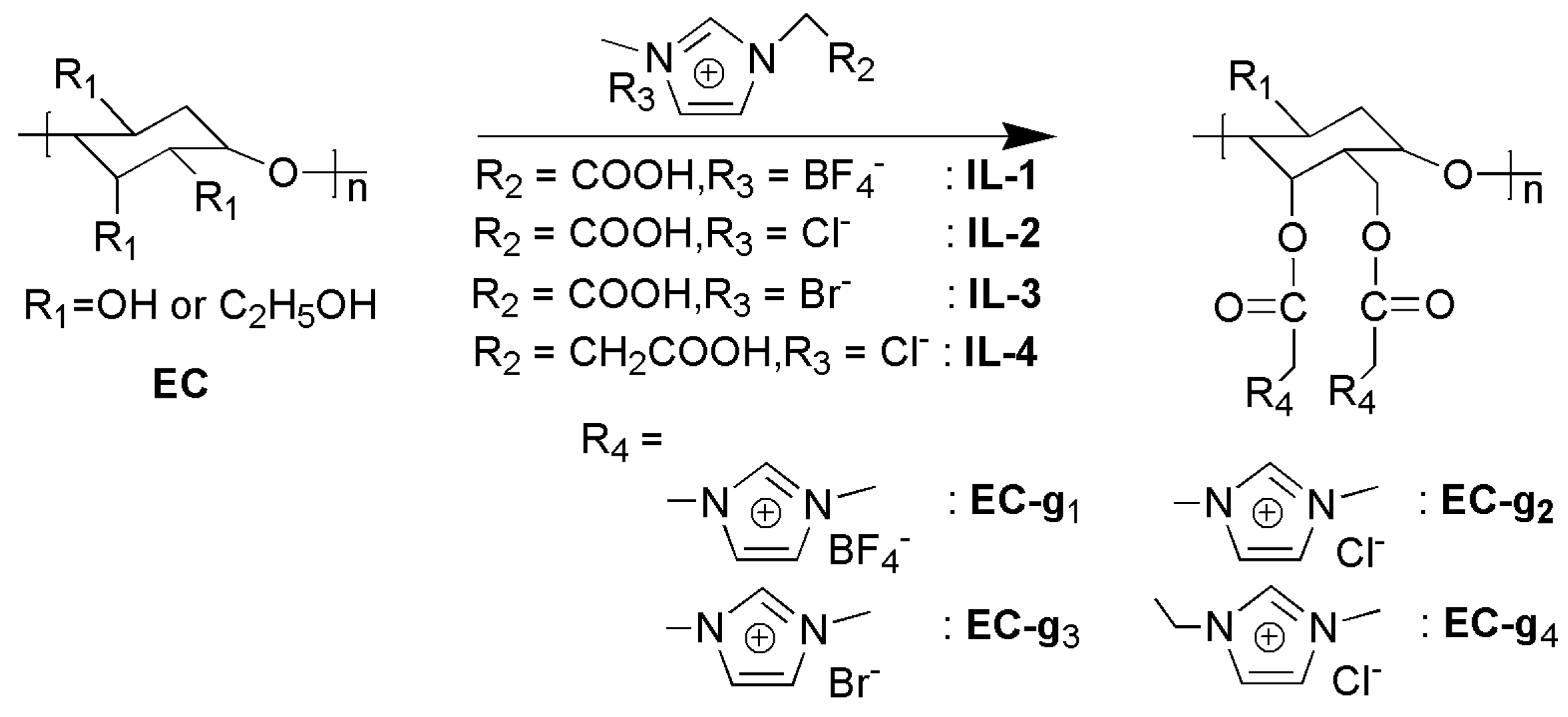
2.2. Preparation of Ethyl Cellulose Grafted with 1-Carboxymethyl-3-Methylimidazolium Tetrafluoroborate (EC-g1)
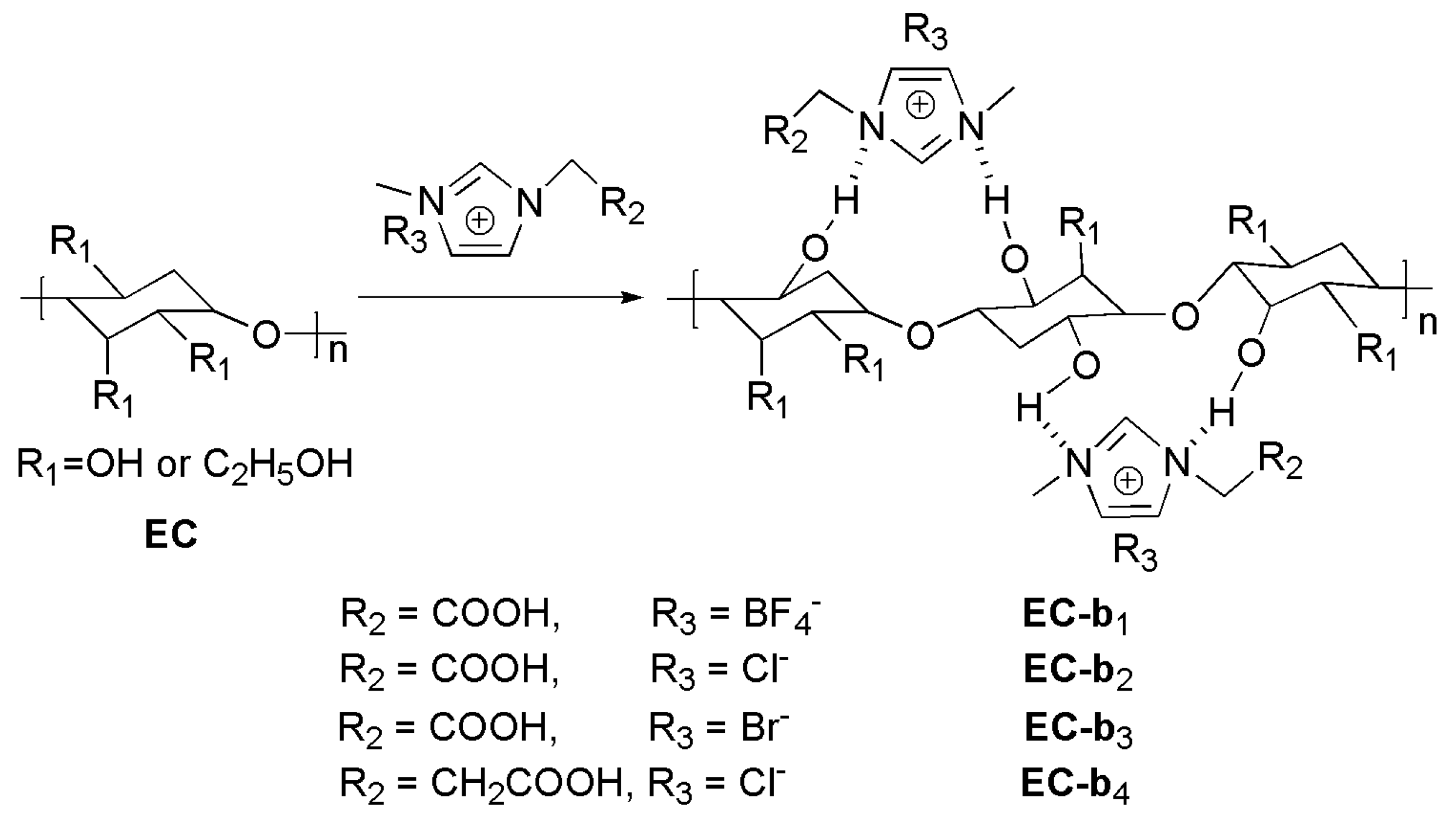
2.3. Preparation of Ethyl Cellulose Blended Ionic Liquids (EC-b1– EC-b4)
2.4. Preparation of Gas Separation Membranes and Mechanical Testing
2.5. Measurements and Calculations of the Gas Permeability Coefficient and the Separation Factor
2.6. Analysis of the Grafting Degree in EC
3. Instruments
4. Results and discussion
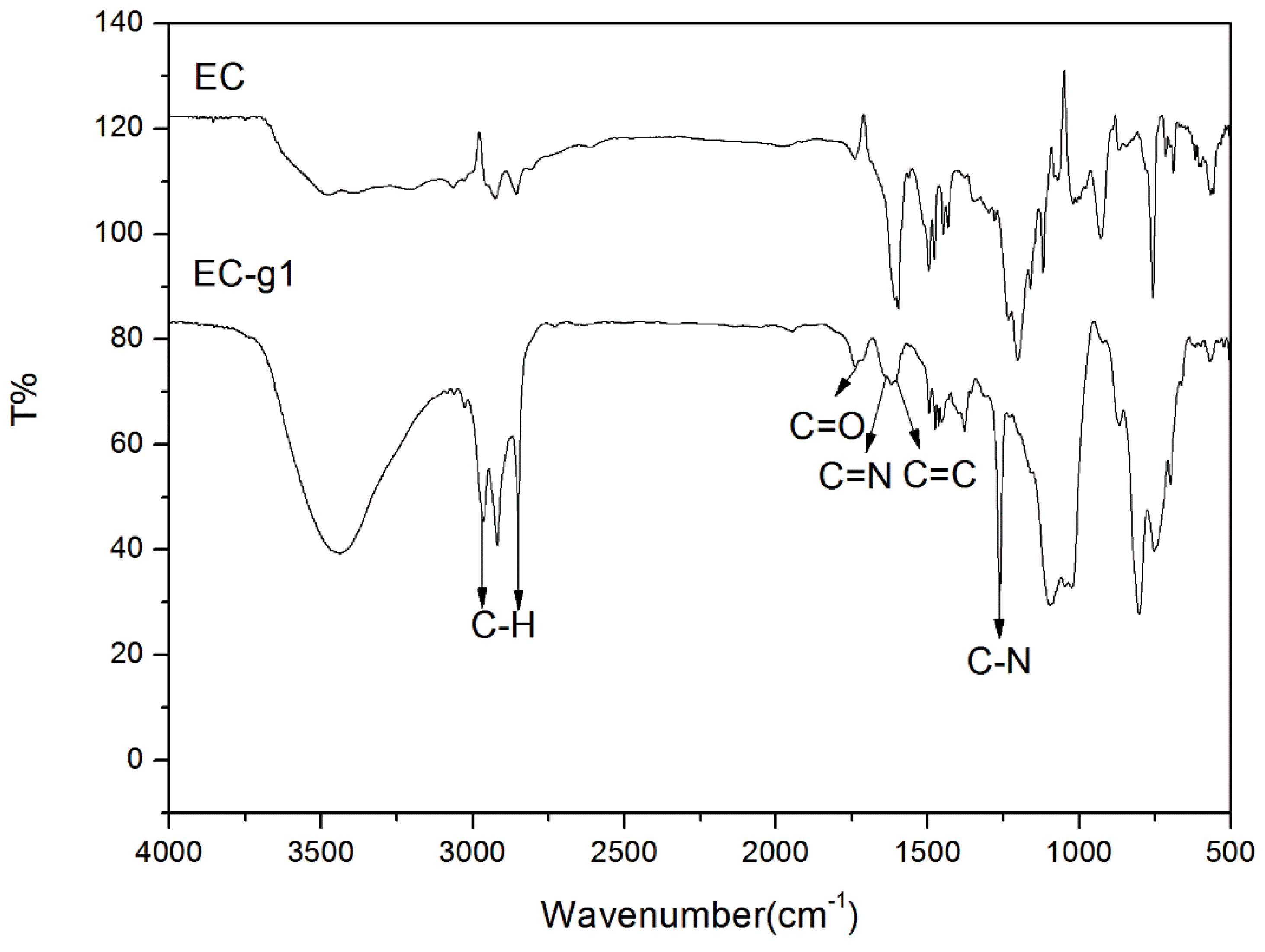
4.1. The Esterification of Ethyl Cellulose
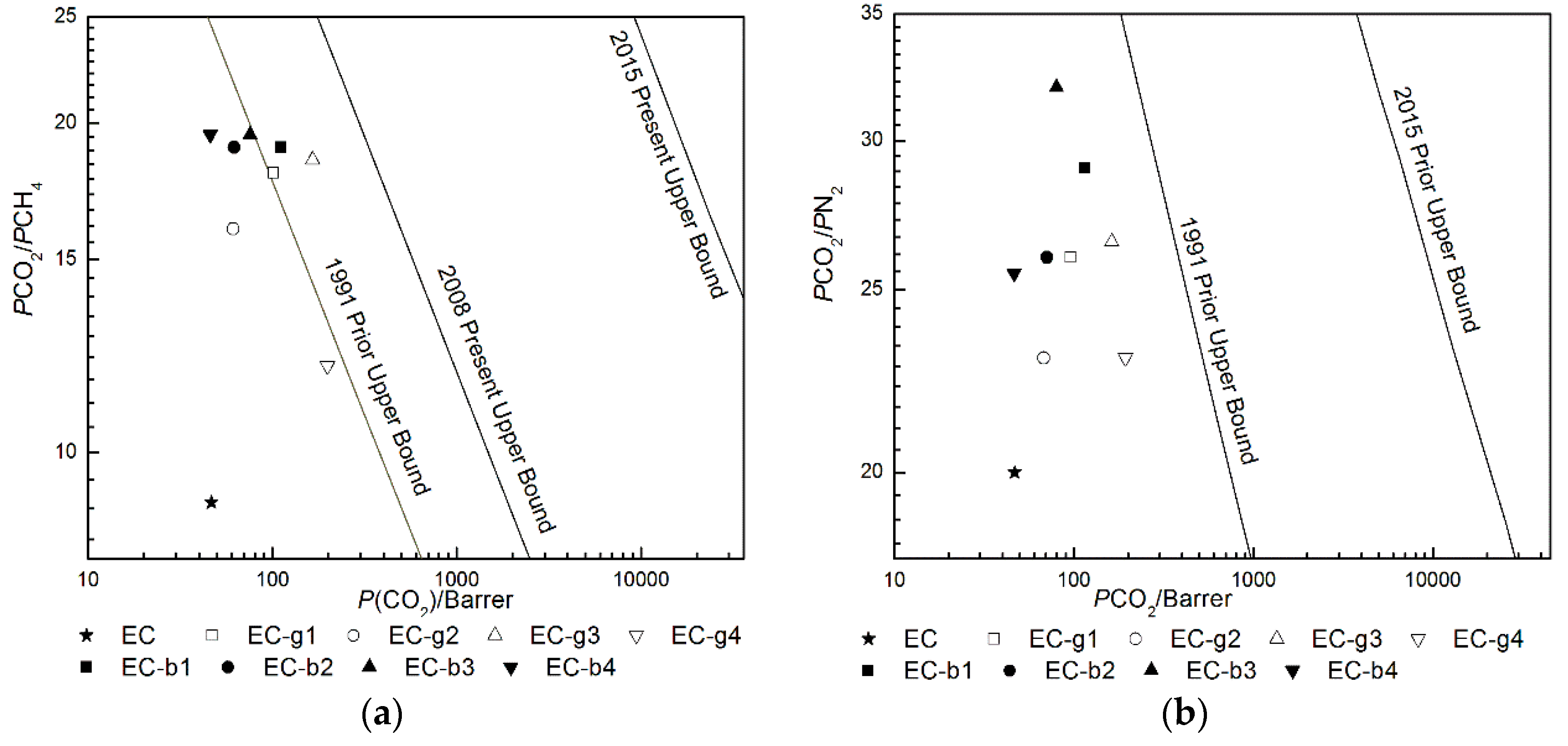
4.2. Gas Permeation Properties of Membranes
4.3. Mechanical Properties of Polymer Membranes
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Klemm, D.; Heublein, B.; Fink, H.; Bohn, A. Cellulose: Fascinating biopolymer and sustainable raw materia. Angew. Chem. Int. Edit. 2005, 36, 3358–3393. [Google Scholar] [CrossRef] [PubMed]
- Houde, A.Y.; Stern, S.A. Solubility and diffusivity of light gases in ethyl cellulose at elevated pressures Effects of ethoxy content. J. Membr. Sci. 1997, 127, 171–183. [Google Scholar] [CrossRef]
- Choi, S.H.; Tasselli, F.; Jansen, J.C. Effect of the preparation conditions on the formation of asymmetric poly (vinylidene fluoride) hollow fibre membranes with a dense skin. Eur. Polym. J. 2010, 46, 1713–1725. [Google Scholar] [CrossRef]
- Shen, Y.; Wang, H.; Liu, J.D. Enhanced performance of a novel polyvinyl amine/chitosan/graphene oxide mixed matrix membrane for CO2 capture. ACS Sustain. Chem. Eng. 2015, 3, 1819–1829. [Google Scholar] [CrossRef]
- Yampolskii, Y.; Alentiev, A.; Bondarenko, G. Intermolecular interactions: New way to govern transport properties of membrane materials. Ind. Eng. Chem. Res. 2010, 49, 12031–12037. [Google Scholar] [CrossRef]
- Li, H.; Huang, Q.; Li, D. The generation of a molecular imprinted membrane by coating cellulose acetate onto a ZrO2-modified alumina membrane for the chiral separation of mandelic acid enantiomers. Org. Process Res. Dev. 2018, 22, 278–285. [Google Scholar] [CrossRef]
- Veronika, V.; Ciahotny, K. Upgrading biogas to biomethane using membrane separation. Energy Fuel. 2017, 31, 9393–9401. [Google Scholar]
- Coombs Obrien, J.; Torrente-Murciano, L.; Mattia, D. Continuous production of cellulose microbeads via membrane emulsification. ACS Sustain. Chem. Eng. 2017, 5, 5931–5939. [Google Scholar] [CrossRef]
- Sirkar, K.K.; Shanbhag, P.V.; Kovvali, A.S. Membrane in a reactor: a functional perspective. Ind. Eng. Chem. Res. 1999, 38, 3715–3737. [Google Scholar] [CrossRef]
- Josephine, O.M.; Kenneth, B.; John, F. Molecular sieving realized with ZIF-8/matrimid mixed-matrix membranes. J. Membr. Sci. 2010, 361, 28–37. [Google Scholar]
- Adams, R.; Carson, C.; Ward, J. Metal organic framework mixed matrix membranes for gas separations. Microporous Mesoporous Mater. 2010, 31, 13–20. [Google Scholar] [CrossRef]
- Lin, H.Q.; Freeman, B.D. Materials selection guidelines for membranes that remove CO2 from gas mixtures. J. Mol. Struct. 2005, 3, 57–74. [Google Scholar] [CrossRef]
- Murtaza, G. Ethylcellulose microparticles: A review. Acta Pol. Pharm. 2012, 69, 11–22. [Google Scholar] [PubMed]
- Xing, Q.; Zhao, F.; Chen, S.M. Porous biocompatible three-dimensional scaffolds of cellulose microfiber/gelatin composites for cell culture. Acta Biomater. 2010, 6, 2132–2139. [Google Scholar] [CrossRef]
- MoonR, J.; Martini, A.; Nairn, J. Cellulose nanomaterials review: Structure, properties and nanocomposites. Chem. Soc. Rev. 2011, 40, 3941–3994. [Google Scholar] [CrossRef]
- Davidovich-Pinhas, M.; Barbut, S.; Marangoni, A.G. Physical structure and thermal behavior of ethylcellulose. Cellulose 2014, 21, 3243–3255. [Google Scholar] [CrossRef]
- Wang, Y.; Ma, X.; Ghanem, B.S. Polymers of intrinsic microporosity for energy-intensive membrane-based gas separations. Mater. Today Nano 2018, 3, 69–95. [Google Scholar] [CrossRef]
- Lu, H.; Wang, Q.; Li, G. Electrospun water-stable zein/ethyl cellulose composite nanofiber and its drug release properties. Mat. Sci. Eng. C-Mater. 2017, 74, 86–93. [Google Scholar] [CrossRef]
- Khan, F.Z.; Sakaguchi, T.; Shiotsuki, M. Synthesis, characterization, and gas permeation properties of silylated derivatives of ethyl cellulose. Macromolecules 2006, 39, 6025–6030. [Google Scholar] [CrossRef]
- Li, X.G.; Kresse, I. Jürgen springer, morphology and gas permselectivity of blend membranes of polyvinylpyridine with ethylcellulose. Polymer 2001, 16, 6859–6869. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, H.; Zhang, Q. Thermotropic liquid crystallinity, thermal decomposition behavior, and aggregated structure of poly (propylene carbonate)/ethyl cellulose blends. J. Appl. Polym. Sci. 2006, 100, 584–592. [Google Scholar] [CrossRef]
- Hu, Q. Poly (amide-imide)/TiO2 nano-composite gas separation membranes: Fabrication and characterization. J. Membr. Sci. 1997, 135, 65–79. [Google Scholar] [CrossRef]
- Moaddeb, M.; Koros, W.J. Gas transport properties of thin polymeric membranes in the presence of silicon dioxide particles. J. Membr. Sci. 1997, 125, 143–163. [Google Scholar] [CrossRef]
- Sablok, A.; Jindal, R. Preparation and applications of room temperature ionic liquids in organic synthesis: a review on recent efforts. Curr. Green Chem. 2015, 2, 135–155. [Google Scholar]
- Andreatta, G.; Lee, L.T.; Lee, F.K. Gas permeability in polymer and surfactant-stabilized bubble films. J. Phys. Chem. B 2006, 11, 19537–19542. [Google Scholar] [CrossRef]
- Nikolaeva, D. The performance of affordable and stable cellulose-based poly-ionic membranes in CO2/N2 and CO2/CH4 gas separation. J. Membr. Sci. 2018, 564, 552–561. [Google Scholar] [CrossRef]
- Wang, J.; Zang, Y.; Yin, G. Facile synthesis of five 2D surface modifiers by highly selective photocyclic aromatization and efficient enhancement of oxygen permselectivities of three polymer membranes by surface modification using a small amount of the 2D surface modifiers. Polymer 2014, 55, 1384–1396. [Google Scholar] [CrossRef]
- Fragaa, S.; Monteleoneb, M. A novel time lag method for the analysis of mixed gas diffusion in polymeric membranes by on-line mass spectrometry: Method development and validation. J. Membr. Sci. 2018, 561, 39–58. [Google Scholar] [CrossRef]
- Beckman, I.; Shalygin, M.; Tepliakov, V. Particularities of membrane gas separation under unsteady state conditions. Mass Transfer in Chemical Engineering Processes; InTech: Rijeka, Croatia, 2011; Volume 1, pp. 205–232. [Google Scholar]
- Li, Y.; Sun, Y.; Deng, X. Graft polymerization of acrylic acid onto polyphenylene sulfide nonwoven initiated by low temperature plasma. J. Appl. Polym. Sci. 2006, 102, 5884–5889. [Google Scholar] [CrossRef]
- Shannon, M.S.; Bara, J.E. Reactive and reversible ionic liquids for CO2 capture and acid gas removal. Sep. Sci. Technol. 2012, 47, 178–188. [Google Scholar] [CrossRef]
- Jia, H.; Luo, J.; Aoki, T. Synthesis and oxygen permselectivity of copoly (substituted acetylene)s with bulky fused polycyclic aliphatic groups. Polymer 2016, 99, 95–703. [Google Scholar] [CrossRef]
- Robeson, L.M. The upper bound revisited. J. Membr. Sci. 2008, 320, 390–400. [Google Scholar] [CrossRef]
- Comesaña-Gándara, B. Redefining the robeson upper bounds for CO2/CH4 and CO2/N2 separations using a series of ultrapermeable benzotriptycene-based polymers of intrinsic microporosity. Energ. Environ. Sci. 2019, 12, 2733–2740. [Google Scholar] [CrossRef]
- Swaidan, R.; Ghanem, B.; Pinnau, I. Fine-tuned Intrinsically ultramicroporous polymers redefine the permeability/selectivity upper bounds of membrane-based air and hydrogen separations. ACS Macro Lett. 2015, 4, 947–951. [Google Scholar] [CrossRef] [Green Version]
- Pathak, B.; Samanta, D.; Ahuja, R. Borane derivatives: A new class of super and hyperhalogens. ChemPhysChem 2011, 12, 2423–2428. [Google Scholar] [CrossRef]
- Li, B.; Xu, D.; Zhang, X. Rubbery polymer inorganic nanocomposite membranes: Free volume characteristics on separation property. Ind. Eng. Chem. Res. 2010, 49, 12444–12451. [Google Scholar] [CrossRef]
| No. | EC Derivatives | Yield (%) | N-element Content (%) | Grafting Degree (%) | Mwa(× 105) | Mw/Mna |
|---|---|---|---|---|---|---|
| 1 | EC | - | - | - | 1.71 | 2.68 |
| 2 | EC-g1 | 64.1 | 0.97 | 7.90 | 1.21 | 2.32 |
| 3 | EC-g2 | 62.5 | 0.94 | 5.93 | 1.39 | 2.20 |
| 4 | EC-g3 | 63.7 | 0.98 | 7.74 | 1.70 | 2.62 |
| 5 | EC-g4 | 62.9 | 0.96 | 6.54 | 1.69 | 2.64 |
| NO. | Membrane | PCO2(Bar) a (PCO2/PCH4) | DCO2b (DCO2/DCH4) c | SCO2d (SCO2/SCH4) e | PCO2(Bar) a (PCO2/PN2) | DCO2b (DCO2/DN2) c | SCO2d (SCO2/SN2) e |
|---|---|---|---|---|---|---|---|
| 1 | EC | 44.1 (9.01) | 9.70 (0.21) | 4.61 (42.9) | 43.2 (20.6) | 14.6 (1.82) | 3.01 (11.8) |
| 2 | EC-g1 | 102 (17.8) | 10.8 (0.07) | 9.42 (262) | 96.0 (25.9) | 41.2 (1.31) | 2.31 (20.5) |
| 3 | EC-g2 | 61.5 (15.8) | 35.6 (0.07) | 1.71 (228) | 67.7 (22.6) | 31.8 (1.41) | 2.11 (15.9) |
| 4 | EC-g3 | 165 (18.8) | 5.31 (0.08) | 31.0 (246) | 157 (26.2) | 18.4 (0.73) | 8.6 (38.7) |
| 5 | EC-g4 | 199 (12.0) | 3.32 (0.08) | 60.8 (147) | 194 (22.5) | 13.6 (0.71) | 14.2 (30.7) |
| 6 | EC-b1 | 110 (18.6) | 9.41 (0.91) | 11.7 (20.0) | 119 (25.8) | 19.3 (2.21) | 6.21 (11.7) |
| 7 | EC-b2 | 62.2 (18.8) | 9.22 (0.71) | 6.81 (26.7) | 70.8 (26.2) | 48.1 (2.21) | 1.51 (11.7) |
| 8 | EC-b3 | 76.0 (19.5) | 23.5 (0.92) | 3.22 (21.9) | 79.7 (31.9) | 13.2 (3.71) | 6.02 (8.71) |
| 9 | EC-b4 | 46.0 (19.2) | 10.3 (0.81) | 4.51 (23.8) | 47.8 (23.9) | 37.2 (2.22) | 1.31 (11.1) |
| No. | Membrane | Thickness (mm) | Elongation (%) | Elasticity Modulus (MPa) |
|---|---|---|---|---|
| 1 | EC | 0.1391 | 21.6 | 207 |
| 2 | EC-g1 | 0.1448 | 4.22 | 648 |
| 3 | EC-g2 | 0.1138 | 8.61 | 560 |
| 4 | EC-g3 | 0.1591 | 3.92 | 644 |
| 5 | EC-g4 | 0.1282 | 5.53 | 593 |
| 6 | EC-b1 | 0.1338 | 16.2 | 117 |
| 7 | EC-b2 | 0.0986 | 10.2 | 177 |
| 8 | EC-b3 | 0.1158 | 8.71 | 119 |
| 9 | EC-b4 | 0.0892 | 15.4 | 143 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, J.; Jia, H.; Yang, N.; Wang, Q.; Yang, G.; Zhang, M.; Xu, S.; Zang, Y.; Ma, L.; Jiang, P.; et al. High Efficiency Gas Permeability Membranes from Ethyl Cellulose Grafted with Ionic Liquids. Polymers 2019, 11, 1900. https://doi.org/10.3390/polym11111900
Xu J, Jia H, Yang N, Wang Q, Yang G, Zhang M, Xu S, Zang Y, Ma L, Jiang P, et al. High Efficiency Gas Permeability Membranes from Ethyl Cellulose Grafted with Ionic Liquids. Polymers. 2019; 11(11):1900. https://doi.org/10.3390/polym11111900
Chicago/Turabian StyleXu, Jingyu, Hongge Jia, Nan Yang, Qingji Wang, Guoxing Yang, Mingyu Zhang, Shuangping Xu, Yu Zang, Liqun Ma, Pengfei Jiang, and et al. 2019. "High Efficiency Gas Permeability Membranes from Ethyl Cellulose Grafted with Ionic Liquids" Polymers 11, no. 11: 1900. https://doi.org/10.3390/polym11111900





