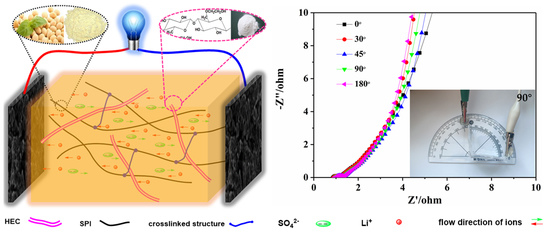
Construction of Polymer Electrolyte Based on Soybean Protein Isolate and Hydroxyethyl Cellulose for a Flexible Solid-State Supercapacitor
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Gel Polymer Electrolyte Based on Crosslinked SPI and HEC
2.3. Fabrication of SSCs
2.4. Characterization of Membrane Samples
2.5. Electrolyte Uptake of Membranes
2.6. Ionic Conductivity of GPEs
2.7. Electrochemical Characterization of SSCs
3. Results and Discussion
3.1. Structural Analysis of Membrane Samples
3.2. Thermal Stability
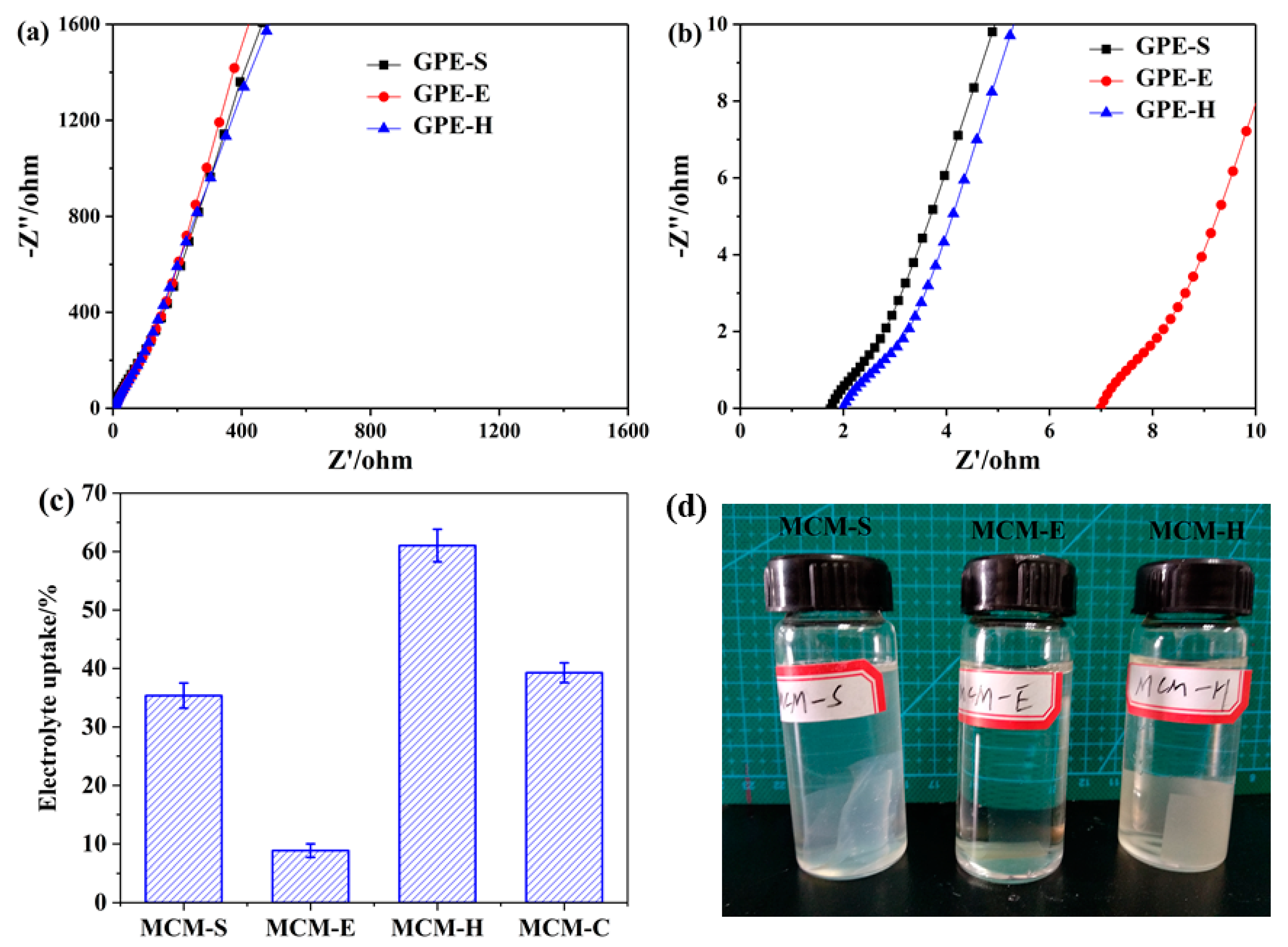
3.3. Electrolyte Uptake, Water Resistance of Membrane Samples, and Ionic Conductivities of GPEs
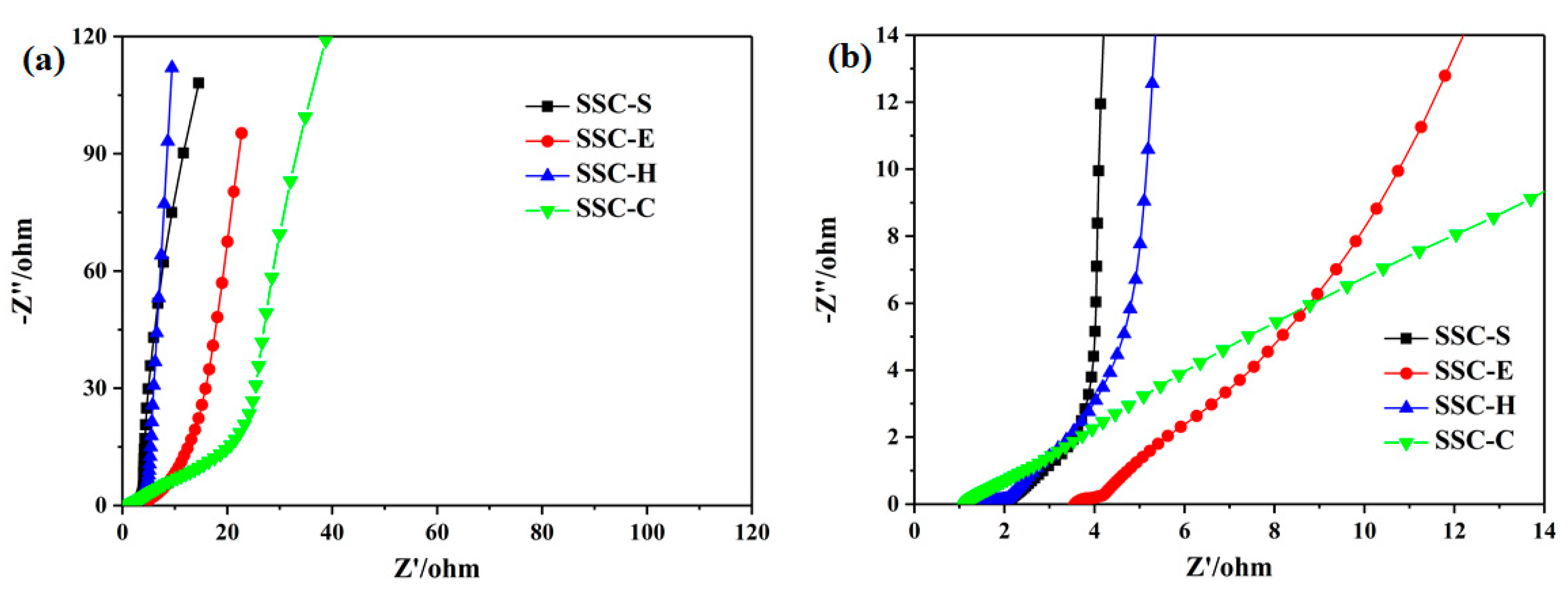
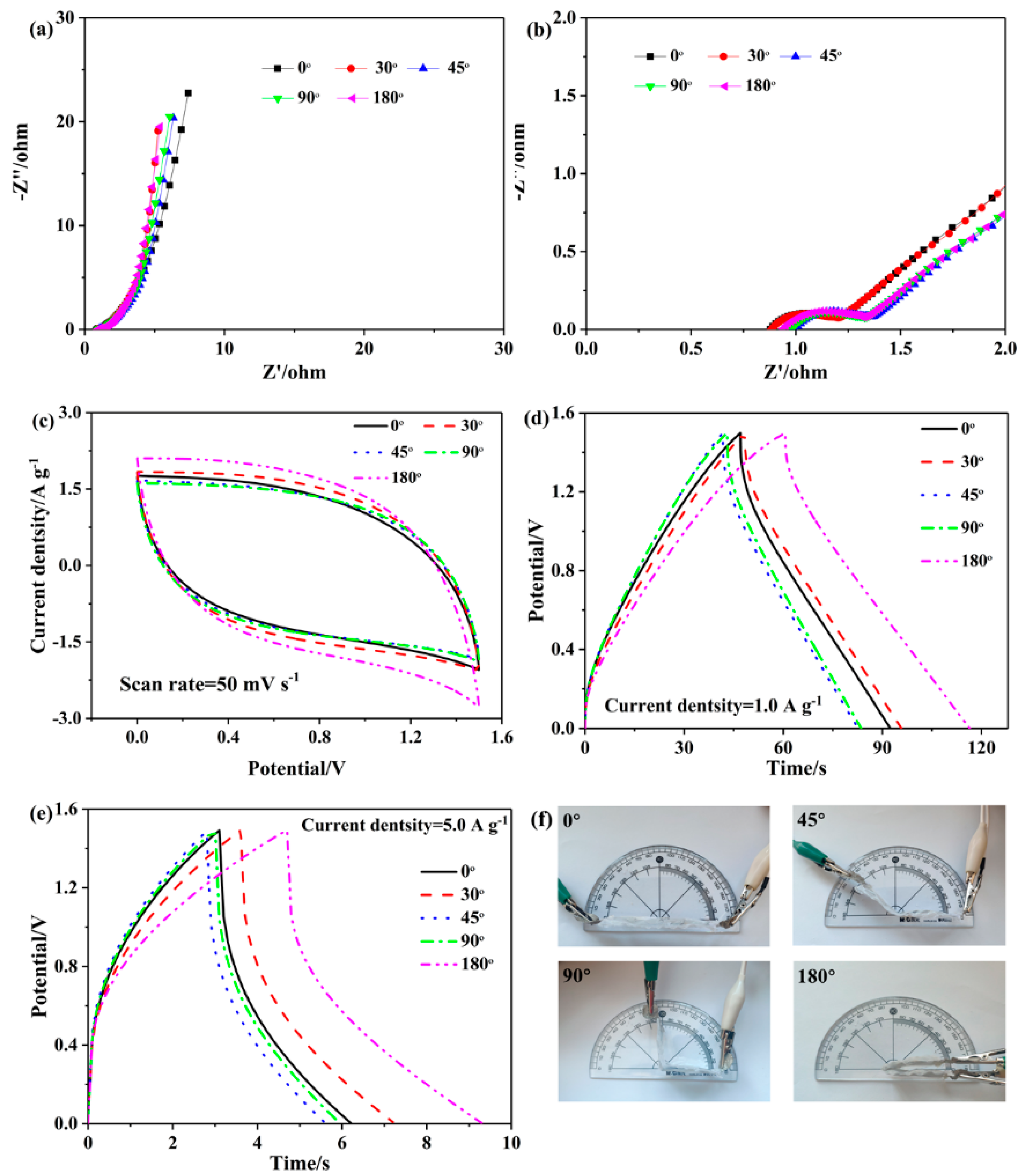
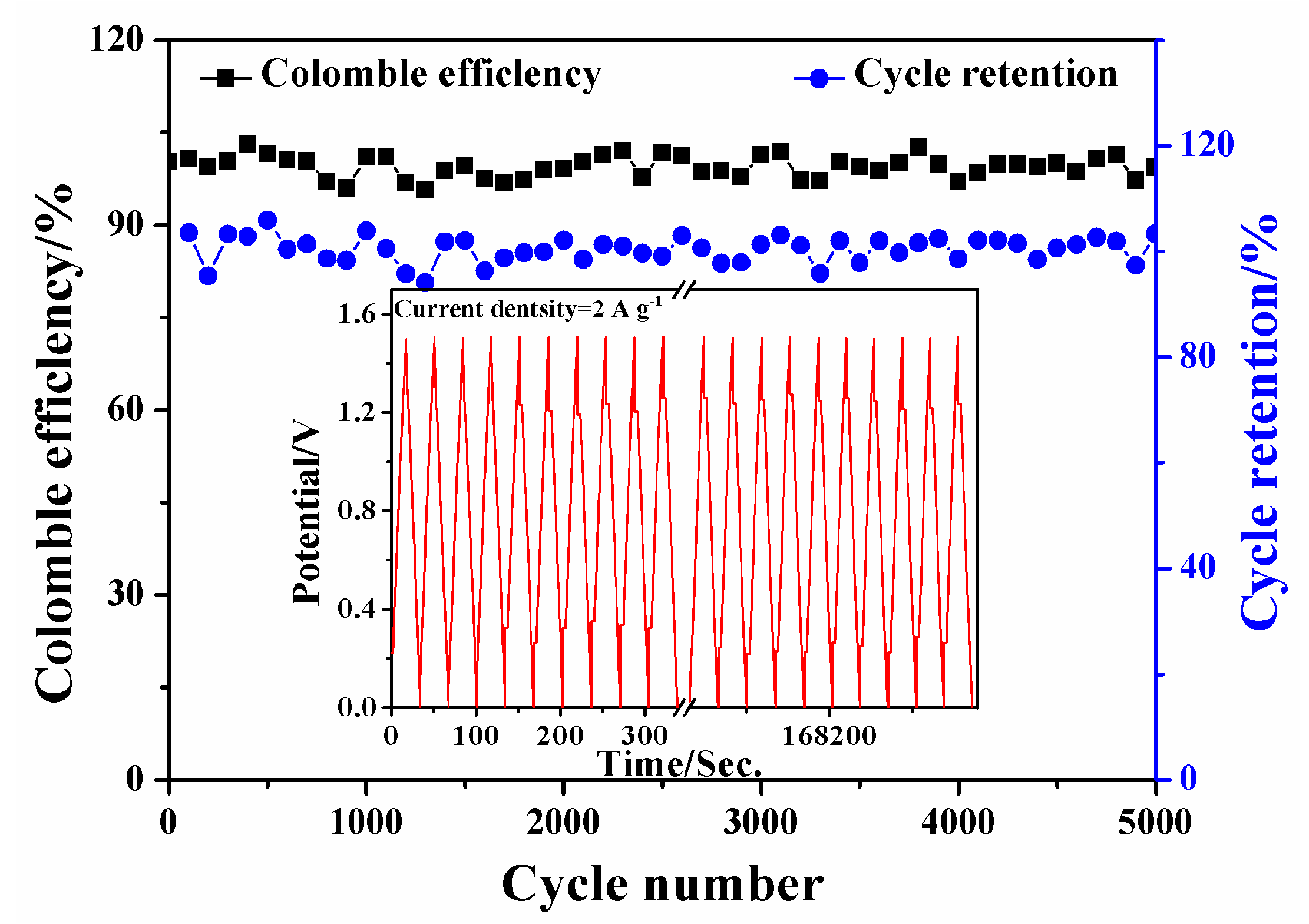
3.4. Electrochemical Performance of SSCs
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Li, Z.; Jiao, X.; Li, C.; Chen, D. Synthesis and application of nanocages in supercapacitors. Chem. Eng. J. 2018, 351, 135–156. [Google Scholar] [CrossRef]
- Na, R.; Lu, N.; Zhang, S.; Huo, G.; Yang, Y.; Zhang, C.; Mu, Y.; Luo, Y.; Wang, G. Facile synthesis of a high-performance, fire-retardant organic gel polymer electrolyte for flexible solid-state supercapacitors. Electrochim. Acta 2018, 290, 262–272. [Google Scholar] [CrossRef]
- Young, C.; Salunkhe, R.R.; Tang, J.; Hu, C.C.; Shahabuddin, M.; Yanmaz, E.; Hossain, M.S.A.; Kim, J.H.; Yamauchi, Y. Zeolitic imidazolate framework (ZIF-8) derived nanoporous carbon: The effect of carbonization temperature on the supercapacitor performance in an aqueous electrolyte. Phys. Chem. Chem. Phys. 2016, 18, 29308–29315. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Liu, F.; Qiu, F.; Lu, C.; Kang, J.; Zhao, D.; Han, S.; Zhuang, X. Cobalt-Doped Porous Carbon Nanosheets Derived from 2D Hypercrosslinked Polymer with CoN4 for High Performance Electrochemical Capacitors. Polymers 2018, 10, 1339. [Google Scholar] [CrossRef] [PubMed]
- Pant, B.; Park, M.; Park, S.J. TiO2 NPs Assembled into a Carbon Nanofiber Composite Electrode by a One-Step Electrospinning Process for Supercapacitor Applications. Polymers 2019, 11, 899. [Google Scholar] [CrossRef]
- Olejnik, P.; Gniadek, M.; Echegoyen, L.; Plonska-Brzezinska, M.E. Nanoforest: Polyaniline Nanotubes Modified with Carbon Nano-Onions as a Nanocomposite Material for Easy-to-Miniaturize High-Performance Solid-State Supercapacitors. Polymers 2018, 10, 1408. [Google Scholar] [CrossRef]
- Tan, S.; Li, J.; Zhou, L.; Chen, P.; Shi, J.; Xu, Z. Modified carbon fiber paper-based electrodes wrapped by conducting polymers with enhanced electrochemical performance for supercapacitors. Polymers 2018, 10, 1072. [Google Scholar] [CrossRef]
- Huang, H.S.; Chang, K.H.; Suzuki, N.; Yamauchi, Y.; Hu, C.C.; Wu, K.C.W. Evaporation-Induced Coating of Hydrous Ruthenium Oxide on Mesoporous Silica Nanoparticles to Develop High-Performance Supercapacitors. Small 2013, 9, 2520–2526. [Google Scholar] [CrossRef]
- Makino, S.; Yamauchi, Y.; Sugimoto, W. Synthesis of electro-deposited ordered mesoporous RuOx using lyotropic liquid crystal and application toward micro-supercapacitors. J. Power Sources 2013, 227, 153–160. [Google Scholar] [CrossRef]
- Bastakoti, B.P.; Huang, H.S.; Chen, L.C.; Wu, K.C.W.; Yamauchi, Y. Block copolymer assisted synthesis of porous α-Ni(OH)2 microflowers with high surface areas as electrochemical pseudocapacitor materials. Chem. Commun. 2012, 48, 9150–9152. [Google Scholar] [CrossRef]
- Xia, L.; Yu, L.; Hu, D.; Chen, G.Z. Electrolytes for electrochemical energy storage. Mater. Chem. Front. 2017, 1, 584–618. [Google Scholar] [CrossRef]
- Na, R.; Su, C.W.; Su, Y.H.; Chen, Y.C.; Chen, Y.M.; Wang, G.; Teng, H. Solvent-free synthesis of an ionic liquid integrated ether-abundant polymer as a solid electrolyte for flexible electric double-layer capacitors. J. Mater. Chem. A 2017, 5, 19703–19713. [Google Scholar] [CrossRef]
- Na, R.; Huo, G.; Zhang, S.; Huo, P.; Du, Y.; Luan, J.; Zhu, K.; Wang, G. A novel poly (ethylene glycol)–grafted poly (arylene ether ketone) blend micro-porous polymer electrolyte for solid-state electric double layer capacitors formed by incorporating a chitosan-based LiClO4 gel electrolyte. J. Mater. Chem. A 2016, 4, 18116–18127. [Google Scholar] [CrossRef]
- Lu, X.; Yu, M.; Wang, G.; Tong, Y.; Li, Y. Flexible solid-state supercapacitors: Design, fabrication and applications. Energy Environ. Sci. 2014, 7, 2160–2181. [Google Scholar] [CrossRef]
- Deng, R.; Xia, Z.; Sun, R.; Wang, S.; Sun, G. Nanostructured ultrathin catalyst layer with ordered platinum nanotube arrays for polymer electrolyte membrane fuel cells. J. Energy Chem. 2019, 43, 33–39. [Google Scholar] [CrossRef]
- Piana, G.; Bella, F.; Geobaldo, F.; Meligrana, G.; Gerbaldi, C. PEO/LAGP hybrid solid polymer electrolytes for ambient temperature lithium batteries by solvent-free, “one pot” preparation. J. Energy Storage 2019, 26, 100947. [Google Scholar] [CrossRef]
- Falco, M.; Simari, C.; Ferrara, C.; Nair, J.R.; Meligrana, G.; Bella, F.; Nicotera, I.; Mustarelli, P.; Winter, M.; Gerbaldi, C. Understanding the effect of UV-induced crosslinking on the physico-chemical properties of highly performing PEO/LiTFSI-based polymer electrolytes. Langmuir 2019, 35, 8210–8219. [Google Scholar] [CrossRef]
- Wang, X.; Hao, X.; Cai, D.; Zhang, S.; Xia, X.; Tu, J. An ultraviolet polymerized 3D gel polymer electrolyte based on multi-walled carbon nanotubes doped double polymer matrices for lithium-sulfur batteries. Chem. Eng. J. 2019, 382, 122714. [Google Scholar] [CrossRef]
- Xun, Z.; Liu, Y.; Gu, J.; Liu, L.; Huo, P. A Biomass-Based Redox Gel Polymer Electrolyte for Improving Energy Density of Flexible Supercapacitor. J. Electrochem. Soc. 2019, 166, A2300–A2312. [Google Scholar] [CrossRef]
- Wang, Y.; Qiao, X.; Zhang, C.; Zhou, X. Development of structural supercapacitors with epoxy based adhesive polymer electrolyte. J. Energy Storage 2019, 26, 100968. [Google Scholar] [CrossRef]
- Wang, Y.; Zhong, W.H.; Schiff, T.; Eyler, A.; Li, B. A Particle-Controlled, High-Performance, Gum-Like Electrolyte for Safe and Flexible Energy Storage Devices. Adv. Energy Mater. 2015, 5, 1400463. [Google Scholar] [CrossRef]
- Moon, W.G.; Kim, G.P.; Lee, M.; Song, H.D.; Yi, J. A Biodegradable Gel Electrolyte for Use in High-Performance Flexible Supercapacitors. ACS Appl. Mater. Interfaces 2015, 7, 3503–3511. [Google Scholar] [CrossRef] [PubMed]
- Sekhon, S.S. Conductivity behaviour of polymer gel electrolytes: Role of polymer. Bull. Mater. Sci. 2003, 26, 321–328. [Google Scholar] [CrossRef]
- Meng, C.; Liu, C.; Chen, L.; Hu, C.; Fan, S. Highly flexible and all-solid-state paperlike polymer supercapacitors. Nano Lett. 2010, 10, 4025–4031. [Google Scholar] [CrossRef] [PubMed]
- Osada, I.; Vries, H.; Scrosati, B.; Passerini, S. Ionic-Liquid-Based Polymer Electrolytes for Battery Applications. Angew. Chem. Int. Ed. 2016, 55, 500–513. [Google Scholar] [CrossRef] [PubMed]
- Kalhoff, J.; Eshetu, G.G.; Bresser, D.; Passerini, S. Safer electrolytes for lithium-ion batteries: State of the art and perspectives. ChemSusChem 2015, 8, 2154–2175. [Google Scholar] [CrossRef]
- Tan, S.; Ji, Y.J.; Zhang, Z.R.; Yang, Y. Recent Progress in Research on High-Voltage Electrolytes for Lithium-Ion Batteries. ChemPhysChem 2014, 15, 1956–1969. [Google Scholar] [CrossRef]
- Jiang, M.; Zhu, J.; Chen, C.; Lu, Y.; Ge, Y.; Zhang, X. Poly (vinyl alcohol) borate gel polymer electrolytes prepared by electrodeposition and their application in electrochemical supercapacitors. ACS Appl. Mater. Interfaces 2016, 8, 3473–3481. [Google Scholar] [CrossRef]
- Kalpana, D.; Renganathan, N.G.; Pitchumani, S. A new class of alkaline polymer gel electrolyte for carbon aerogel supercapacitors. J. Power Sources 2006, 157, 621–623. [Google Scholar] [CrossRef]
- Cheng, X.; Pan, J.; Zhao, Y.; Liao, M.; Peng, H. Gel Polymer Electrolytes for Electrochemical Energy Storage. Adv. Energy Mater. 2018, 8, 1702184. [Google Scholar] [CrossRef]
- Ponez, L.; Sentanin, F.C.; Majid, S.R.; Arof, A.K.; Pawlicka, A. Ion-conducting membranes based on gelatin and containing LiI/I2 for electrochromic devices. Mol. Cryst. Liq. Cryst. 2012, 554, 239–251. [Google Scholar] [CrossRef]
- Ma, L.; Yang, Y.; Yao, J.; Shao, Z.; Chen, X. Robust soy protein films obtained by slight chemical modification of polypeptide chains. Polym. Chem. 2013, 4, 5425–5431. [Google Scholar] [CrossRef]
- Wang, X.; Fu, X.; Wang, Y.; Zhong, W. A protein-reinforced adhesive composite electrolyte. Polymer 2016, 106, 43–52. [Google Scholar] [CrossRef] [Green Version]
- Zhu, M.; Tan, C.; Fang, Q.; Gao, L.; Sui, G.; Yang, X. High performance and biodegradable skeleton material based on soy protein isolate for gel polymer electrolyte. ACS Sustain. Chem. Eng. 2016, 4, 4498–4505. [Google Scholar] [CrossRef]
- Zhu, M.; Wu, J.; Zhong, W.H.; Lan, J.; Sui, G.; Yang, X. A Biobased Composite Gel Polymer Electrolyte with Functions of Lithium Dendrites Suppressing and Manganese Ions Trapping. Adv. Energy Mater. 2018, 8, 1702561. [Google Scholar] [CrossRef]
- González, A.; Tártara, L.I.; Palma, S.D.; Igarzabal, C.I.A. Crosslinked soy protein films and their application as ophthalmic drug delivery system. Mater. Sci. Eng. C 2015, 51, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Boy, R.; Maness, C.; Kotek, R. Properties of chitosan/soy protein blended films with added plasticizing agent as a function of solvent type at acidic pH. Int. J. Polym. Mater. Polym. Biomater. 2016, 65, 11–17. [Google Scholar] [CrossRef]
- Wang, L.; Li, J.; Zhang, S.; Shi, J. Preparation and characterization of all-biomass soy protein isolate-based films enhanced by epoxy castor oil acid sodium and hydroxypropyl cellulose. Materials 2016, 9, 193. [Google Scholar] [CrossRef] [Green Version]
- Zhu, C.Y.; Liu, H.F.; Fu, M.; Zhao, X.H. Structure and property changes of soybean protein isolates resulted from the glycation and cross-linking by transglutaminase and a degraded chitosan. CyTA J. Food 2016, 14, 138–144. [Google Scholar] [CrossRef] [Green Version]
- Huo, P.; Ni, S.; Hou, P.; Xun, Z.; Liu, Y.; Gu, J. A Crosslinked Soybean Protein Isolate Gel Polymer Electrolyte Based on Neutral Aqueous Electrolyte for a High-Energy-Density Supercapacitor. Polymers 2019, 11, 863. [Google Scholar] [CrossRef] [Green Version]
- Zhou, J.; Qin, Y.; Liu, S.; Zhang, L. Homogenous synthesis of hydroxyethylcellulose in NaOH/urea aqueous solution. Macromol. Biosci. 2006, 6, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; He, M.; Zhao, L.; Wang, S.; Li, Y.; Gan, L.; Li, M.; Xu, L.; Chang, P.R.; Anderson, D.P.; et al. Epichlorohydrin-cross-linked hydroxyethyl cellulose/soy protein isolate composite films as biocompatible and biodegradable implants for tissue engineering. ACS Appl. Mater. Interfaces 2016, 8, 2781–2795. [Google Scholar] [CrossRef] [PubMed]
- Ciannamea, E.M.; Stefani, P.M.; Ruseckaite, R.A. Physical and mechanical properties of compression molded and solution casting soybean protein concentrate based films. Food Hydrocoll. 2014, 38, 193–204. [Google Scholar] [CrossRef]
- Lei, H.; Du, G.; Wu, Z.; Xi, X.; Dong, Z. Cross-linked soy-based wood adhesives for plywood. Int. J. Adhes. Adhes. 2014, 50, 199–203. [Google Scholar] [CrossRef]
- Xu, F.; Dong, Y.; Zhang, W.; Zhang, S.; Li, L.; Li, J. Preparation of cross-linked soy protein isolate-based environmentally-friendly films enhanced by PTGE and PAM. Ind. Crop. Prod. 2015, 67, 373–380. [Google Scholar] [CrossRef]
- Chen, J.; Chen, X.; Zhu, Q.; Chen, F.; Zhao, X.; Ao, Q. Determination of the domain structure of the 7s and 11s globulins from soy proteins by XRD and FTIR. J. Sci. Food Agric. 2013, 93, 1687–1691. [Google Scholar] [CrossRef]
- Tõnurist, K.; Thomberg, T.; Jänes, A.; Romann, T.; Sammelselg, V.; Lust, E. Influence of separator properties on electrochemical performance of electrical double-layer capacitors. J. Electroanal. Chem. 2013, 689, 8–20. [Google Scholar] [CrossRef]
- Tu, Q.M.; Fan, L.Q.; Pan, F.; Huang, J.L.; Gu, Y.; Lin, J.M.; Huang, M.L.; Huang, Y.F.; Wu, J.H. Design of a novel redox-active gel polymer electrolyte with a dual-role ionic liquid for flexible supercapacitors. Electrochim. Acta 2018, 268, 562–568. [Google Scholar] [CrossRef]
- Sun, X.; Zhang, X.; Zhang, H.; Zhang, D.; Ma, Y. A comparative study of activated carbon-based symmetric supercapacitors in Li2SO4 and KOH aqueous electrolytes. J. Solid State Electr. 2012, 16, 2597–2603. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, L.; Zhang, F.; Zhang, T.; Huang, Y.; Chen, Y. A high-performance all-solid-state supercapacitor with graphene-doped carbon material electrodes and a graphene oxide-doped ion gel electrolyte. Carbon 2014, 72, 381–386. [Google Scholar] [CrossRef]
- Sun, X.Z.; Zhang, X.; Huang, B.; Ma, Y.W. Effects of separator on the electrochemical performance of electrical double-layer capacitor and hybrid battery-supercapacitor. Acta Phys. Chim. Sin. 2014, 30, 485–491. [Google Scholar] [CrossRef]
- Pandey, G.P.; Hashmi, S.A. Performance of solid-state supercapacitors with ionic liquid 1-ethyl-3-methylimidazolium tris (pentafluoroethyl) trifluorophosphate based gel polymer electrolyte and modified MWCNT electrodes. Electrochim. Acta 2013, 105, 333–341. [Google Scholar] [CrossRef]

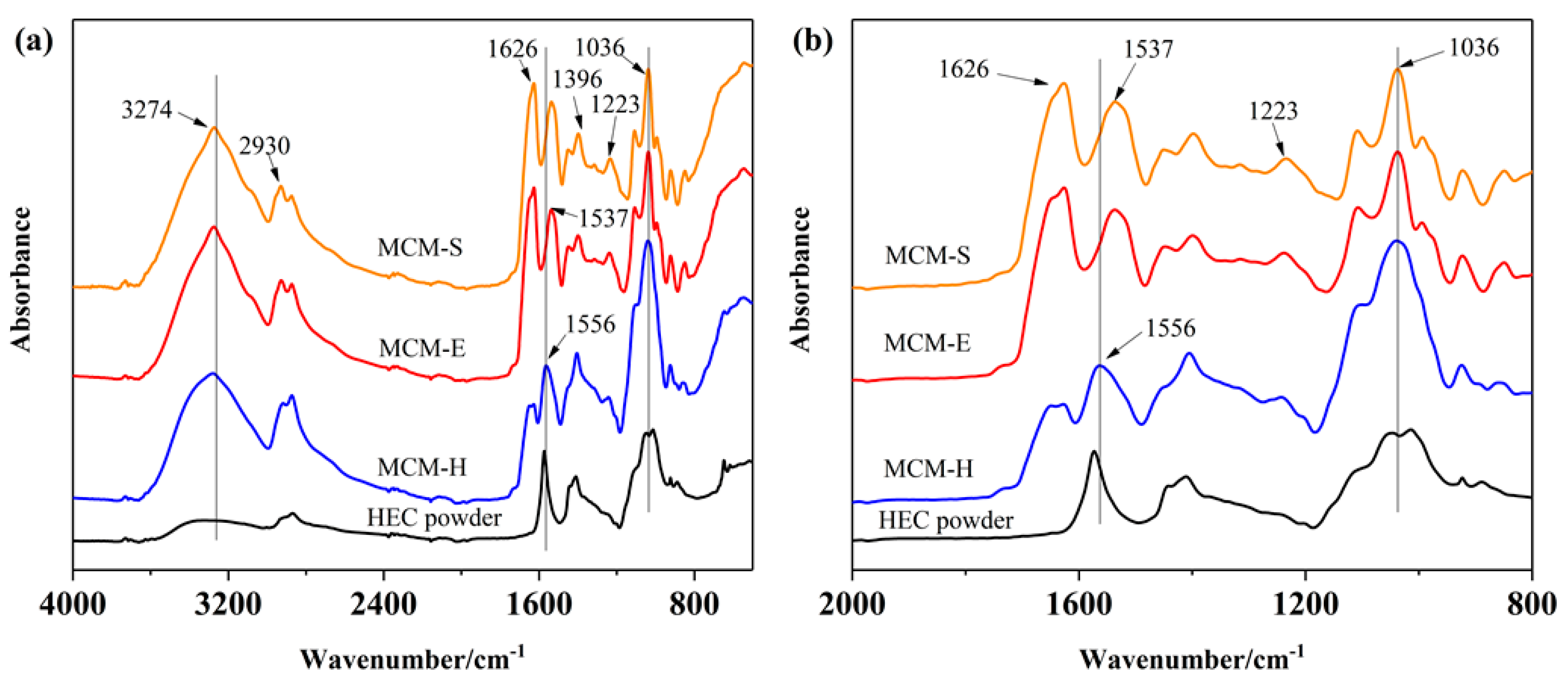

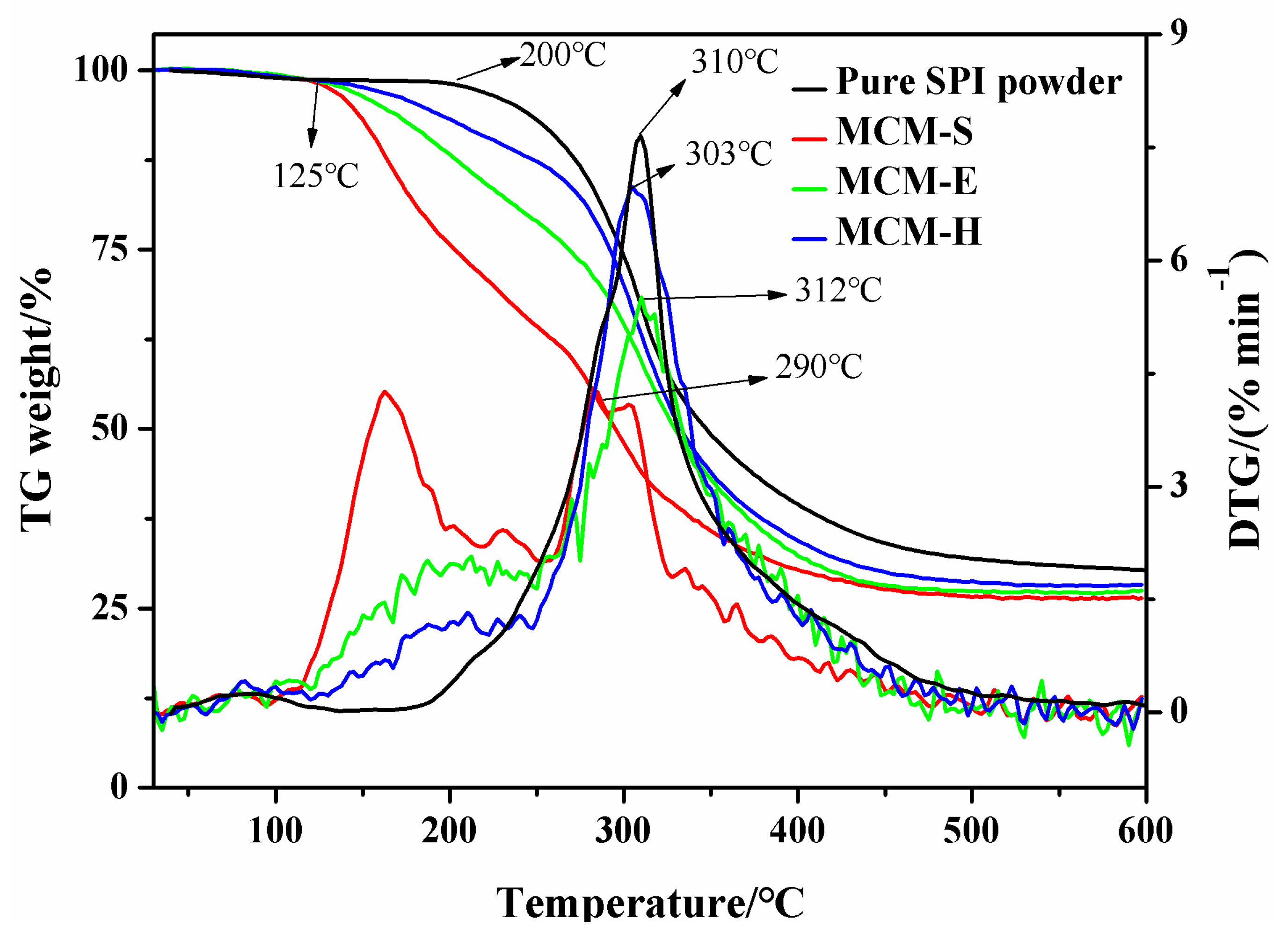



| Samples | SPI (5 wt % Solution, g) | Glycerol (g) | EGDE (g) | HEC (5 wt % Solution, g) |
|---|---|---|---|---|
| MCM-S a | 12 | 0.3 | 0 | 0 |
| MCM-E b | 12 | 0.3 | 0.12 | 0 |
| MCM-H c | 8.4 | 0.21 | 0.12 | 3.6 |
| GPE Sample | Thickness of GPEs (cm) | Ionic Conductivity (10−3 S cm−1) | Membrane Samples | Electrolyte Uptake (%) |
|---|---|---|---|---|
| GPE-S a | 0.0133 | 7.58 | MCM-S | 35.35 ± 2.16 |
| GPE-E b | 0.0157 | 2.25 | MCM-E | 8.84 ± 1.15 |
| GPE-H c | 0.0168 | 8.40 | MCM-H | 61.03 ± 2.80 |
| GPE-C d | 0.0138 | 9.40 | MCM-C | 49.27 ± 1.68 |
| Sample | Current Density (A g−1) | Specific Capacitance (Csp, F g−1) | Energy Density (Ecell, W h kg−1) | Power Density (Pcell, W kg−1) |
|---|---|---|---|---|
| SSC-S a | 1 | 100.35 | 7.84 | 771.15 |
| 5 | 95.27 | 7.44 | 5351.76 | |
| SSC-E b | 1 | 100.55 | 7.86 | 862.68 |
| 5 | 65.98 | 5.15 | 6621.43 | |
| SSC-H c | 1 | 98.91 | 7.73 | 786.10 |
| 5 | 91.79 | 7.17 | 4780.00 | |
| SSC-C d | 1 | 86.22 | 6.74 | 786.89 |
| 5 | 69.9 | 5.46 | 5312.43 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xun, Z.; Ni, S.; Gao, Z.; Zhang, Y.; Gu, J.; Huo, P. Construction of Polymer Electrolyte Based on Soybean Protein Isolate and Hydroxyethyl Cellulose for a Flexible Solid-State Supercapacitor. Polymers 2019, 11, 1895. https://doi.org/10.3390/polym11111895
Xun Z, Ni S, Gao Z, Zhang Y, Gu J, Huo P. Construction of Polymer Electrolyte Based on Soybean Protein Isolate and Hydroxyethyl Cellulose for a Flexible Solid-State Supercapacitor. Polymers. 2019; 11(11):1895. https://doi.org/10.3390/polym11111895
Chicago/Turabian StyleXun, Zhiyu, Shoupeng Ni, Zhenhua Gao, Yanhua Zhang, Jiyou Gu, and Pengfei Huo. 2019. "Construction of Polymer Electrolyte Based on Soybean Protein Isolate and Hydroxyethyl Cellulose for a Flexible Solid-State Supercapacitor" Polymers 11, no. 11: 1895. https://doi.org/10.3390/polym11111895