Revealing Adsorption Behaviors of Amphoteric Polyacrylamide on Cellulose Fibers and Impact on Dry Strength of Fiber Networks
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of AmPAM Polymers
2.3. Preparation of Handsheet Treated with AmPAMs and Strength Characterization
2.4. Preparation of Cellulose Model Films
2.5. Adsorption Behaviors Monitored by QCM-D Technique
3. Results and Discussion
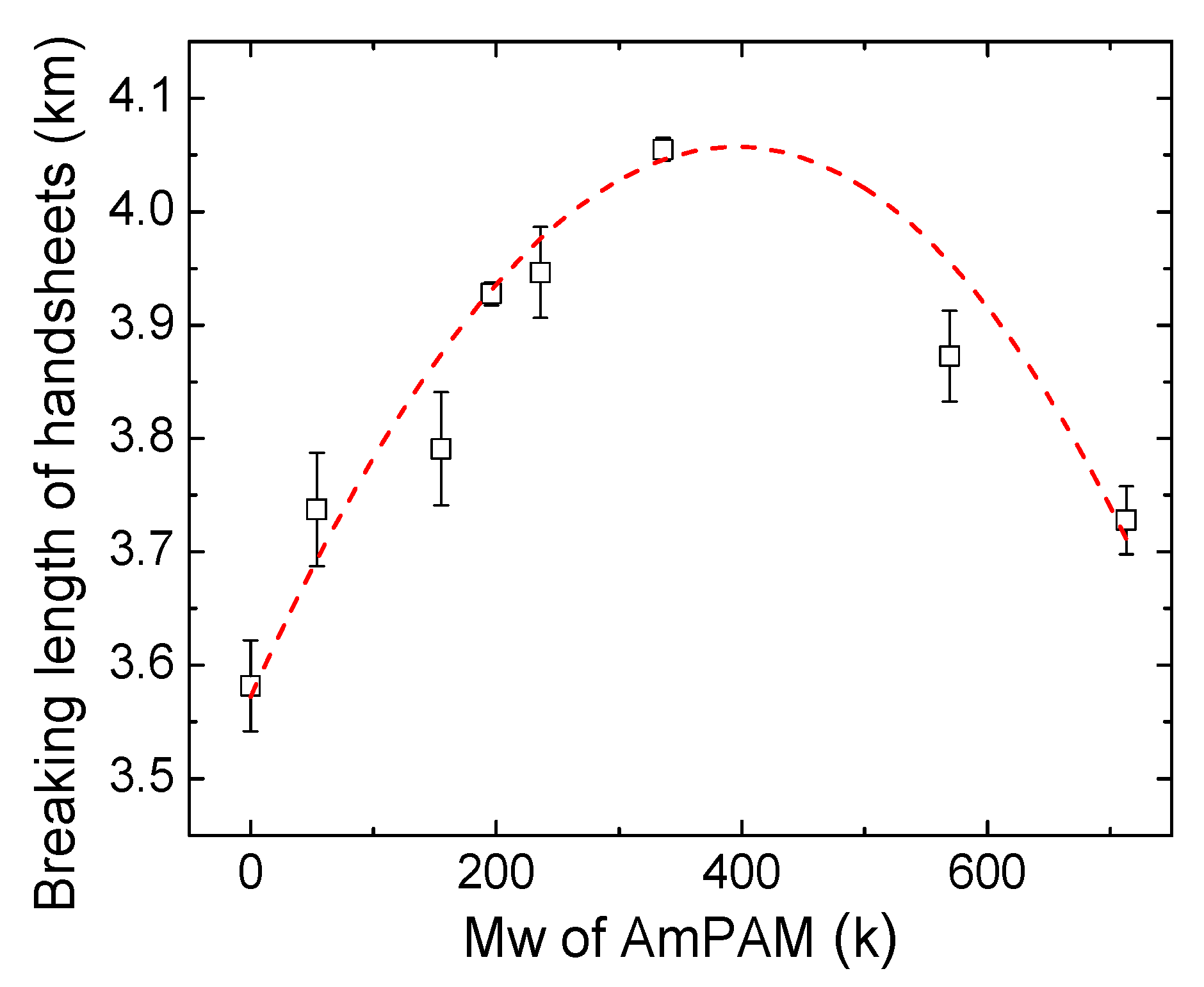
3.1. Dry Strength of Handsheets Treated with AmPAM of Various MWs
3.2. Adsorption Behaviors of AmPAMs
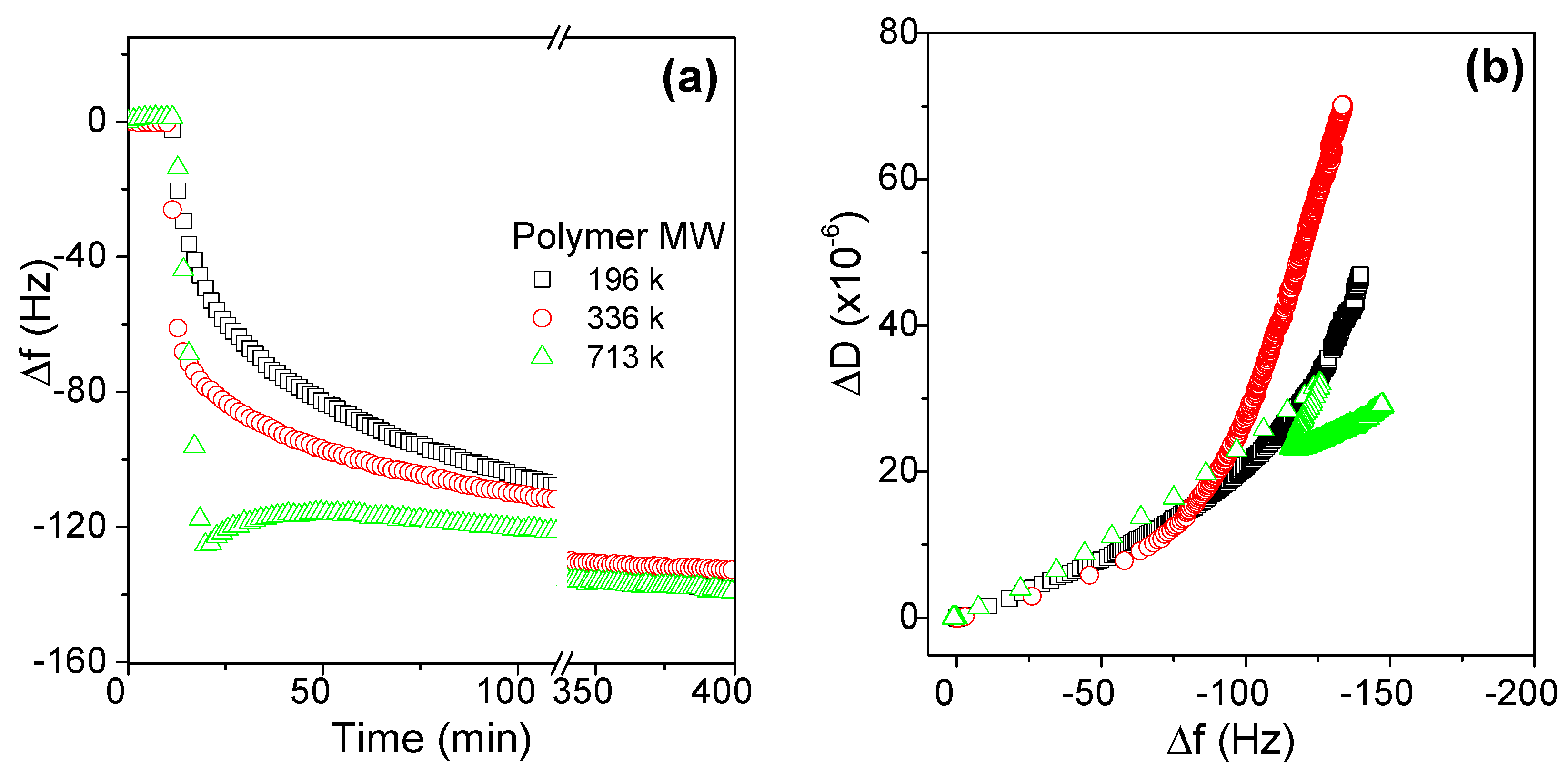
3.2.1. Effects of Molecular Weight on the Adsorption Behaviors of AmPAMs
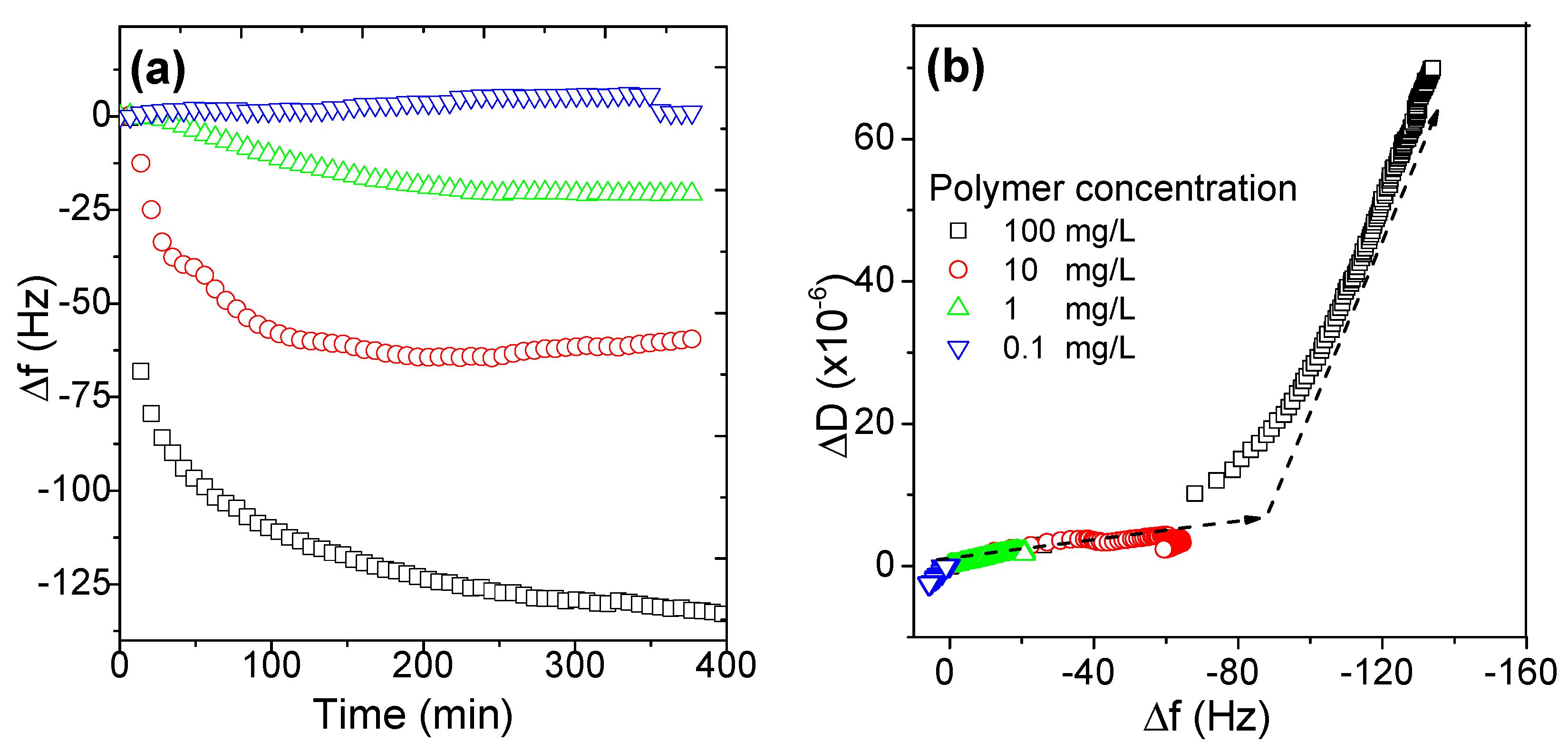
3.2.2. Effects of Polymer Concentration on the Adsorption Behaviors of AmPAMs
3.2.3. Effects of Medium pH on the Adsorption Behaviors of AmPAMs
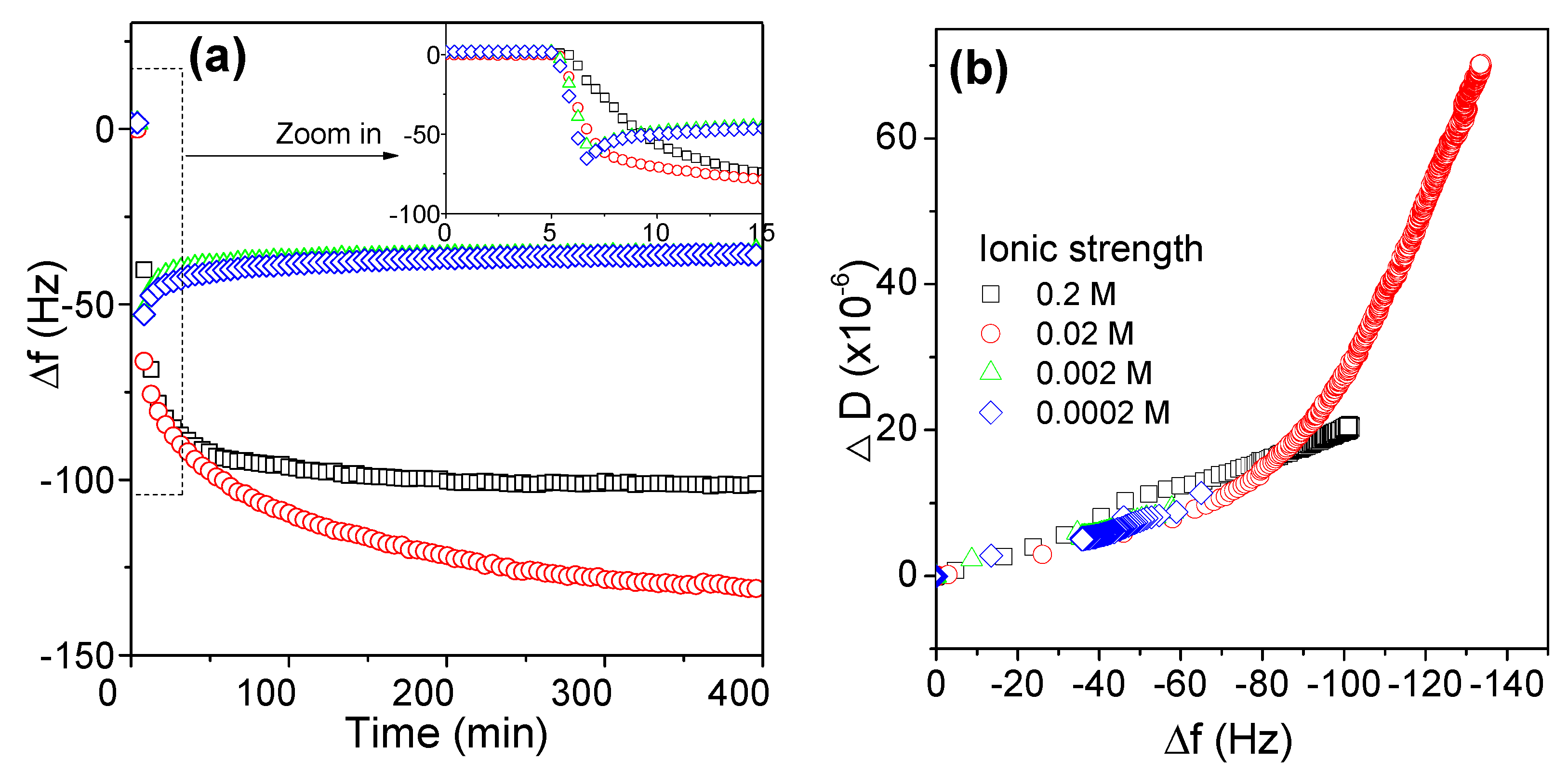
3.2.4. Effects of Ionic Strength on the Adsorption Behaviors of AmPAM
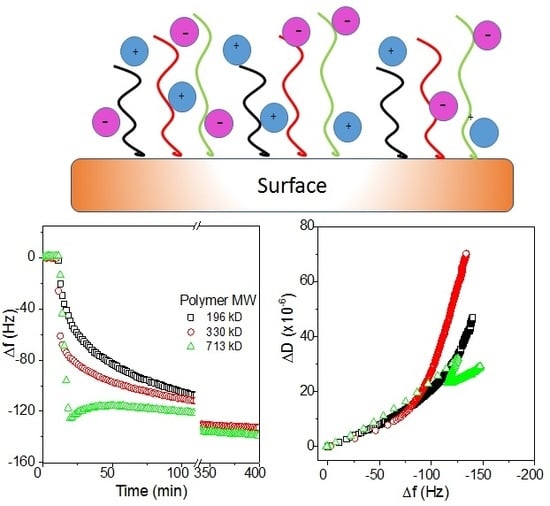
3.2.5. Effects of Substrate Surface on the Adsorption Behaviors of AmPAMs
3.3. Elucidation of Impact of AmPAM MW on Dry Strength of Handsheets
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dobrynin, A.V.; Colby, R.H.; Rubinstein, M. Polyampholytes. J. Polym. Sci. B 2004, 42, 3513–3538. [Google Scholar] [CrossRef]
- Wang, P.; Zhu, Y.; Wang, X.; Zhang, X.; Zhu, W.; Yao, C.; Song, J. Application of Amphoteric Polyacrylamide Solely or with the Combination of Cationic Starch for Paper Strength Improvement. BioResources 2018, 13, 7864–7872. [Google Scholar] [CrossRef]
- Hubbe, M.A.; Rojas, O.J.; Argyropoulos, D.S.; Wang, Y.; Song, J.; Sulic, N.; Sezaki, T. Charge and the dry-strength performance of polyampholytes—Part 2. Colloidal effects. Colloid Surf. A 2007, 301, 23–32. [Google Scholar] [CrossRef]
- Wang, Y.; Hubbe, M.A.; Rojas, O.J.; Argyropoulos, D.S.; Wang, X.; Sezaki, T. Charge and the dry-strength performance of polyampholytes. Part 3: Streaming potential analysis. Colloid Surf. A 2007, 301, 33–40. [Google Scholar] [CrossRef]
- Valley, B.; Jing, B.; Ferreira, M.; Zhu, Y. Rapid and Efficient Coacervate Extraction of Cationic Industrial Dyes from Wastewater. ACS Appl. Mater. Interface USA 2019, 11, 7472–7478. [Google Scholar] [CrossRef]
- Blackman, L.D.; Gunatillake, P.A.; Cass, P.; Locock, K.E.S. An introduction to zwitterionic polymer behavior and applications in solution and at surfaces. Chem. Soc. Rev. 2019, 48, 757–770. [Google Scholar] [CrossRef]
- Zhang, W.; Cheng, W.; Ziemann, E.; Be’er, A.; Lu, X.; Elimelech, M.; Bernstein, R. Functionalization of ultrafiltration membrane with polyampholyte hydrogel and graphene oxide to achieve dual antifouling and antibacterial properties. J. Membr. Sci. 2018, 565, 293–302. [Google Scholar] [CrossRef]
- Batchelor, M.; Paci, E. Helical Polyampholyte Sequences Have Unique Thermodynamic Properties. J. Phys. Chem. B 2018, 122, 11784–11791. [Google Scholar] [CrossRef]
- Dimassi, S.; Tabary, N.; Chai, F.; Blanchemain, N.; Martel, B. Sulfonated and sulfated chitosan derivatives for biomedical applications: A review. Carbohydr. Polym. 2018, 202, 382–396. [Google Scholar] [CrossRef]
- Calabrese, V.; da Silva, M.A.; Schmitt, J.; Munoz-Garcia, J.C.; Gabrielli, V.; Scott, J.L.; Angulo, J.; Khimyak, Y.Z.; Edler, K.J. Surfactant controlled zwitterionic cellulose nanofibril dispersions. Soft Matter 2018, 14, 7793–7800. [Google Scholar] [CrossRef]
- Lin, L.; Luo, Y.; Li, X. Synthesis of Diblock Polyampholyte PAMPS-b-PMAPTAC and Its Adsorption on Bentonite. Polymers 2019, 11, 49. [Google Scholar] [CrossRef]
- Duan, C.; Meng, X.; Meng, J.; Khan, I.H.; Dai, L.; Khan, A.; An, X.; Zhang, J.; Hua, T.; Ni, Y. Chitosan as A Preservative for Fruits and Vegetables: A Review on Chemistry and Antimicrobial Properties. J. Bioresour. Bioprod. 2019, 4, 11–21. [Google Scholar]
- Carr, M.E.; Hofreiter, B.T.; Schulte, M.I.; Russell, C.R. Starch polyampholyte for paper. Tappi 1977, 60, 66–69. [Google Scholar]
- Hubbe, M.A.; Rojas, O.J.; Sulic, N.; Sezaki, T. Unique behaviour of polyampholytes as dry-strength additives. Appita J. 2007, 60, 106–111. [Google Scholar]
- Sezaki, T.; Hubbe, M.A.; Heitmann, J.A.; Argyropoulos, D.S. Colloidal effects of acrylamide polyampholytes—Part 2: Adsorption onto cellulosic fibers. Colloid Surface A 2006, 289, 89–95. [Google Scholar] [CrossRef]
- Song, J.; Wang, Y.; Hubbe, M.A.; Rojas, O.J.; Sulic, N.; Sezaki, T. Charge and the dry-strength performance of polyampholytes. Part 1: Handsheet properties and polymer solution viscosity. J. Pulp Pap. Sci. 2006, 32, 156–162. [Google Scholar]
- Sezaki, T.; Hubbe, M.A.; Heitmann, J.A.; Argyropoulos, D.S.; Wang, X. Colloidal effects of acrylamide polyampholytes. Colloid Surf. A 2006, 281, 74–81. [Google Scholar] [CrossRef]
- Zhu, Y.; Jin, E.-s.; Yang, F.; Li, X.; Zhu, W.; Yao, C.; Song, J. Molecular weight of amphoteric polyacrylamide: How it is influenced by the variables in synthesis, and its impacts on the dry strength of paper sheets. BioResources 2016, 11, 3709–3718. [Google Scholar] [CrossRef]
- Liu, Q.; Lei, Y.H.; Bai, Y.Y.; Yang, Y.; He, W.T.; Yao, C.L. Optimizing the preparation conditions of amphoteric polyacrylamide as a strength additive for recycled paper. Nord. Pulp Pap. Res. J. 2018, 33, 297–308. [Google Scholar] [CrossRef]
- Ertas, D.; Kantor, Y. Randomly charged polymers, random walks, and their extremal properties. Phys. Rev. E 1996, 53, 846–860. [Google Scholar] [CrossRef]
- Gutin, A.M.; Shakhnovich, E.I. Effect of a Net Charge on the Conformation of Polyampholytes. Phys. Rev. E 1994, 50, 3322–3325. [Google Scholar] [CrossRef]
- Kantor, Y.; Kardar, M. Instabilities of Charged Polyampholytes. Phys. Rev. E 1995, 51, 1299–1312. [Google Scholar] [CrossRef]
- Lee, N.; Thirumalai, D. Dynamics of collapse of flexible polyampholytes. J. Chem. Phys. 2000, 113, 5126–5129. [Google Scholar] [CrossRef]
- Yamakov, V.; Milchev, A.; Limbach, H.J.; Dunweg, B.; Everaers, R. Conformations of random polyampholytes. Phys. Rev. Lett. 2000, 85, 4305–4308. [Google Scholar] [CrossRef]
- Bratko, D.; Chakraborty, A.K. A numerical study of polyampholyte configuration. J. Phys. Chem. 1996, 100, 1164–1173. [Google Scholar] [CrossRef]
- Schiessel, H.; Blumen, A. Conformations of freely jointed polyampholytes in external fields. J. Chem. Phys. 1996, 104, 6036–6040. [Google Scholar] [CrossRef]
- Srivastava, D.; Muthukumar, M. Sequence dependence of conformations of polyampholytes. Macromolecules 1996, 29, 2324–2326. [Google Scholar] [CrossRef]
- Ozon, F.; di Meglio, J.M.; Joanny, J.F. Adsorption of polyampholytes on charged surfaces. Eur. Phys. J. E 2002, 8, 321–330. [Google Scholar] [CrossRef]
- Tran, Y.; Perrin, P.; Deroo, S.; Lafuma, F. Adsorption of randomly annealed polyampholytes at the silica-water interface. Langmuir 2006, 22, 7543–7551. [Google Scholar] [CrossRef]
- Kamiyama, Y.; Israelachvili, J. Effect of pH and salt on the adsorption and interactions of an amphoteric polyelectrolyte. Macromolecules 1992, 25, 5081–5088. [Google Scholar] [CrossRef]
- Song, J.L.; Yamagushi, T.; Silva, D.J.; Hubbe, M.A.; Rojas, O.J. Effect of Charge Asymmetry on Adsorption and Phase Separation of Polyampholytes on Silica and Cellulose Surfaces. J. Phys. Chem. B 2010, 114, 719–727. [Google Scholar] [CrossRef] [PubMed]
- Abdelmouleh, M.; Boufi, S.; ben Salah, A.; Belgacem, M.N.; Gandini, A. Interaction of silane coupling agents with cellulose. Langmuir 2002, 18, 3203–3208. [Google Scholar] [CrossRef]
- Edvardsson, M.; Rodahl, M.; Kasemo, B.; Hook, F. A dual-frequency QCM-D setup operating at elevated oscillation amplitudes. Anal. Chem. 2005, 77, 4918–4926. [Google Scholar] [CrossRef] [PubMed]
- Marx, K.A.; Zhou, T.; Sarma, R. Quartz crystal microbalance measurement of self-assembled micellar tubules of the amphiphilic decyl ester of D-tyrosine and their enzymatic polymerization. Biotechnol. Prog. 1999, 15, 522–528. [Google Scholar] [CrossRef] [PubMed]
- Rodahl, M.; Kasemo, B. Frequency and dissipation-factor responses to localized liquid deposits on a QCM electrode. Sens. Actuators B 1996, 37, 111–116. [Google Scholar] [CrossRef]
- Rodahl, M.; Kasemo, B. On the measurement of thin liquid overlayers with the quartz-crystal microbalance. Sens. Actuators A 1996, 54, 448–456. [Google Scholar] [CrossRef]
- Song, J.L.; Liang, J.; Liu, X.M.; Krause, W.E.; Hinestroza, J.P.; Rojas, O.J. Development and characterization of thin polymer films relevant to fiber processing. Thin Solid Films 2009, 517, 4348–4354. [Google Scholar] [CrossRef]
- Beck-Candanedo, S.; Roman, M.; Gray, D.G. Effect of reaction conditions on the properties and behavior of wood cellulose nanocrystal suspensions. Biomacromolecules 2005, 6, 1048–1054. [Google Scholar] [CrossRef]
- Song, J.; Li, Y.; Hinestroza, J.P.; Rojas, O.J. Tools to Probe Nanoscale Surface Phenomena in Cellulose Thin Films—Applications in the Area of Adsorption and Friction. In The Nanoscience and Technology of Renewable Biomaterials; Lucia, L., Rojas, O.J., Eds.; Wiley-Blackwell: New York, NY, USA, 2009; pp. 91–122. [Google Scholar]
- Sauerbrey, G. The use of quartz oscillators for weighing thin layers and for microweighing. Z. Angew. Phys. 1959, 155, 206–222. [Google Scholar]
- Rodahl, M.; Hook, F.; Krozer, A.; Brzezinski, P.; Kasemo, B. Quartz-crystal microbalance setup for frequency and Q-factor measurements in gaseous and liquid environments. Rev. Sci. Instrum. 1995, 66, 3924–3930. [Google Scholar] [CrossRef] [Green Version]
- Wågberg, L.; Hägglund, R. Kinetics Of Polyelectrolyte Adsorption On Cellulosic Fibers. Langmuir 2001, 17, 1096–1103. [Google Scholar] [CrossRef]
- Silva, D.J.; Rojas, O.J.; Hubbe, M.A.; Park, S.W.; Yamaguchi, T.; Song, J. Polyampholytes: Their use in papermaking and their solution and adsorption behaviors. O Papel (Braz.) 2009, 70, 40–50. [Google Scholar]
- Khan, M.O.; Akesson, T.; Jonsson, B. Adsorption of polyampholytes to charged surfaces. Macromolecules 2001, 34, 4216–4221. [Google Scholar] [CrossRef]
- Khan, M.O.; Akesson, T.; Jonsson, B. Simple approach to polyampholytes based on chain polarizabilities. J. Chem. Phys. 2002, 116, 3917–3924. [Google Scholar] [CrossRef]
- Radtchenko, I.L.; Papastavrou, G.; Borkovec, M. Direct force measurements between cellulose surfaces and colloidal silica particles. Biomacromolecules 2005, 6, 3057–3066. [Google Scholar] [CrossRef] [PubMed]
- Habibi, Y.; Lucia, L.A.; Rojas, O.J. Cellulose Nanocrystals: Chemistry, Self-Assembly, and Applications. Chem. Rev. 2010, 110, 3479–3500. [Google Scholar] [CrossRef]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Zhu, Y.; Wang, X.; Wang, P.; Tian, J.; Zhu, W.; Song, J.; Xiao, H. Revealing Adsorption Behaviors of Amphoteric Polyacrylamide on Cellulose Fibers and Impact on Dry Strength of Fiber Networks. Polymers 2019, 11, 1886. https://doi.org/10.3390/polym11111886
Zhang X, Zhu Y, Wang X, Wang P, Tian J, Zhu W, Song J, Xiao H. Revealing Adsorption Behaviors of Amphoteric Polyacrylamide on Cellulose Fibers and Impact on Dry Strength of Fiber Networks. Polymers. 2019; 11(11):1886. https://doi.org/10.3390/polym11111886
Chicago/Turabian StyleZhang, Xinyu, Yangyang Zhu, Xiaoyan Wang, Peipei Wang, Jing Tian, Wenyuan Zhu, Junlong Song, and Huining Xiao. 2019. "Revealing Adsorption Behaviors of Amphoteric Polyacrylamide on Cellulose Fibers and Impact on Dry Strength of Fiber Networks" Polymers 11, no. 11: 1886. https://doi.org/10.3390/polym11111886