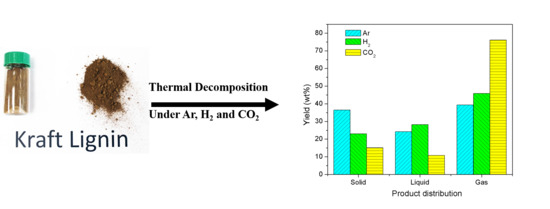
Thermal Decomposition of Kraft Lignin under Gas Atmospheres of Argon, Hydrogen, and Carbon Dioxide
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. FTIR Spectroscopy
2.3. Thermal Decomposition
3. Results and Discussion
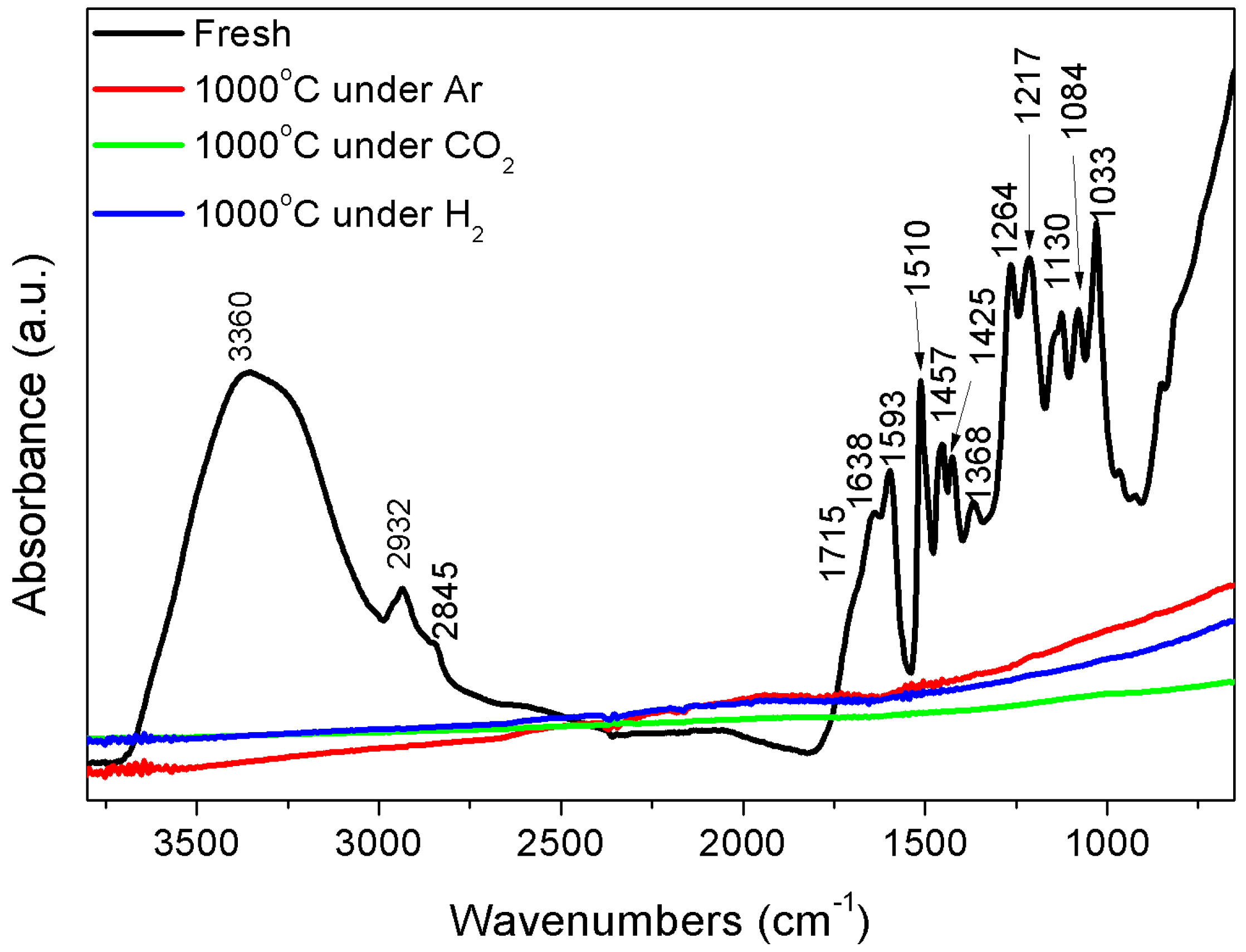
3.1. FT-IR
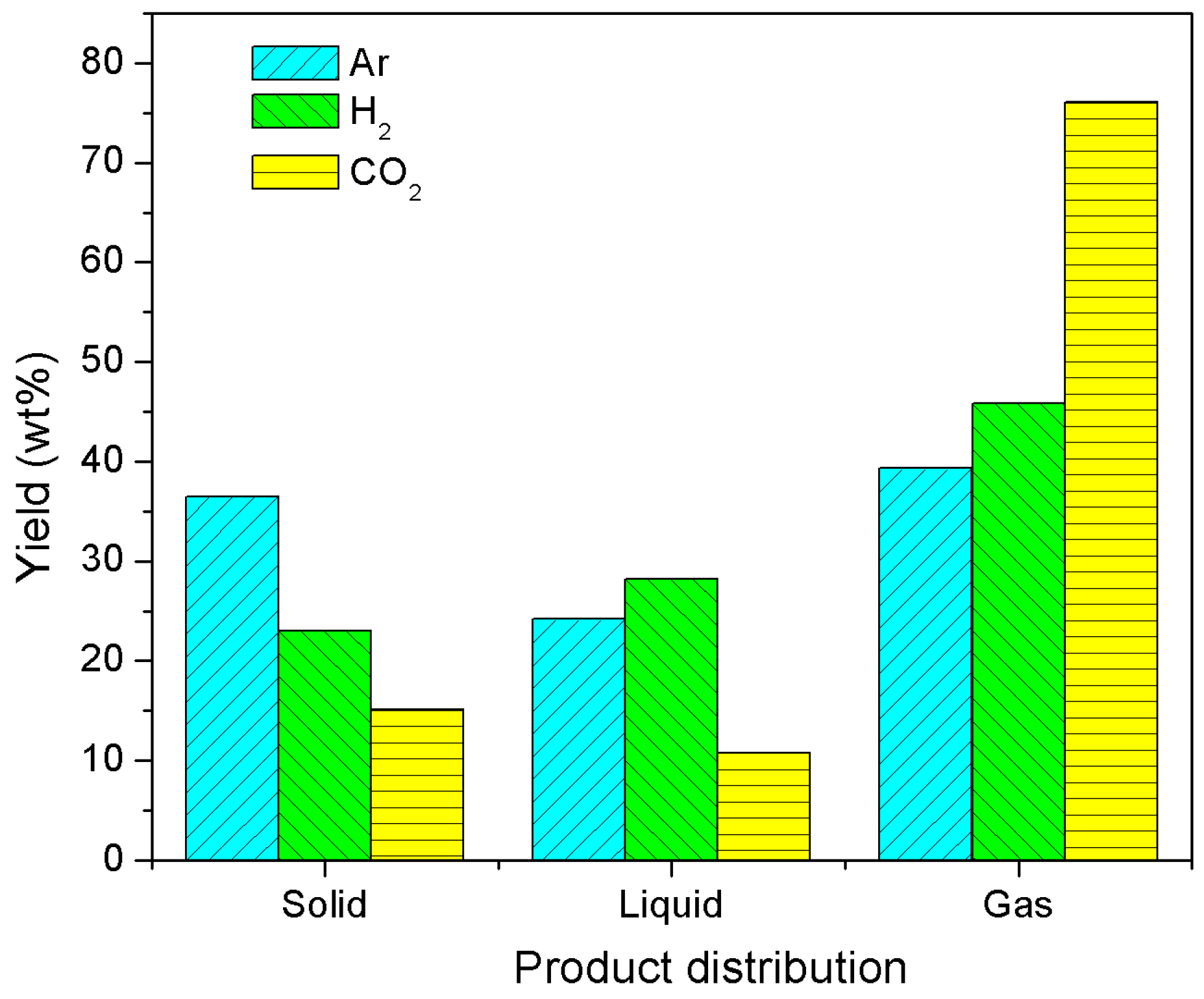
3.2. Product Distribution
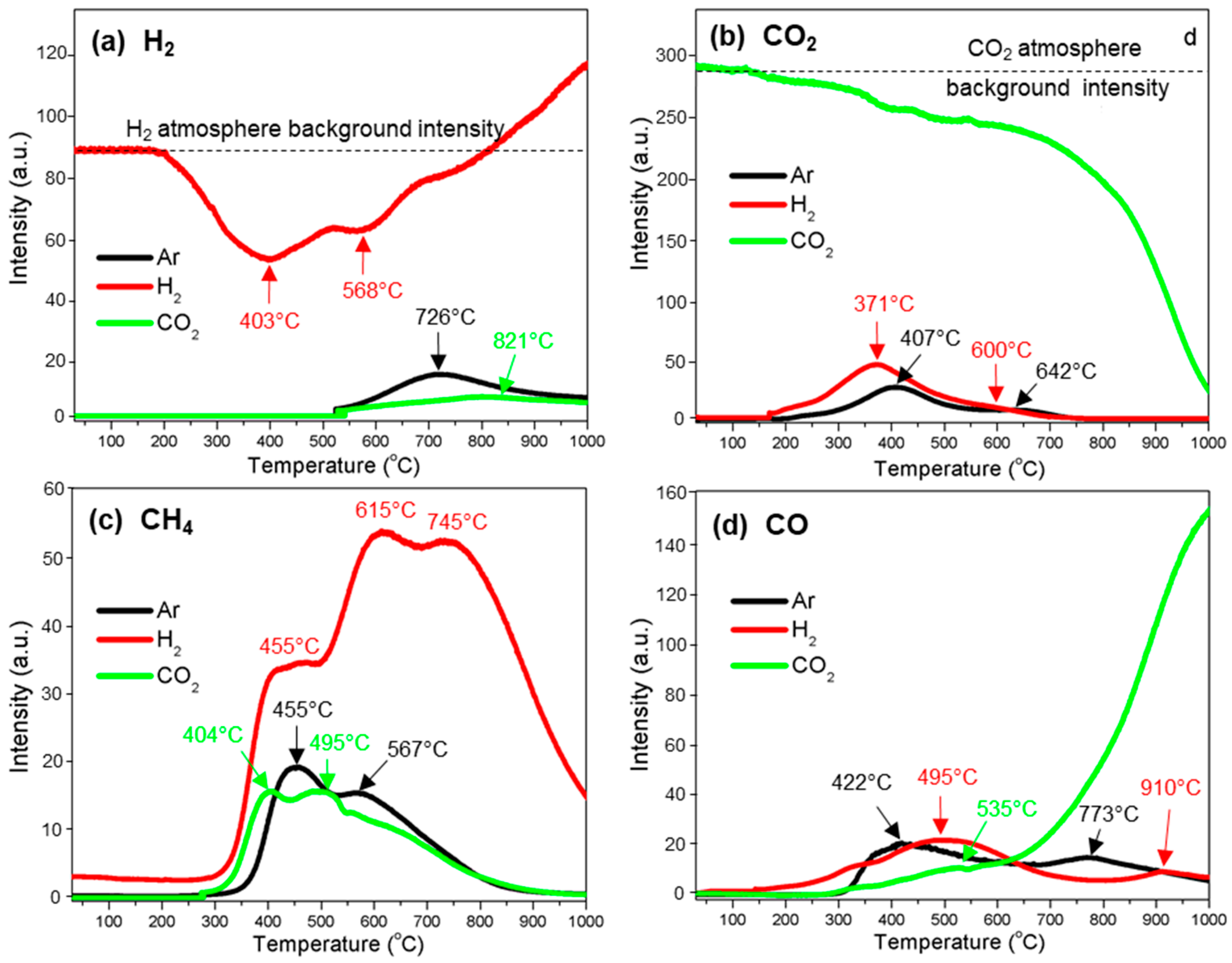
3.3. Gas Evolution
3.3.1. H2
3.3.2. CO2
3.3.3. CH4
3.3.4. CO
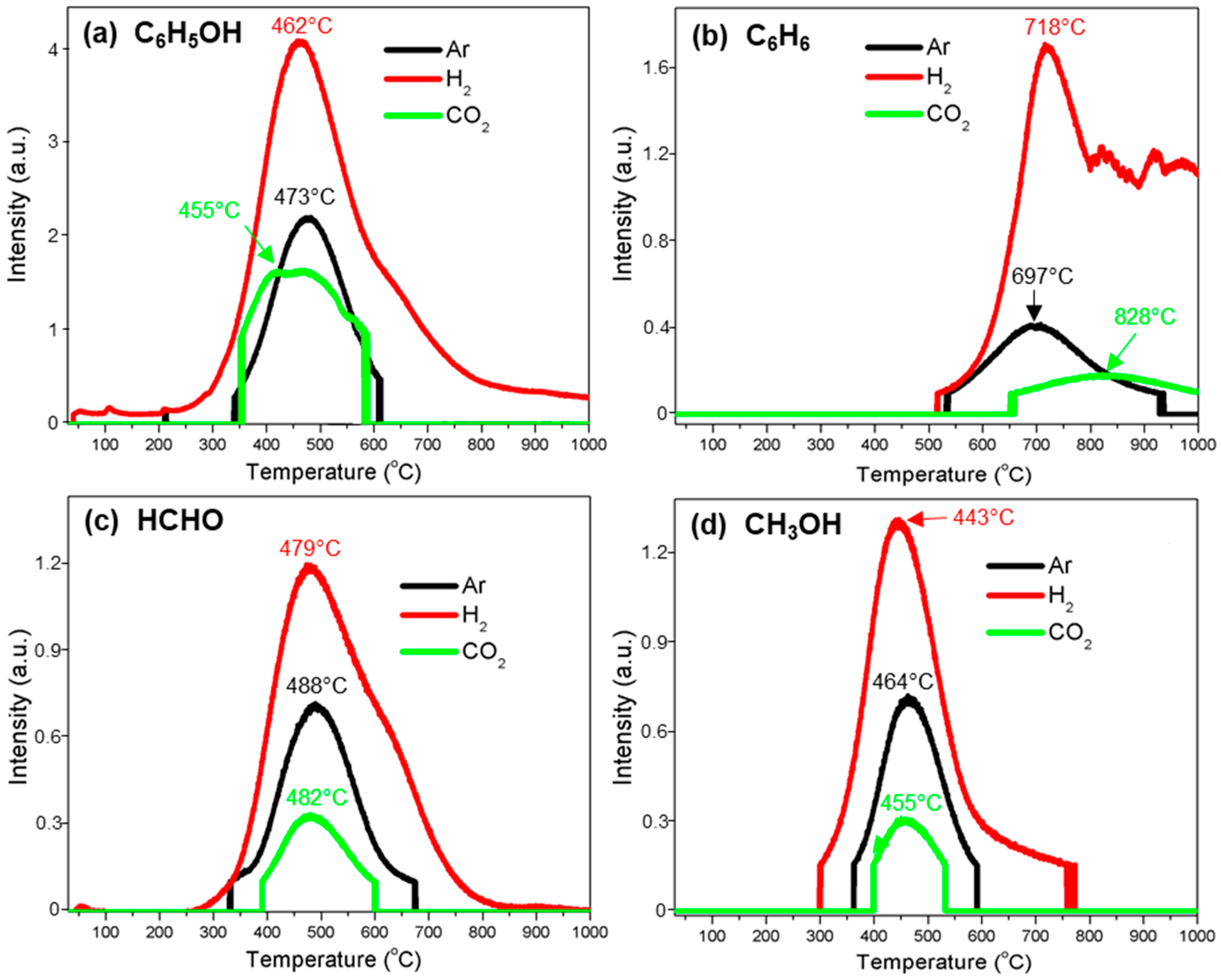
3.3.5. Phenol (C6H5OH)
3.3.6. Benzene (C6H6)
3.3.7. Formaldehyde (HCHO)
3.3.8. Methanol (CH3OH)
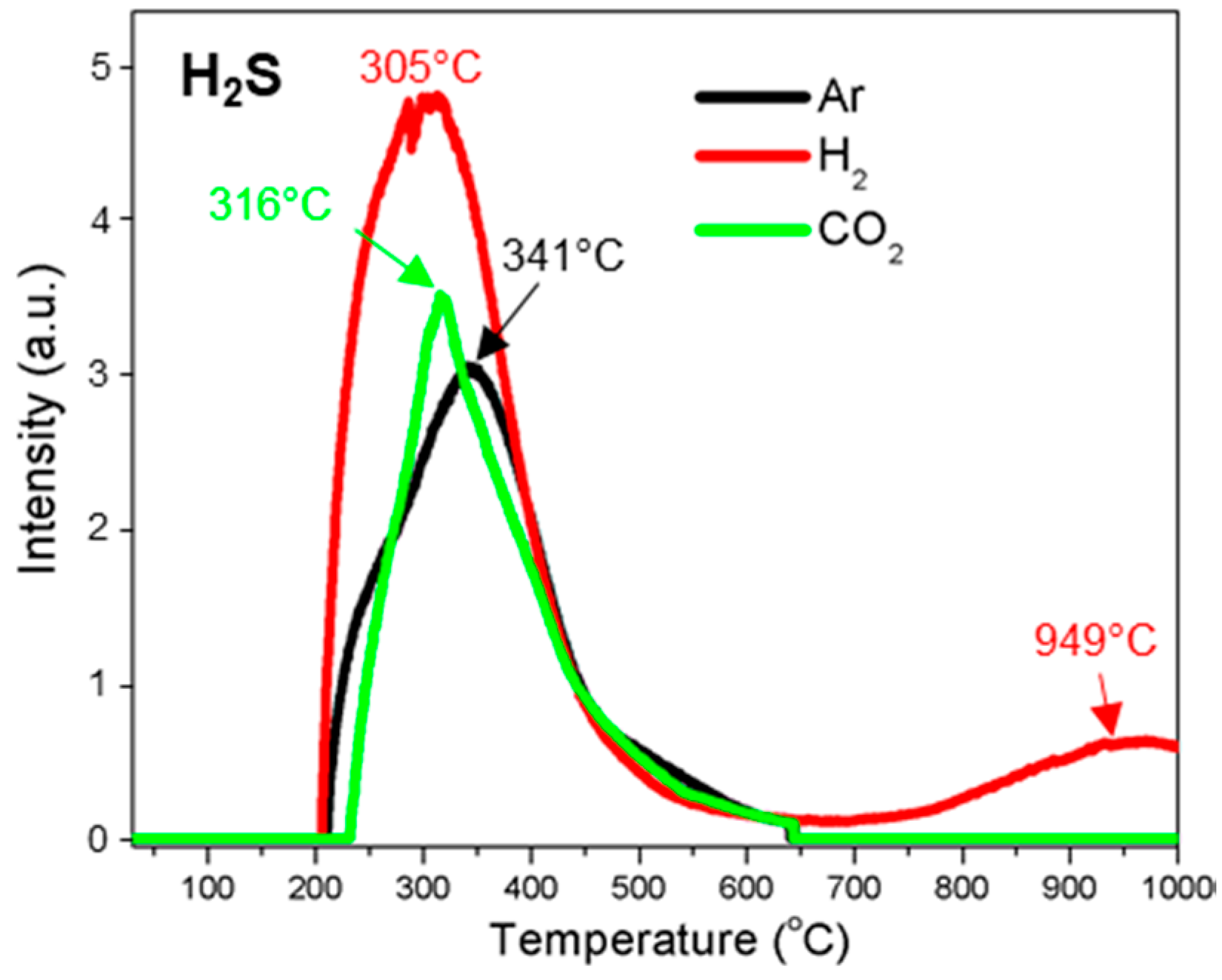
3.3.9. Hydrogen Sulfide (H2S)
3.4. Liquid Phase Analysis
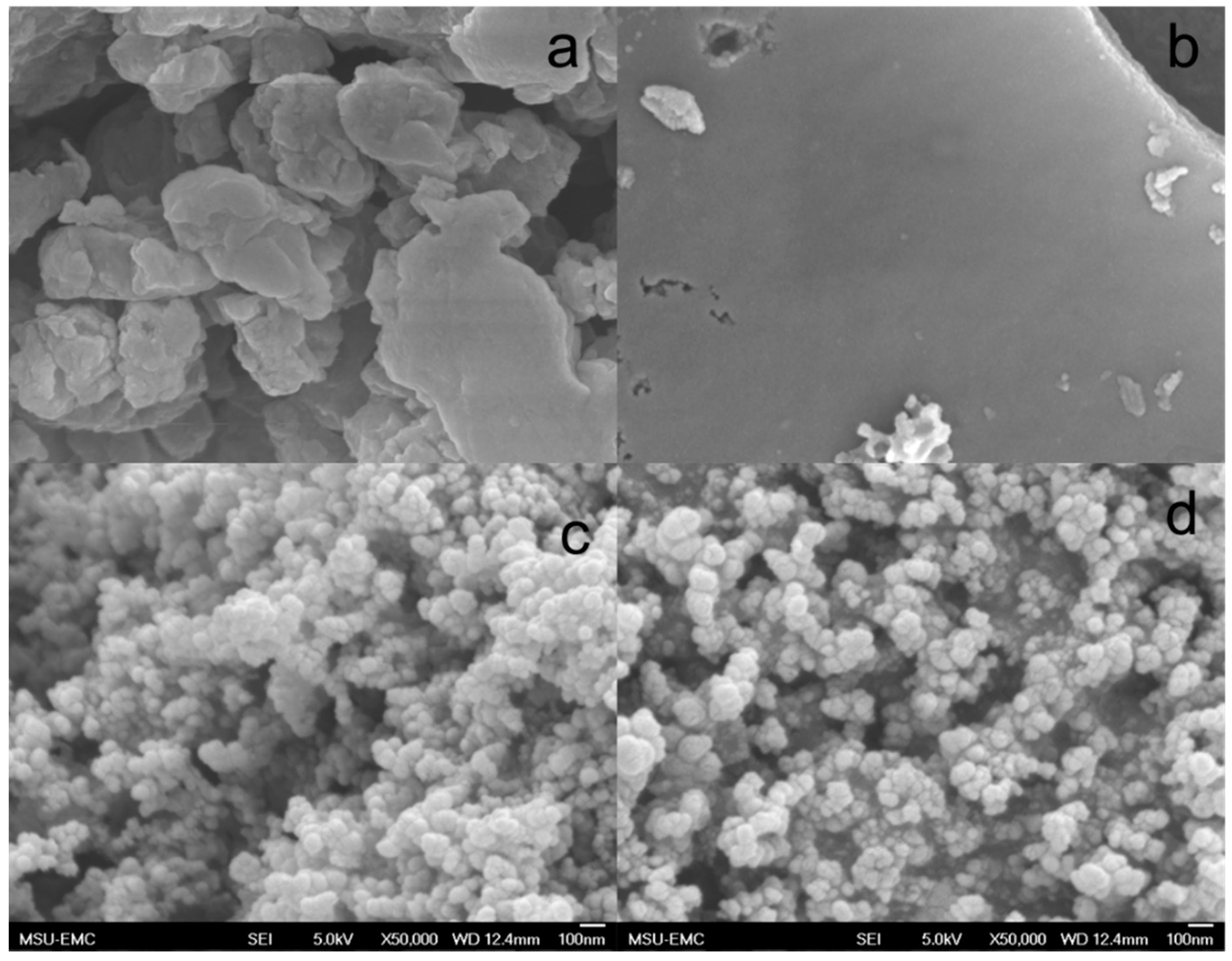
3.5. Analysis and Characterization of Solid Products
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Isikgor, F.H.; Becer, C.R. Lignocellulosic biomass: A sustainable platform for the production of bio-based chemicals and polymers. Polym. Chem. 2015, 6, 4497–4559. [Google Scholar] [CrossRef]
- Zhang, X.; Yan, Q.; Hassan, E.B.; Li, J.; Cai, Z.; Zhang, J. Temperature effects on formation of carbon-based nanomaterials from kraft lignin. Mater. Lett. 2017, 203, 42–45. [Google Scholar] [CrossRef]
- Satheesh Kumar, M.N.; Mohanty, A.K.; Erickson, L.; Misra, M. Lignin and its applications with polymers. J. Biobased Mater. Bioenergy 2009, 3, 1–24. [Google Scholar] [CrossRef]
- Zhang, X.; Yan, Q.; Li, J.; Zhang, J.; Cai, Z. Effects of physical and chemical states of iron-based catalysts on formation of carbon-encapsulated iron nanoparticles from kraft lignin. Materials 2018, 11, 139. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, N.; Yuan, Z.; Schmidt, J.; Xu, C. Production of polyols via direct hydrolysis of kraft lignin: Effect of process parameters. Bioresour. Technol. 2013, 139, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yan, Q.; Leng, W.; Li, J.; Zhang, J.; Cai, Z.; Hassan, E.B. Carbon nanostructure of kraft lignin thermally treated at 500 to 1000 °C. Materials 2017, 10, 975. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Jae, J.; Ha, J.-M.; Suh, D.J. Hydro- and solvothermolysis of kraft lignin for maximizing production of monomeric aromatic chemicals. Bioresour. Technol. 2016, 203, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhao, X.; Wang, A.; Huber, G.W.; Zhang, T. Catalytic transformation of lignin for the production of chemicals and fuels. Chem. Rev. 2015, 115, 11559–11624. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Su, D.; Luo, S.; Jiang, H.; Qian, M.; Zhou, H.; Street, J.; Luo, Y.; Xu, Q. Pyrolysis gas as a carbon source for biogas production via anaerobic digestion. RSC Adv. 2017, 7, 41889–41895. [Google Scholar] [CrossRef] [Green Version]
- Zakzeski, J.; Bruijnincx, P.C.A.; Jongerius, A.L.; Weckhuysen, B.M. The Catalytic Valorization of Lignin for the Production of Renewable Chemicals. Chem. Rev. 2010, 110, 3552–3599. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Yan, R.; Chen, H.; Lee, D.H.; Zheng, C. Characteristics of hemicellulose, cellulose and lignin pyrolysis. Fuel 2007, 86, 1781–1788. [Google Scholar] [CrossRef]
- Snell, G.J.; Huibers, D.T.A. Lignin Cracking Process Using Fast Fluidized Bed Reactions. U.S. Patent 4,409,416, 11 October 1982. [Google Scholar]
- Rodríguez-Mirasol, J.; Bedia, J.; Cordero, T.; Rodríguez, J.J. Influence of Water Vapor on the Adsorption of VOCs on Lignin-Based Activated Carbons. Sep. Sci. Technol. 2005, 40, 3113–3135. [Google Scholar] [CrossRef]
- Rodríguez-Mirasol, J.; Cordero, T.; Rodríguez, J.J. High-temperature carbons from kraft lignin. Carbon 1996, 34, 43–52. [Google Scholar] [CrossRef]
- Hu, S.; Hsieh, Y.-L. Ultrafine microporous and mesoporous activated carbon fibers from alkali lignin. J. Mater. Chem. A 2013, 1, 11279. [Google Scholar] [CrossRef] [Green Version]
- Ruiz-Rosas, R.; Valero-Romero, M.J.; Salinas-Torres, D.; Rodríguez-Mirasol, J.; Cordero, T.; Morallón, E.; Cazorla-Amorós, D. Electrochemical Performance of Hierarchical Porous Carbon Materials Obtained from the Infiltration of Lignin into Zeolite Templates. ChemSusChem 2014, 7, 1458–1467. [Google Scholar] [CrossRef] [PubMed]
- Mun, S.P.; Cai, Z.; Zhang, J. Fe-catalyzed thermal conversion of sodium lignosulfonate to grapheme. Mater. Lett. 2013, 100, 180–183. [Google Scholar] [CrossRef]
- Asadullah, M.; Rahman, M.A.; Ali, M.M.; Motin, M.A.; Sultan, M.B.; Alam, M.R.; Rahman, M.S. Jute stick pyrolysis for bio-oil production in fluidized bed reactor. Bioresour. Technol. 2008, 99, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Shen, D.K.; Gu, S.; Luo, K.H.; Wang, S.R.; Fang, M.X. The pyrolytic degradation of wood-derived lignin from pulping process. Bioresour. Technol. 2010, 101, 6136–6146. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Xiao, G.; Xu, X.; Shen, D.; Jin, B. Study on carbonization of lignin by TG-FTIR and high-temperature carbonization reactor. Fuel Process. Technol. 2013, 106, 41–47. [Google Scholar] [CrossRef]
- Fox, S.C.; McDonald, A.G. Chemical and thermal charcterization of three industrial lignins and their corresponding lignin esters. BioResources 2010, 5, 990–1009. [Google Scholar] [CrossRef]
- Tsujiyama, S. Differential scanning calorimetric analysis of the lignin-carbohydrate complex degraded by wood-rotting fungi. J. Wood Sci. 2001, 47, 497–510. [Google Scholar] [CrossRef]
- Chen, C.L. Characterization of lignin by oxidative degradation: Use of gas chromatography-mass spectrometry technique. Meth. Enzymol. 1988, 161, 110–136. [Google Scholar]
- Kale, S.K.; Deshmukh, A.G.; Dudhare, M.S.; Patil, V.B. Microbial degradation of plastic: A review. J. Biochem. Technol. 2015, 6, 952–961. [Google Scholar]
- Dobele, G.; Rossinskaja, G.; Dizhbite, T.; Telysheva, G.; Meier, D.; Faix, O. Application of catalysts for obtaining 1,6-anhydrosaccharides from cellulose and wood by fast pyrolysis. J. Anal. Appl. Pyrolysis 2005, 74, 401–405. [Google Scholar] [CrossRef]
- Predel, M.; Kaminsky, W. Pyrolysis of rape-seed in a fluidised-bed reactor. Bioresour. Technol. 1998, 66, 113–117. [Google Scholar] [CrossRef]
- Heo, H.S.; Park, H.J.; Yim, J.-H.; Sohn, J.M.; Park, J.; Kim, S.-S.; Ryu, C.; Jeon, J.-K.; Park, Y.-K. Influence of operation variables on fast pyrolysis of Miscanthus sinensis var. purpurascens. Bioresour. Technol. 2010, 101, 3672–3677. [Google Scholar] [CrossRef] [PubMed]
- He, R.; Ye, X.P.; English, B.C.; Satrio, J.A. Influence of pyrolysis condition on switchgrass bio-oil yield and physicochemical properties. Bioresour. Technol. 2009, 100, 5305–5311. [Google Scholar] [CrossRef] [PubMed]
- Brebu, M.; Tamminen, T.; Spiridon, I. Thermal degradation of various lignins by TG-MS/FTIR and Py-GC-MS. J. Anal. Appl. Pyrolysis 2013, 104, 531–539. [Google Scholar] [CrossRef]
- Shen, D.; Hu, J.; Xiao, R.; Zhang, H.; Li, S.; Gu, S. Online evolved gas analysis by Thermogravimetric-Mass Spectroscopy for thermal decomposition of biomass and its components under different atmospheres: Part I. Lignin. Bioresour. Technol. 2013, 130, 449–456. [Google Scholar] [CrossRef] [PubMed]
- Yan, Q.; Toghiani, H.; Yu, F.; Cai, Z.; Zhang, J.; Yan, Q. Effects of pyrolysis conditions on yield of bio-chars from pine chips. For. Prod. J. 2011, 61, 367–371. [Google Scholar] [CrossRef]
- Mamoru Kaiho, Y.T. Change in thermoplastic properties of coal under pressure of various gases. Fuel 1979, 58, 397–398. [Google Scholar] [CrossRef]
- ASTM D4442-07(2007). Standard Test Methods for Direct Moisture Content Measurement of Wood and Wood-Based Materials; ASTM Int.: West Conshohocken, PA, USA, 2007. [Google Scholar]
- ASTM D1102-84(2007). Standard Test Method for Ash in Wood; ASTM Int.: West Conshohocken, PA, USA, 2007. [Google Scholar]
- Faix, O. Classification of lignins from different botanical origins by FT-IR spectroscopy. Holzforschung 1991, 45, 21–27. [Google Scholar] [CrossRef]
- Brebu, M.; Vasile, C. Thermal degradation of lignin—A review. Cellul. Chem. Technol. 2010, 44, 353–363. [Google Scholar]
- Yamaguchi, A.; Mimura, N.; Shirai, M.; Sato, O. Bond cleavage of lignin model compounds into aromatic monomers using supported metal catalysts in supercritical water. Sci. Rep. 2017, 7, 46172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsuchiya, K.; Huang, J.D.; Tominaga, K.I. Reverse Water-Gas Shift Reaction Catalyzed by Mononuclear Ru Complexes. ACS Catal. 2013, 3, 2865–2868. [Google Scholar] [CrossRef]
- Wang, S.; Lin, H.; Ru, B.; Sun, W.; Wang, Y.; Luo, Z. Comparison of the pyrolysis behavior of pyrolytic lignin and milled wood lignin by using TG–FTIR analysis. J. Anal. Appl. Pyrolysis 2014, 108, 78–85. [Google Scholar] [CrossRef]
- Zhang, H.; Xiao, R.; Wang, D.; He, G.; Shao, S.; Zhang, J.; Zhong, Z. Biomass fast pyrolysis in a fluidized bed reactor under N2, CO2, CO, CH4 and H2 atmospheres. Bioresour. Technol. 2011, 102, 4258–4264. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wang, K.; Liu, Q.; Gu, Y.; Luo, Z.; Cen, K.; Fransson, T. Comparison of the pyrolysis behavior of lignins from different tree species. Biotechnol. Adv. 2009, 27, 562–567. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Wang, S.; Zheng, Y.; Luo, Z.; Cen, K. Mechanism study of wood lignin pyrolysis by using TG–FTIR analysis. J. Anal. Appl. Pyrolysis 2008, 82, 170–177. [Google Scholar] [CrossRef]
- Kishimoto, T.; Uraki, Y.; Ubukata, M. Chemical synthesis of β-O-4 type artificial lignin. Org. Biomol. Chem. 2006, 4, 1343–1347. [Google Scholar] [CrossRef] [PubMed]
- Babij, N.R.; McCusker, E.O.; Whiteker, G.T.; Canturk, B.; Choy, N.; Creemer, L.C.; Amicis, C.V.D.; Hewlett, N.M.; Johnson, P.L.; Knobelsdorf, J.A.; et al. NMR chemical shifts of trace impurities: Industrially preferred solvents used in process and green chemistry. Org. Process Res. Dev. 2016, 20, 661–667. [Google Scholar] [CrossRef]
- Zhao, J.; Xiuwen, W.; Hu, J.; Liu, Q.; Shen, D.; Xiao, R. Thermal degradation of softwood lignin and hardwood lignin by TG-FTIR and Py-GC/MS. Polym. Degrad. Stab. 2014, 108, 133–138. [Google Scholar] [CrossRef]
- Sjostrom, E. Wood Chemistry: Fundamentals and Applications; Elsevier: Amsterdam, The Netherlands, 2013; ISBN 978-0-08-092589-9. [Google Scholar]
- Dondi, D.; Zeffiro, A.; Speltini, A.; Tomasi, C.; Vadivel, D.; Buttafava, A. The role of inorganic sulfur compounds in the pyrolysis of Kraft lignin. J. Anal. Appl. Pyrolysis 2014, 107, 53–58. [Google Scholar] [CrossRef]
| Gas/Volatile | Gas Atmosphere | ||
|---|---|---|---|
| Argon | Hydrogen °C | Carbon Dioxide | |
| H2 | 522–1000 (722) | 205–527 (403) * 527–800 (568) * 800–1000 | 821 (522–1000) |
| CO2 | 185–583 (407) 583–791 (642) | 162–554 (371) 654–770 (600) | 585–1000 * |
| CH4 | 236–532 (455) 532–915 (567) | 268–495 (455) 495–688 (615) 688–1000 (745) | 266–443 (404) 445–920 (495) |
| CO | 246–679 (422) 679–1000 (773) | 155–780 (495) 780–1000 (910) | 279–547 (535) 547–1000 (1000) |
| C6H5OH | 338–610 (473) | 239–854 (462) | 345–600 (445) |
| C6H6 | 528–938 (697) | 510–799 (718) 799–1000 | 656–1000 (828) |
| HCHO | 329–678 (488) | 249–850 (479) | 390–601 (482) |
| CH3OH | 361–595 (464) | 299–778 (443) | 395–537 (455) |
| H2S | 210–646 (341) | 199–656 (305) 704–1000 (949) | 228–645 (316) |
| Aqueous Phase Components | Ar | H2 | CO2 |
|---|---|---|---|
| Water content | 90.1 | 85.3 | 95.5 |
| Compounds in aqueous phase | |||
| Acetic acid | 27.8 | 25.5 | 29.1 |
| Acetone | 8.5 | 8.6 | 9.1 |
| Hydroxyacetaldehyde | 6.7 | 6.6 | 5.7 |
| Methanol | 2.5 | 4.5 | 2.1 |
| Phenols | 24.7 | 27.6 | 24.3 |
| Other acids | 8.5 | 6.1 | 10.7 |
| Other alcohols | 2.7 | 2.9 | 1.5 |
| Other ketones | 5.9 | 5.8 | 5 |
| Other aldehydes | 4.3 | 4.8 | 4 |
| Esters | 1.1 | 0.8 | 1.3 |
| Nonidentified | 7.3 | 6.8 | 7.2 |
| Thermally Treated Condition | C | H | O | N | S |
|---|---|---|---|---|---|
| Untreated | 65.2 ± 0.2 | 6.1 ± 0.2 | 27.4 ± 0.9 | 0.1 ± 0.05 | 0.8 ± 0.2 |
| Under Ar | 92.3 ± 0.7 | 0.9 ± 0.1 | 1.8 ± 0.5 | - | 0.1 ± 0.1 |
| Under H2 | 96.3 ± 0.5 | 1.0 ± 0.2 | 1.2 ± 0.3 | - | - |
| Under CO2 | 97.5 ± 0.5 | 0.5 ± 0.1 | 1.5 ± 0.3 | - | - |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, Q.; Li, J.; Zhang, J.; Cai, Z. Thermal Decomposition of Kraft Lignin under Gas Atmospheres of Argon, Hydrogen, and Carbon Dioxide. Polymers 2018, 10, 729. https://doi.org/10.3390/polym10070729
Yan Q, Li J, Zhang J, Cai Z. Thermal Decomposition of Kraft Lignin under Gas Atmospheres of Argon, Hydrogen, and Carbon Dioxide. Polymers. 2018; 10(7):729. https://doi.org/10.3390/polym10070729
Chicago/Turabian StyleYan, Qiangu, Jinghao Li, Jilei Zhang, and Zhiyong Cai. 2018. "Thermal Decomposition of Kraft Lignin under Gas Atmospheres of Argon, Hydrogen, and Carbon Dioxide" Polymers 10, no. 7: 729. https://doi.org/10.3390/polym10070729