Novel Kinetic Models of Xylan Dissolution and Degradation during Ethanol Based Auto-Catalyzed Organosolv Pretreatment of Bamboo
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Ethanol Based Auto-Catalyzed Organosolv (EACO) Pretreatment
2.3. Analysis of Pretreatment Hydrolysates and Solid Substrates
2.3.1. Compositional Analysis of Raw Material and Substrates
2.3.2. Measurement of Degradations Concentration in Pretreatment Hydrolysates
3. Results and Discussion
3.1. Development of Kinetic Models
3.1.1. Kinetic Model of Xylan Dissolution
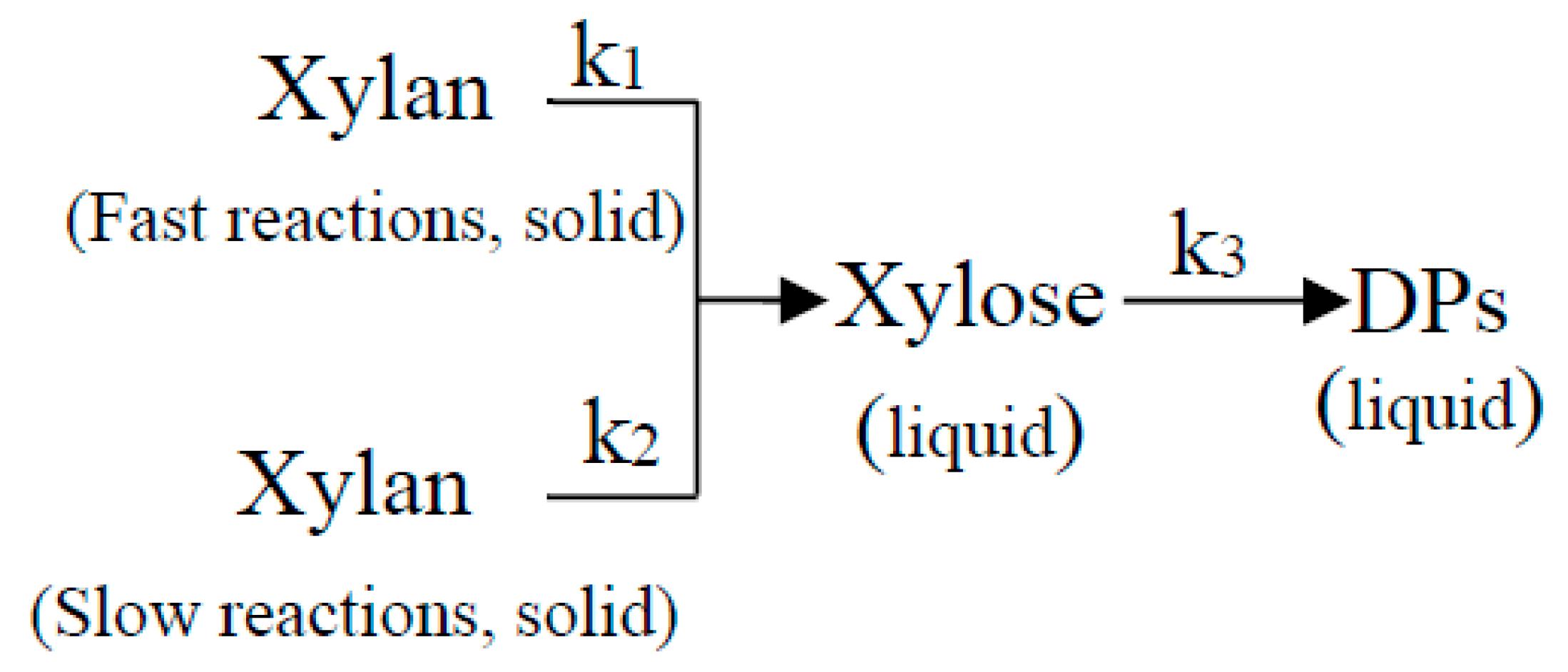
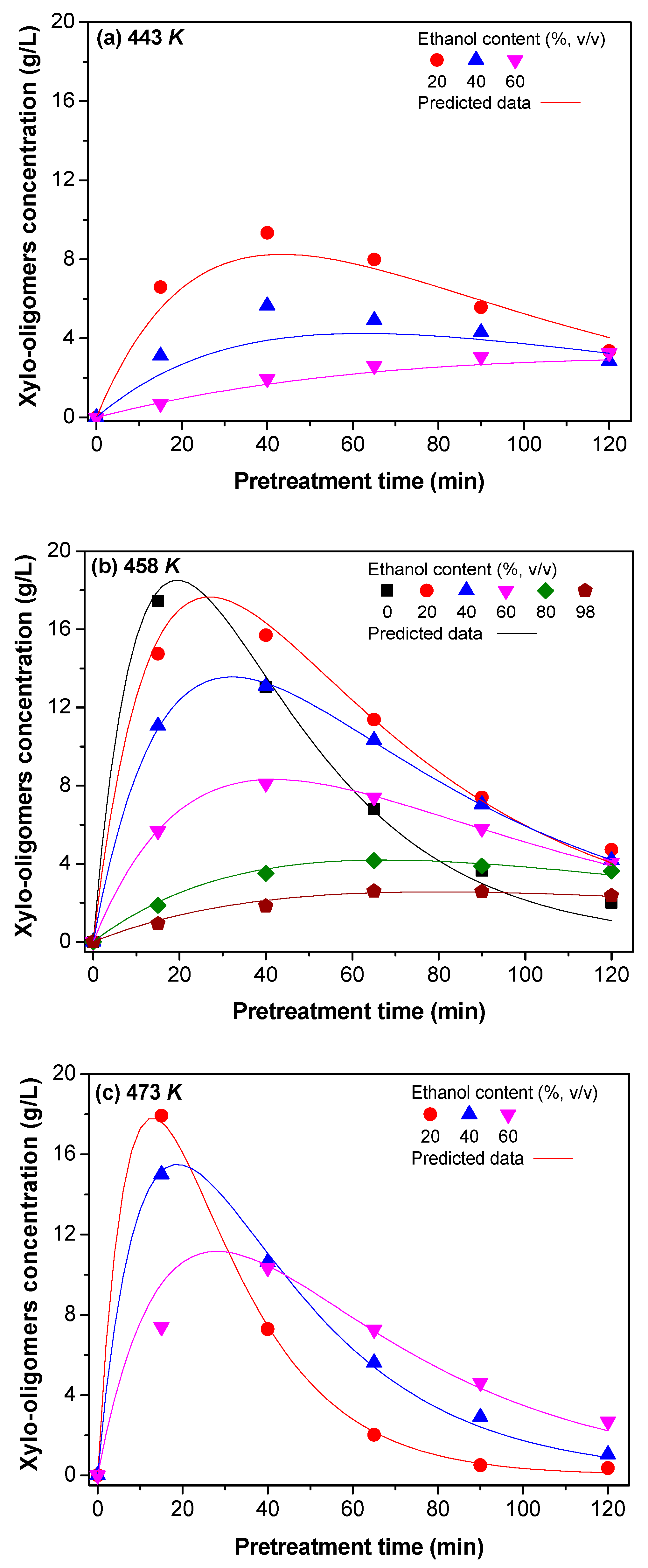
3.1.2. Kinetic Model of Xylo-Oligosaccharides Formation
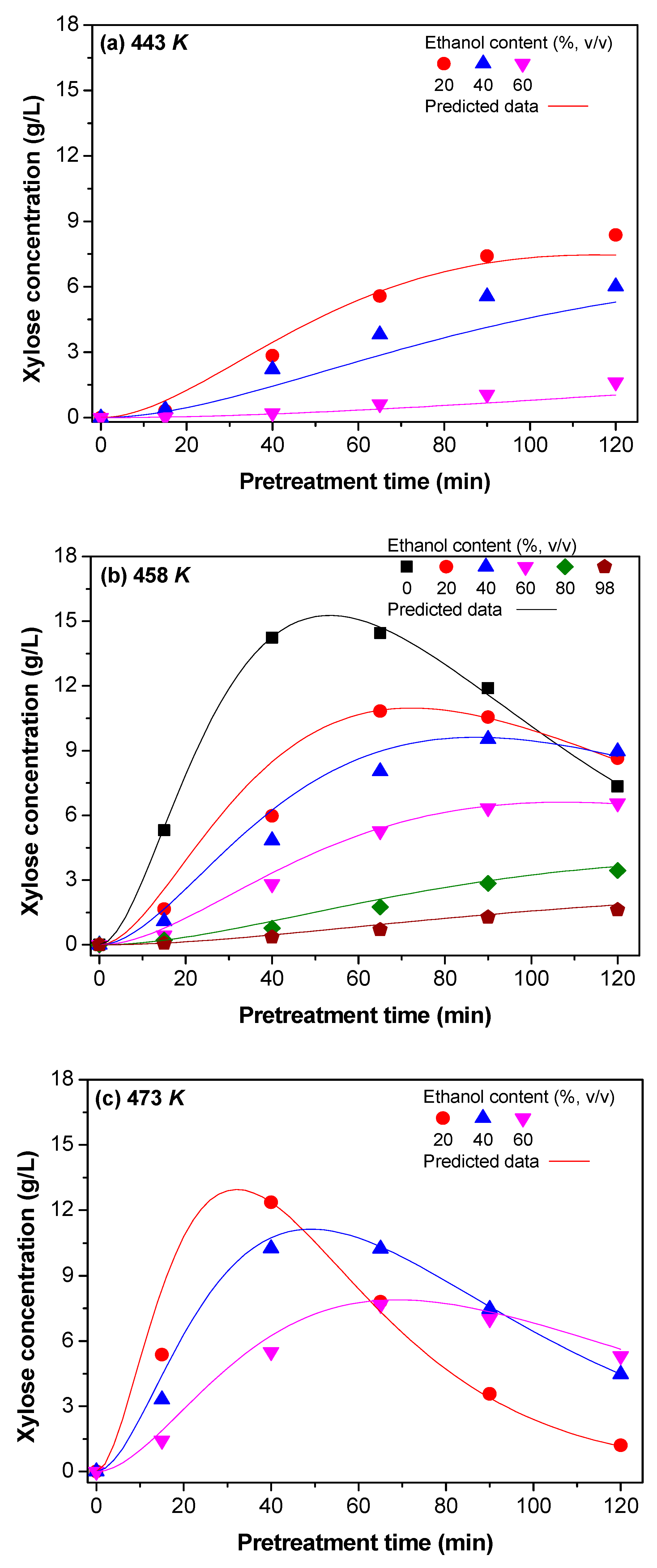
3.1.3. Kinetic Model of Xylose Formation
3.2. Determination of Kinetic Constants
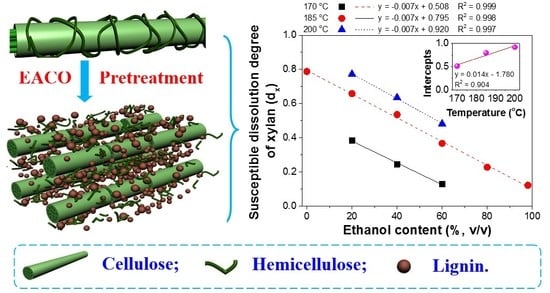
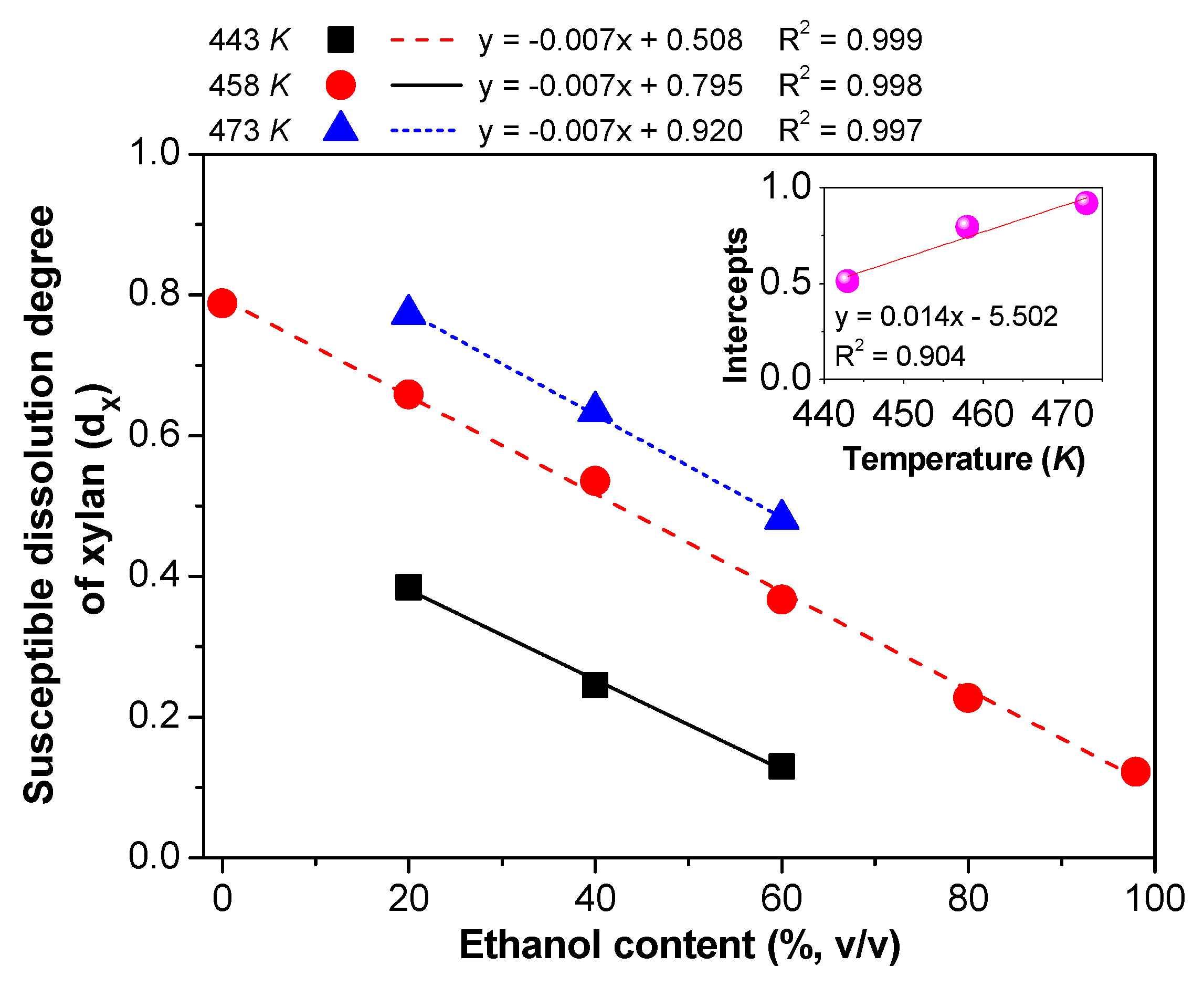
3.2.1. The dx and Reaction Rate Constants
3.2.2. Activation Energy and Pre-Exponential Factor
3.3. The Relationship between the dX and Pretreatment Conditions
3.4. Predicting the Formations of Furfural and Acetic Acid
3.5. The Relationships between Xylan and Other Main Component Removal
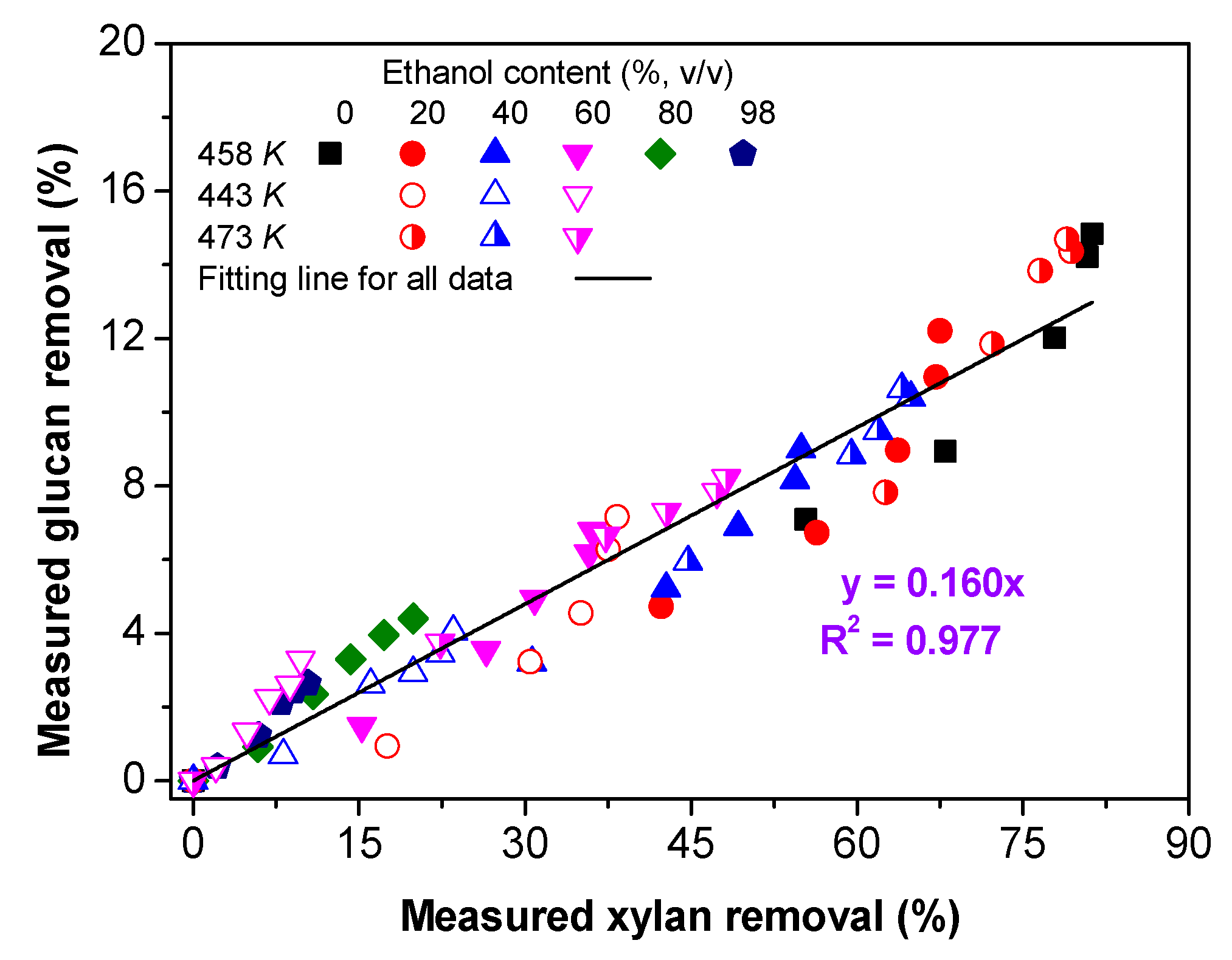
3.5.1. The Relationship between Xylan and Glucan Removal
3.5.2. The Relationship between Xylan and Klason Lignin Removal
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Li, M.F.; Yang, S.; Sun, R.C. Recent advances in alcohol and organic acid fractionation of lignocellulosic biomass. Bioresour. Technol. 2015, 200, 971–980. [Google Scholar] [CrossRef] [PubMed]
- Ragauskas, A.J.; Williams, C.K.; Davison, B.H.; Britovsek, G.; Cairney, J.; Eckert, C.A.; Frederick, W.J., Jr.; Hallett, J.P.; Leak, D.J.; Liotta, C.L.; et al. The path forward for biofuels and biomaterials. Science 2006, 311, 484–489. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Pei, Z.; Wang, D. Organic solvent pretreatment of lignocellulosic biomass for biofuels and biochemicals: A review. Bioresour. Technol. 2016, 199, 21–33. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.Y.; Pan, X.; Zalesny, R.S. Pretreatment of woody biomass for biofuel production: Energy efficiency, technologies, and recalcitrance. Appl. Microbiol. Biotechnol. 2010, 87, 847–857. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.B.; Li, S.M.; Wu, R.C.; Liu, D.H. Organosolv fractionating pretreatment of lignocellulosic biomass for efficient enzymatic saccharification: Chemistry, kinetics, and substrate structures. Biofuels Bioprod. Biorefin. 2017, 11, 567–590. [Google Scholar] [CrossRef]
- Zhang, Z.Y.; Harrison, M.D.; Rackemann, D.W.; Doherty, W.O.S.; O’Hara, I.M. Organosolv pretreatment of plant biomass for enhanced enzymatic saccharification. Green Chem. 2016, 47, 360–381. [Google Scholar] [CrossRef] [Green Version]
- Shuai, L.; Luterbacher, J. Organic Solvent Effects in Biomass Conversion Reactions. ChemSusChem 2015, 9, 133–155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, X.B.; Cheng, K.; Liu, D.H. Organosolv pretreatment of lignocellulosic biomass for enzymatic hydrolysis. Appl. Microbiol. Biotechnol. 2009, 82, 815–827. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.J.; Arato, C.; Gilkes, N.; Gregg, D.J.; Mabee, W.; Pye, E.K.; Xiao, Z.; Zhang, X.; Saddler, J.N. Biorefining of softwoods using ethanol organosolv pulping—Preliminary evaluation of process streams for manufacture of fuel-grade ethanol and co-products. Biotechnol. Bioeng. 2005, 90, 473–481. [Google Scholar] [CrossRef] [PubMed]
- Ghose, T.K.; Tyagi, R.D. Rapid ethanol fermentation of cellulose hydrolysate. I. Batch versus continuous systems. Biotechnol. Bioeng. 2010, 21, 1387–1400. [Google Scholar] [CrossRef]
- Ando, H.; Ohba, H.; Sakaki, T.; Takamine, K.; Kamino, Y.; Moriwaki, S.; Bakalova, R.; Uemura, Y.; Hatate, Y. Hot-compressed-water decomposed products from bamboo manifest a selective cytotoxicity against acute lymphoblastic leukemia cells. Toxicol. In Vitro 2004, 18, 765–771. [Google Scholar] [CrossRef] [PubMed]
- Winkelhausen, E.; Kuzmanova, S. Microbial conversion of D-xylose to xylitol. J. Ferment. Bioeng. 1998, 86, 1–14. [Google Scholar] [CrossRef]
- Schutyser, W.; Renders, T.; Van, D.B.S.; Koelewijn, S.F.; Beckham, G.T.; Sels, B.F. Chemicals from lignin: An interplay of lignocellulose fractionation, depolymerisation, and upgrading. Chem. Soc. Rev. 2018, 47, 852–908. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.Q.; Wu, S.B.; Xu, S.H. Enhancement of enzymatic saccharification of bagasse by ethanol-based organosolv auto-catalyzed pretreatment. J. Chem. Technol. Biotechnol. 2017, 92, 580–587. [Google Scholar] [CrossRef]
- Wen, J.L.; Xue, B.L.; Sun, S.L.; Sun, R.C. Quantitative structural characterization and thermal properties of birch lignins after auto-catalyzed organosolv pretreatment and enzymatic hydrolysis. J. Chem. Technol. Biotechnol. 2013, 88, 1663–1671. [Google Scholar] [CrossRef]
- Oliet, M.; Rodríguez, F.; Santos, A.; Gilarranz, M.A.; Félix Garcíaochoa, A.; Tijero, J. Organosolv delignification of eucalyptus globulus: kinetic study of autocatalyzed ethanol pulping. Ind. Eng. Chem. Res. 2000, 39, 34–39. [Google Scholar] [CrossRef]
- Shatalov, A.A.; Pereira, H. Kinetics of organosolv delignification of fibre crop Arundo donax L. Ind. Crops Prod. 2005, 21, 203–210. [Google Scholar] [CrossRef]
- Sidiras, D.; Koukios, E. Simulation of acid-catalysed organosolv fractionation of wheat straw. Bioresour. Technol. 2004, 94, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Saeman, J.F. Kinetics of wood saccharification: Hydrolysis of cellulose and decomposition of sugars in dilute acid at high temperature. Ind. Eng. Chem. 1945, 37, 42–52. [Google Scholar] [CrossRef]
- Lavarack, B.P.; Griffin, G.J.; Rodman, D. The acid hydrolysis of sugarcane bagasse hemicellulose to produce xylose, arabinose, glucose and other products. Biomass Bioenerg. 2002, 23, 367–380. [Google Scholar] [CrossRef]
- Montane, D.; Salvado, J.; Torras, C.; Farriol, X. High-temperature dilute-acid hydrolysis of olive stones for furfural production. Biomass Bioenerg. 2002, 22, 295–304. [Google Scholar] [CrossRef]
- Caparrós, S.; Garrote, G.; Ariza, J.; López, F. Autohydrolysis of Arundo donax L., a kinetic assessment. Ind. Eng. Chem. Res. 2006, 45, 8909–8920. [Google Scholar] [CrossRef]
- Schwiderski, M.; Kruse, A.; Grandl, R.; Dockendorf, D. Comparison of the influence of a Lewis acid AlCl3 and a Brønsted acid HCl on the organosolv pulping of beech wood. Green Chem. 2013, 16, 1569–1578. [Google Scholar] [CrossRef]
- Garrote, G.; Domı́Nguez, H.; Parajo, J.C. Kinetic modelling of corncob autohydrolysis. Process Biochem. 2001, 36, 571–578. [Google Scholar] [CrossRef]
- Guerra-Rodríguez, E.; Portilla-Rivera, O.M.; Jarquín-Enríquez, L.; Ramírez, J.A.; Vázquez, M. Acid hydrolysis of wheat straw: A kinetic study. Biomass Bioenerg. 2012, 36, 346–355. [Google Scholar] [CrossRef]
- Rodrı́Guez-Chong, A.; Ramı́Rez, J.A.; Garrote, G.; Vázquez, M. Hydrolysis of sugar cane bagasse using nitric acid: A kinetic assessment. J. Food Eng. 2004, 61, 143–152. [Google Scholar] [CrossRef]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D.; Crocker, D. Determination of Structural Carbohydrates and Lignin in Biomass; Laboratory Analytical Procedure (LAP), Technical Report (NREL/TP-510-42618); National Renewable Energy Laboratory: Golden, CO, USA, 2008. [Google Scholar]
- Pan, X.J.; Gilkes, N.; Kadla, J.; Pye, K.; Saka, S.; Ehara, K.; Gregg, D.; Xie, D.; Lam, D.; Saddler, J. Bioconversion of hybrid poplar to ethanol and co-products using an organosolv fractionation Process: Optimization of process yields. Biotechnol. Bioeng. 2006, 94, 851–861. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.H.; Liu, J.; Li, M.; Huang, L.L.; Chen, L.H.; Luo, X.L. Acid-free ethanol-water pretreatment with low ethanol concentration for robust enzymatic saccharification of cellulose in bamboo. BioEnergy Res. 2018, 11, 665–676. [Google Scholar] [CrossRef]
- Lu, Y.; Mosier, N.S. Kinetic modeling analysis of maleic acid-catalyzed hemicellulose hydrolysis in corn stover. Biotechnol. Bioeng. 2010, 101, 1170–1181. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Li, M.; Luo, X.L.; Chen, L.H.; Huang, L.L. Effect of hot-water extraction (HWE) severity on bleached pulp based biorefinery performance of eucalyptus during the hwe-kraft-ecf bleaching process. Bioresour. Technol. 2015, 181, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Vroom, K.E. The “H” factor: A means of expressing cooking times and temperatures as a single variable. Pulp Paper Mag. Can. 1957, 58, 228–231. [Google Scholar]
- Tunc, M.S.; van Heiningen, A.R.P. Autohydrolysis of mixed southern hardwoods: Effect of P-factor. Nord. Pulp Pap. Res. J. 2009, 24, 46–51. [Google Scholar] [CrossRef]
- Lin, C.K. Prehydrolysis-Alkaline Pulping of Sweetgum Wood. Ph.D. Thesis, Department of Wood and Paper Science, North Carolina State University, Raleigh, NC, USA , 1979. [Google Scholar]
- Liu, J.; Li, R.Q.; Shuai, L.; You, J.H.; Zhao, Y.B.; Chen, L.; Li, M.; Chen, L.H.; Huang, L.L.; Luo, X.L. Comparison of liquid hot water (LHW) and high boiling alcohol/water (HBAW) pretreatments for improving enzymatic saccharification of cellulose in bamboo. Ind. Crop. Prod. 2017, 107, 139–148. [Google Scholar] [CrossRef]
- Ni, Y.; Hu, Q. Alcell® lignin solubility in ethanol–water mixtures. J. Appl. Polym. Sci. 1995, 57, 1441–1446. [Google Scholar] [CrossRef]
- Shuai, L.; Amiri, M.T.; Questellsantiago, Y.M.; Héroguel, F.; Li, Y.; Kim, H.; Meilan, R.; Chapple, C.; Ralph, J.; Luterbacher, J. Formaldehyde stabilization facilitates lignin monomer production during biomass depolymerization. Science 2016, 354, 329–333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sannigrahi, P.; Kim, D.H.; Jung, S.; Ragauskas, A. Pseudo-lignin and pretreatment chemistry. Energ. Environ. Sci. 2011, 4, 1306–1310. [Google Scholar] [CrossRef]
- Hu, F.; Jung, S.; Ragauskas, A. Pseudo-lignin formation and its impact on enzymatic hydrolysis. Bioresour. Technol. 2012, 117, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Pan, X.J. Correlation between lignin physicochemical properties and inhibition to enzymatic hydrolysis of cellulose. Biotechnol. Bioeng. 2015, 113, 1213–1224. [Google Scholar] [CrossRef] [PubMed]
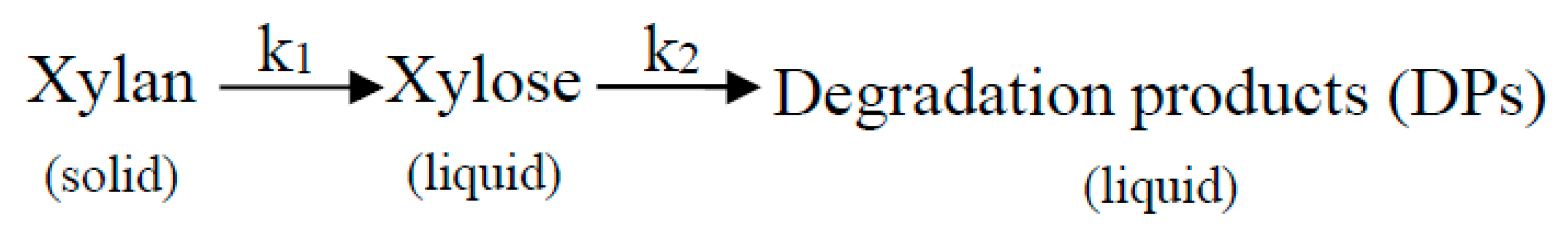
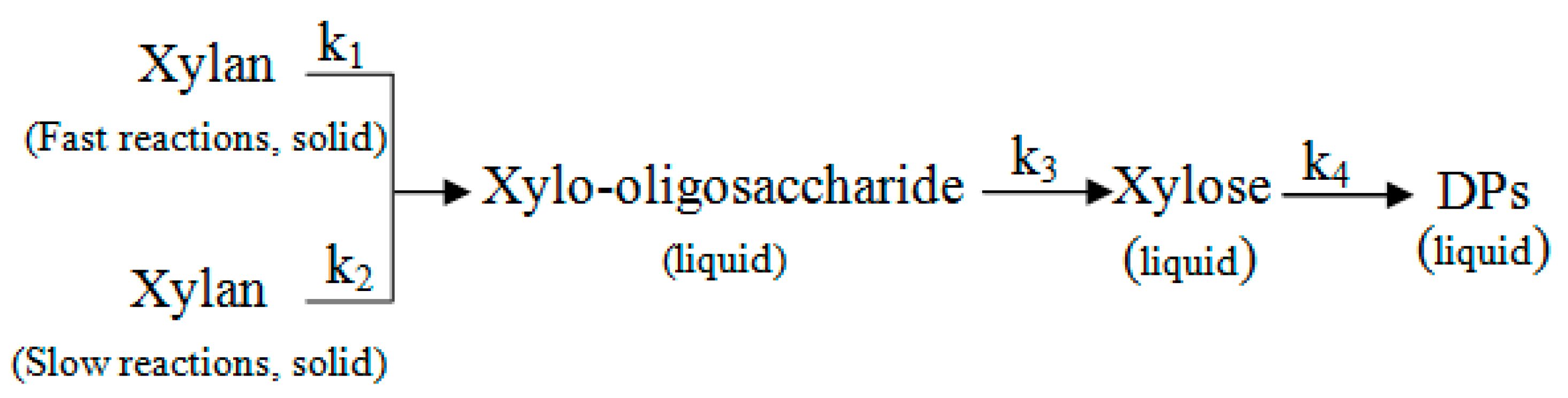
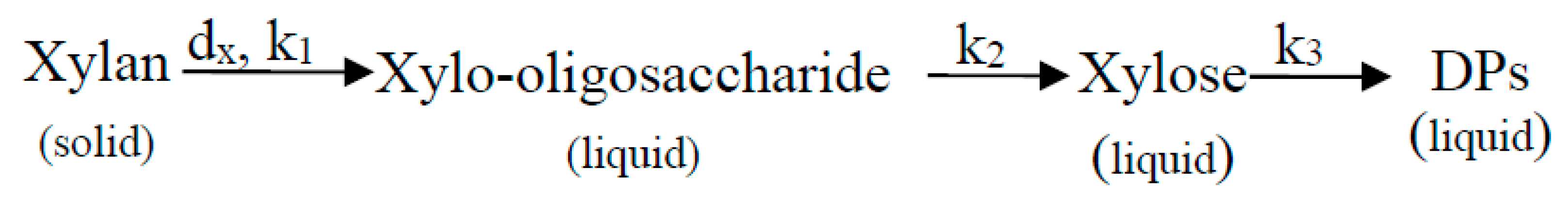


| Ethanol content (%, v/v) | Pretreatment temperature (K) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 443 | 458 | 473 | ||||||||||
| dx | k1 | k2 | k3 | dx | k1 | k2 | k3 | dx | k1 | k2 | k3 | |
| 0 | NA | NA | NA | NA | 0.7878 | 0.0735 | 0.0344 | 0.0214 | NA | NA | NA | NA |
| 20 | 0.3844 | 0.0297 | 0.0175 | 0.0101 | 0.6586 | 0.0623 | 0.0199 | 0.0189 | 0.7723 | 0.1077 | 0.0530 | 0.0431 |
| 40 | 0.2450 | 0.0165 | 0.0156 | 0.0037 | 0.5355 | 0.0486 | 0.0185 | 0.0148 | 0.6346 | 0.0794 | 0.0336 | 0.0267 |
| 60 | 0.1293 | 0.0093 | 0.0046 | 0.0010 | 0.3670 | 0.0327 | 0.0169 | 0.0121 | 0.4812 | 0.0387 | 0.0243 | 0.0221 |
| 80 | NA | NA | NA | NA | 0.2272 | 0.0162 | 0.0135 | 0.0103 | NA | NA | NA | NA |
| 98 | NA | NA | NA | NA | 0.1218 | 0.0158 | 0.0098 | 0.0090 | NA | NA | NA | NA |
| k | k0 (min−1) | Ea (kJ/mol) | α | F value | p value |
|---|---|---|---|---|---|
| k1 | 2.72 × 109 | 83.16 | 0.88 | 59.1280 | 0.0000 |
| k2 | 1.12 × 107 | 68.19 | 0.63 | 20.1566 | 0.0010 |
| k3 | 4.38 × 1013 | 126.84 | 0.69 | 18.2701 | 0.0006 |
| Degradation products (DPs) | Parameters in empirical model (Equation (40)) | |||
|---|---|---|---|---|
| MP0 (min) | ∆MP | δ | ε | |
| Furfural (F) | 12,984.6 | 3474.0 | 0.0760 | 7.4865 |
| Acetic acid (AA) | 10,933.0 | 4025.4 | 0.0689 | 7.8696 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Gong, Z.; Yang, G.; Chen, L.; Huang, L.; Zhou, Y.; Luo, X. Novel Kinetic Models of Xylan Dissolution and Degradation during Ethanol Based Auto-Catalyzed Organosolv Pretreatment of Bamboo. Polymers 2018, 10, 1149. https://doi.org/10.3390/polym10101149
Liu J, Gong Z, Yang G, Chen L, Huang L, Zhou Y, Luo X. Novel Kinetic Models of Xylan Dissolution and Degradation during Ethanol Based Auto-Catalyzed Organosolv Pretreatment of Bamboo. Polymers. 2018; 10(10):1149. https://doi.org/10.3390/polym10101149
Chicago/Turabian StyleLiu, Jing, Zhenggang Gong, Guangxu Yang, Lihui Chen, Liulian Huang, Yonghui Zhou, and Xiaolin Luo. 2018. "Novel Kinetic Models of Xylan Dissolution and Degradation during Ethanol Based Auto-Catalyzed Organosolv Pretreatment of Bamboo" Polymers 10, no. 10: 1149. https://doi.org/10.3390/polym10101149