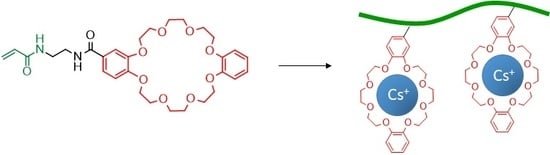
Synthesis and Characterization of Alkali Metal Ion-Binding Copolymers Bearing Dibenzo-24-crown-8 Ether Moieties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Measurements
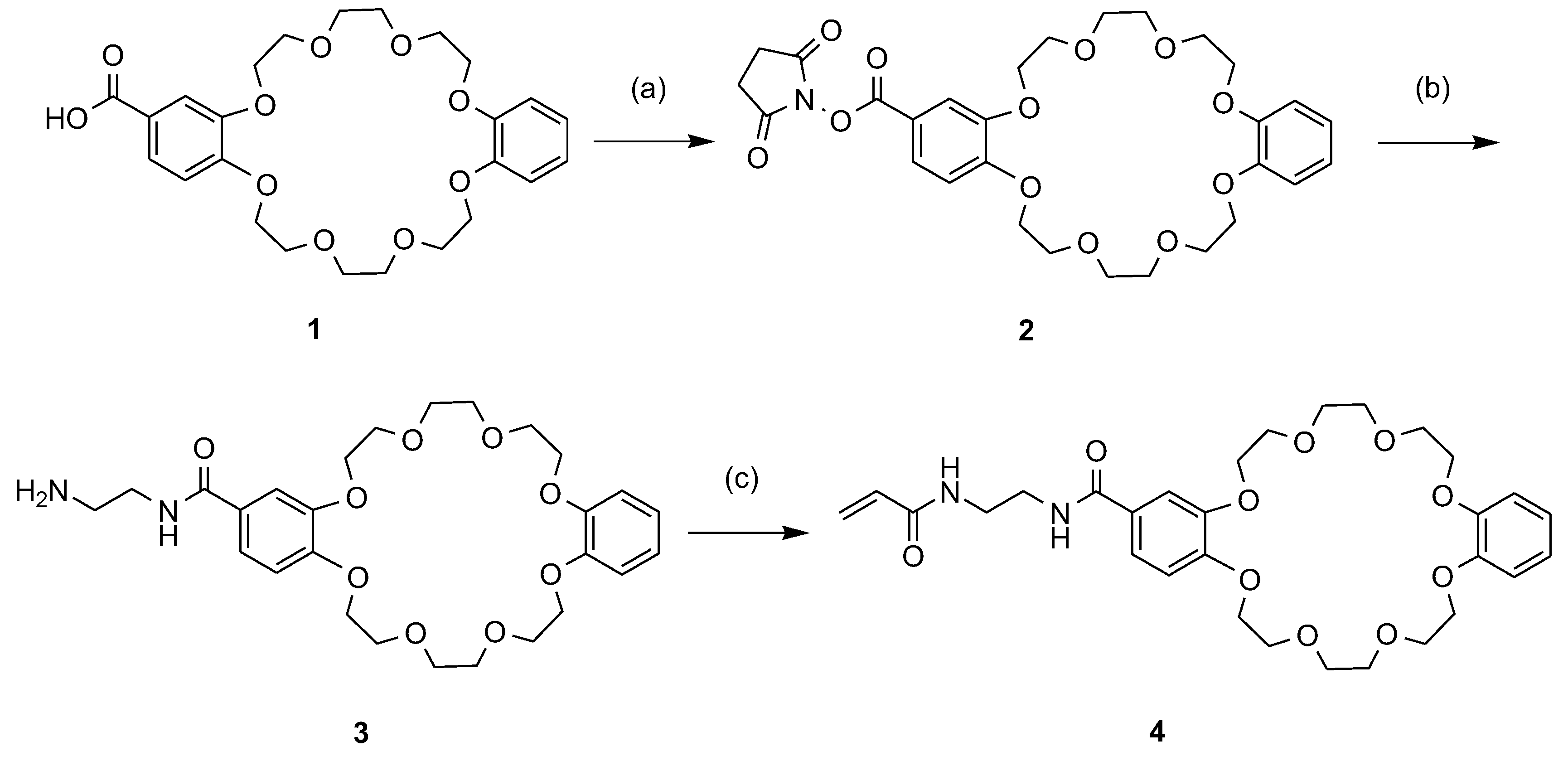
2.3. Synthesis of DB24C8 Monomer
N-(2-Carboxydibenzo-24-crown-8)-succinimide (2) [28,29]
2-[(2-Aminoethyl) carbamoyl]dibenzo-24-crown-8 (3)
2-(1,6-Dioxo-2,5-diaza-7-oxamyl)dibenzo-24-crown-8 (4)
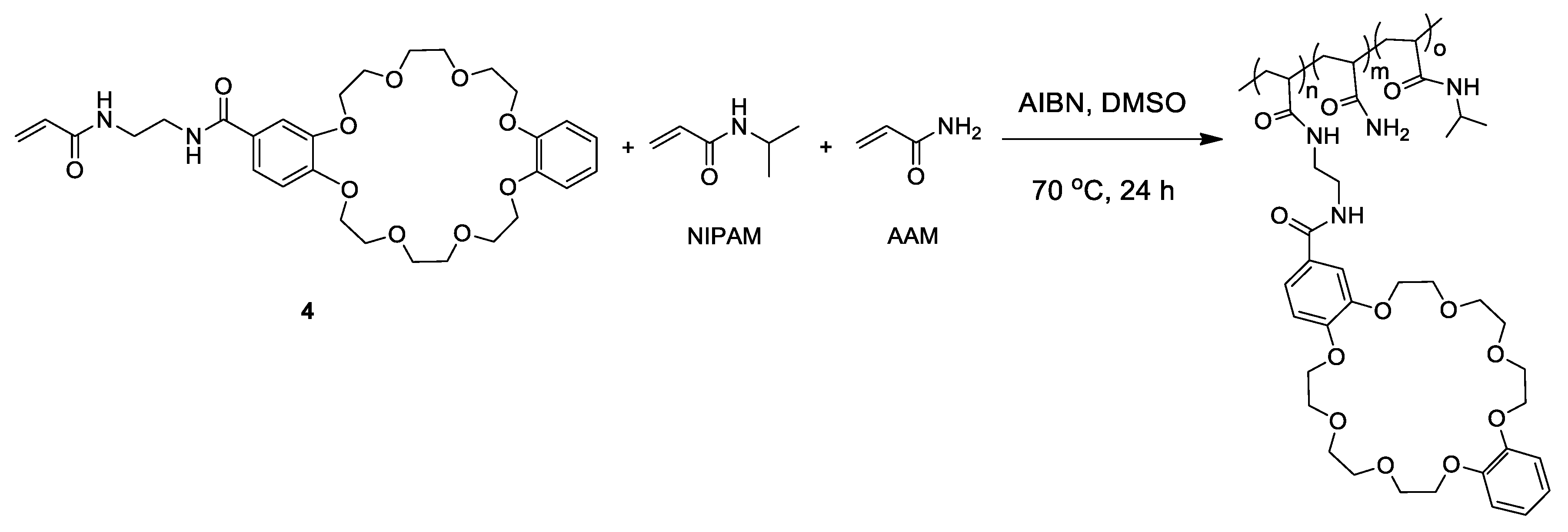
2.4. Synthesis of DB24C8-Bearing Copolymers
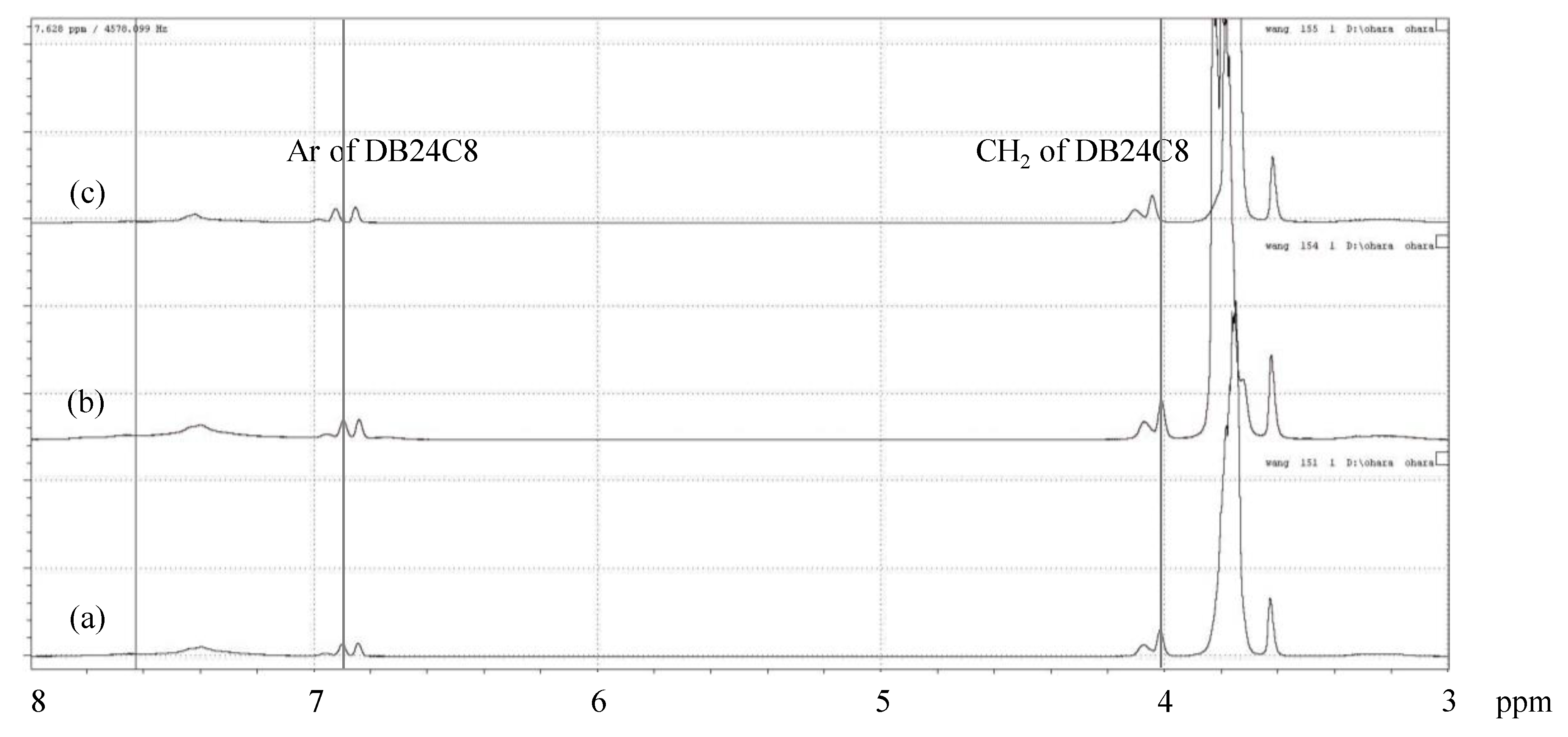
2.5. Binding Test of Copolymers with Metal Ions by 1H NMR
3. Results and Discussion
3.1. Synthesis of DB24C8-Bearing Copolymers
3.2. Cloud Points Analysis of DB24C8-Bearing Copolymers
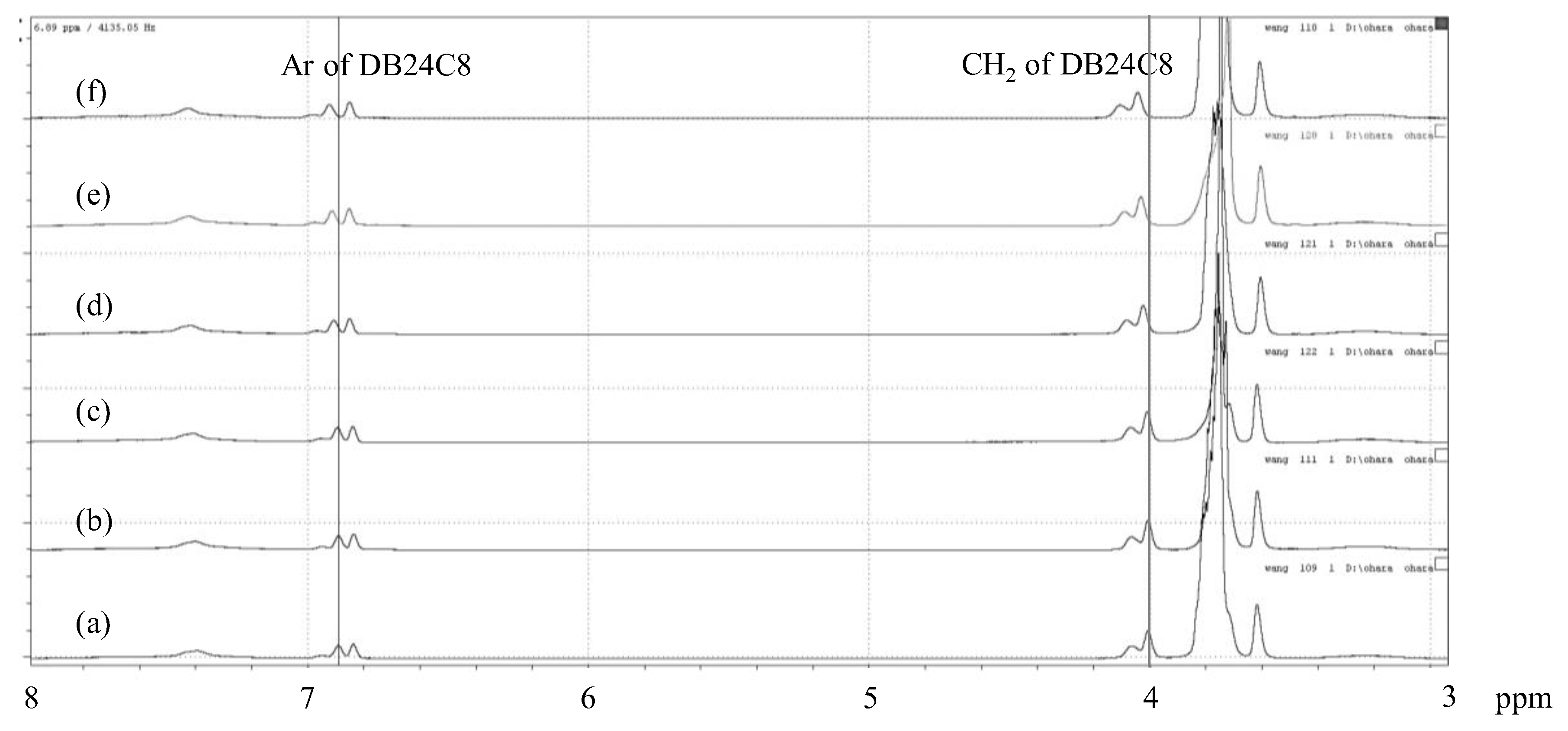
3.3. Cesium Ion Binding to the DB24C8-Bearing Copolymers
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Yin, J.; Hu, Y.; Yoon, J. Fluorescent probes and bioimaging: Alkali metals, alkaline earth metals and pH. Chem. Soc. Rev. 2015, 44, 4619–4644. [Google Scholar] [CrossRef] [PubMed]
- Shamsipur, M.; Rajabi, H.R. Flame photometric determination of cesium ion after its preconcentration with nanoparticles imprinted with the cesium-dibenzo-24-crown-8 complex. Microchim. Acta 2013, 180, 243–252. [Google Scholar] [CrossRef]
- Choi, T.A.; Costes, S.V.; Abergel, R.J. Understanding the health impacts and risks of exposure to radiation. In Reflections on the Fukushima Daiichi Nuclear Accident: Toward Social-Scientific Literacy and Engineering Resilience; Ahn, J., Carson, C., Jensen, M., Juraku, K., Nagasaki, S., Tanaka, S., Eds.; Springer: London, UK, 2015; pp. 259–281. ISBN 978-3-319-12089-8. [Google Scholar]
- Yu, H.-R.; Hu, J.-Q.; Liu, Z.; Ju, X.-J.; Xie, R.; Wang, W.; Chu, L.-Y. Ion-recognizable hydrogels for efficient removal of cesium ions from aqueous environment. J. Hazard. Mater. 2017, 323, 632–640. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, C.J. The Discovery of Crown Ethers (Noble Lecture). Angew. Chem. Int. Ed. 1988, 27, 1021–1027. [Google Scholar] [CrossRef]
- Gokel, G.W.; Leevy, W.M.; Weber, M.E. Crown ethers: Sensors for ions and molecular scaffolds for materials and biological models. Chem. Rev. 2004, 104, 2723–2750. [Google Scholar] [CrossRef] [PubMed]
- Sakata, Y.; Kobayashi, S.; Akine, S. Two-step modulation of ion recognition using a bis(saloph)-macrocyclic host having a 24-crown-8-like cavity. Chem. Commun. 2017, 53, 6363–6366. [Google Scholar] [CrossRef] [PubMed]
- Takeda, Y. The solvent extraction of uni- and bivalent metal picrates by dibenzo-24-crown-8. Bull. Chem. Soc. Jpn. 1979, 52, 2501–2504. [Google Scholar] [CrossRef]
- Shamsipur, M.; Rounaghi, G.; Popov, A.I. Sodium-23, cesium-133 and thallium-205 NMR study of sodium, cesium and thallium complexes with large crown ethers in nonaqueous solutions. J. Solut. Chem. 1980, 9, 701–714. [Google Scholar] [CrossRef]
- Shinkai, S.; Kinda, H.; Manabe, O. Photoresponsive complexation of metal cations with an azobenzene-crown-azobenzene bridge immobilized in polymer supports. J. Am. Chem. Soc. 1982, 104, 2933–2934. [Google Scholar] [CrossRef]
- Tawarah, K.M.; Mizyed, S.A. A conductance study of the association of alkali cations with 1,13-dibenzo-24-crown-8 in acetonitrile. J. Solut. Chem. 1989, 18, 387–401. [Google Scholar] [CrossRef]
- Srivastava, S.K.; Gupta, V.K.; Dwivedi, M.K.; Jain, S. Caesium PVC–crown (dibenzo-24-crown-8) based membrane sensor. Anal. Proc. Incl. Anal. Commun. 1995, 32, 21–23. [Google Scholar] [CrossRef]
- Rofouei, M.K.; Taghdiri, M.; Shamsipur, M.; Alizadeh, K. 133Cs NMR study of Cs+ ion complexes with dibenzo-24-crown-8, dicyclohexano-24-crown-8 and dibenzo-30-crown-10 in binary acetonitrile-nitromethane mixtures. J. Solut. Chem. 2010, 39, 1350–1359. [Google Scholar] [CrossRef]
- Vibhute, R.G.; Khopkar, S.M. Solvent extraction separation of cesium with dibenzo-24-crown-8 from picrate solution. J. Radioanal. Nucl. Chem. 1991, 152, 487–496. [Google Scholar] [CrossRef]
- Awual, M.R.; Yaita, T.; Taguchi, T.; Shiwaku, H.; Suzuki, S.; Okamoto, Y. Selective cesium removal from radioactive liquid waste by crownether immobilized new class conjugate adsorbent. J. Hazard. Mater. 2014, 278, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, M.; Okano, T.; Winnik, F.M. Poly(N-isopropylacrylamide)-based smart surfaces for cell sheet tissue engineering. Mater. Matters 2010, 5, 56. [Google Scholar]
- Irie, M.; Misumi, Y.; Tanaka, T. Stimuli-responsive polymers: Chemical induced reversible phase separation of an aqueous solution of poly(N-isopropylacrylamide) with pendent crown ether groups. Polymer 1993, 34, 4531–4535. [Google Scholar] [CrossRef]
- Qiu, Y.; Park, K. Environment-sensitive hydrogels for drug delivery. Adv. Drug Deliv. Rev. 2001, 53, 321–339. [Google Scholar] [CrossRef]
- Yi, R.; Ye, G.; Lv, D.; Chen, J. Novel thermo-responsive hydrogel microspheres with calixcrown host molecules as cross-links for highly specific binding and controllable release of cesium. RSC Adv. 2015, 5, 55277–55284. [Google Scholar] [CrossRef]
- Cheng, J.; Shan, G.; Pan, P. Temperature and pH-dependent swelling and copper(II) adsorption of poly(Nisopropylacrylamide) copolymer hydrogel. RSC Adv. 2015, 5, 62091–62100. [Google Scholar] [CrossRef]
- Deng, M.; Wang, Y.; Men, G.; Shang, H.; Jiang, S. Reusable highly sensitive and selective fluorescent sensor for Hg2+ detection in water based on a thermoresponsive copolymer. Sens. Actuators B Chem. 2016, 234, 609–615. [Google Scholar] [CrossRef]
- Tanaka, T.; Okamoto, M. Reversible Temperature-responsive and Lectin-recognizing Glycosylated Block Copolymers Synthesized by RAFT Polymerization. Polym. J. 2018, 50, 523–531. [Google Scholar] [CrossRef]
- Tanaka, T.; Okamoto, M. Lectin and Temperature Dual-responsive Glycosylated Block Copolymers Synthesized by Consecutive RAFT Polymerization Reactions. Bull. Chem. Soc. Jpn. 2018, 91, 727–777. [Google Scholar] [CrossRef]
- Zhang, B.; Ju, X.-J.; Xie, R.; Liu, Z.; Pi, S.-W.; Chu, L.-Y. Comprehensive effects of metal ions on responsive characteristics of P(NIPAM-co-B18C6Am). J. Phys. Chem. B 2012, 116, 5527–5536. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.-R.; Hu, J.-Q.; Lu, X.-H.; Ju, X.-J.; Liu, Z.; Xie, R.; Wang, W.; Chu, L.-Y. Insights into the effects of 2:1 “sandwich-type” crown-ether/metal-ion complexes in responsive host-guest systems. J. Phys. Chem. B 2015, 119, 1696–1705. [Google Scholar] [CrossRef] [PubMed]
- Ju, X.-J.; Chu, L.-Y.; Mi, P.; Song, H.; Lee, Y.M. Synthesis and characterization of a novel thermo-sensitive copolymer of N-isopropylacrylamide and dibenzo-18-crown -6-diacrylamide. Macromol. Rapid Commun. 2006, 27, 2072–2077. [Google Scholar] [CrossRef]
- Liu, D.; Wang, D.; Wang, M.; Zheng, Y.; Koynov, K.; Auernhammer, G.K.; Butt, H.-J.; Ikeda, T. Supramolecular organogel based on crown ether and secondary ammoniumion functionalized glycidyl triazole polymers. Macromolecules 2013, 46, 4617–4625. [Google Scholar] [CrossRef]
- Iqbal, P.; Rawson, F.J.; Ho, W.K.-W.; Lee, S.-F.; Leung, K.C.-F.; Wang, X.; Beri, A.; Preece, J.A.; Ma, J.; Mendes, P.M. Surface molecular tailoring using pH-switchable supramolecular dendron-ligand assemblies. ACS Appl. Mater. Interfaces 2014, 6, 6264–6274. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.-M.; Tanaka, T.; Aoki, T.; Aso, Y.; Minami, H.; Ohara, H. Synthesis of dibenzo-24-crown-8 conjugated chitosan with different amide bond coupling methods. Lett. Org. Chem. 2018, 15, 214–220. [Google Scholar] [CrossRef]
- Jones, J.W.; Bryant, W.S.; Bosman, A.W.; Janssen, R.A.J.; Meijer, E.W.; Gibson, H.W. Crowned dendrimers: pH-responsive pseudorotaxane formation. J. Org. Chem. 2003, 68, 2385–2389. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh, N.; Roomiani, A. Kinetic study of charge transfer complexes of iodine with some crown ethers in nonaqueous solvents. J. Chil. Chem. Soc. 2012, 57, 1130–1133. [Google Scholar] [CrossRef]
- Chamsaz, M.; Rounaghi, G.H.; Sovizi, M.R. Polarographic study of the interaction of Tl+, Pb2+ and Cd2+ cations with 18-crown-6 in binary non-aqueous solvents. Russ. J. Inorg. Chem. 2005, 50, 413–417. [Google Scholar]
- Pedersen, C.J. Cyclic polyethers and their complexes with metal salts. J. Am. Chem. Soc. 1967, 89, 7017–7036. [Google Scholar] [CrossRef]


| Polymer | Feed Molar Ratio of NIPAM/4/AAM | Conv (%) a | Yield (%) b | Mw c (×10−3) | Mw/Mn c | Molar Ratio of NIPAM/4/AAM in Polymer a |
|---|---|---|---|---|---|---|
| P1 | 100/0/0 | 97 | 94 | 167 | 2.87 | 100/0/0 |
| P2 | 97.0/3.0/0 | 92 | 95 | 248 | 1.62 | 97.1/2.9/0 |
| P3 | 95.0/5.0/0 | 82 | 63 | 158 | 2.43 | 95.8/4.2/0 |
| P4 | 93.5/6.5/0 | 95 | 76 | 210 | 2.02 | 93.7/6.3/0 |
| P5 | 85.0/0/15 | 97 | 89 | 166 | 2.93 | 83.3/0/16.7 |
| P6 | 82.0/3.0/15 | 98 | 80 | 244 | 1.54 | 85.7/2.4/11.9 |
| P7 | 80.0/5.0/15 | 87 | 94 | 197 | 2.00 | 85.6/5.0/9.4 |
| P8 | 77.5/7.5/15 | 98 | 84 | 176 | 2.95 | 81.7/7.6/10.7 |
| P9 | 75.0/10/15 | 85 | 93 | 127 | 2.47 | 82.6/10.5/6.9 |
| Polymer | Without Cs+ (°C) | With Cs+ (°C) |
|---|---|---|
| P7 | 16.2 | 16.9 |
| P8 | 4–5 a | 9.2 |
| P9 | −3–0 b | 0–1 b |
| None | Li+ | Na+ | K+ | Rb+ | Cs+ | |
|---|---|---|---|---|---|---|
| Cation diameter (Å) [33] | - | 1.20 | 1.90 | 2.66 | 2.96 | 3.34 |
| δ (ppm) | 4.005 | 4.006 | 4.008 | 4.022 | 4.030 | 4.042 |
| Δδ (ppm) | - | 0.001 | 0.003 | 0.017 | 0.025 | 0.037 |
| None | NaCl | KCl | CsCl | CsCl + NaCl (CsCl:NaCl = 1:1) | CsCl + NaCl (CsCl:NaCl = 1:5) | CsCl + KCl (CsCl:KCl = 1:1) | CsCl + KCl (CsCl:KCl = 1:5) | |
|---|---|---|---|---|---|---|---|---|
| δ (ppm) | 4.005 | 4.008 | 4.022 | 4.042 | 4.043 | 4.040 | 4.047 | 4.054 |
| Δδ (ppm) | - | 0.003 | 0.017 | 0.037 | 0.038 | 0.035 | 0.042 | 0.049 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, D.-M.; Aso, Y.; Ohara, H.; Tanaka, T. Synthesis and Characterization of Alkali Metal Ion-Binding Copolymers Bearing Dibenzo-24-crown-8 Ether Moieties. Polymers 2018, 10, 1095. https://doi.org/10.3390/polym10101095
Wang D-M, Aso Y, Ohara H, Tanaka T. Synthesis and Characterization of Alkali Metal Ion-Binding Copolymers Bearing Dibenzo-24-crown-8 Ether Moieties. Polymers. 2018; 10(10):1095. https://doi.org/10.3390/polym10101095
Chicago/Turabian StyleWang, Da-Ming, Yuji Aso, Hitomi Ohara, and Tomonari Tanaka. 2018. "Synthesis and Characterization of Alkali Metal Ion-Binding Copolymers Bearing Dibenzo-24-crown-8 Ether Moieties" Polymers 10, no. 10: 1095. https://doi.org/10.3390/polym10101095