Synthesis and 3D Network Architecture of 1- and 16-Hydrated Salts of 4-Dimethylaminopyridinium Decavanadate, (DMAPH)6[V10O28]·nH2O
Abstract
:1. Introduction
2. Results and Discussion
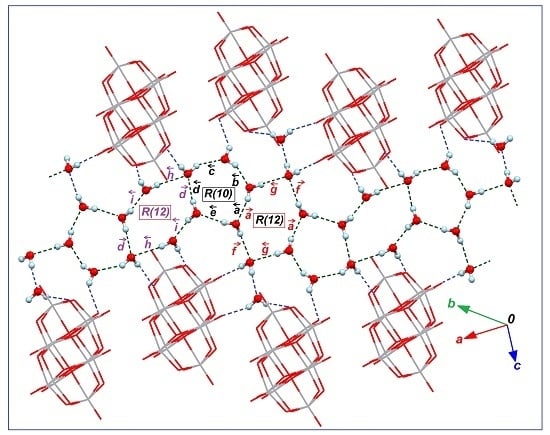
2.1. Features of Crystalline Structures of 1 and 2
2.2. FT-IR and FT-Raman Spectroscopic Studies
2.3. Thermal Analysis
3. Experimental Section
Synthesis of (DMAPH)6[V10O28]·H2O, (1) and (DMAPH)6[V10O28]·16H2O (2)
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| DMAP | 4-Dimethylaminopyridine |
| DMAPH | 4-Dimethylaminopiridinium |
| POV | Polyoxovanadate |
References and Notes
- Hayashi, Y. Hetero and lacunary polyoxovanadate chemistry: Synthesis, reactivity and structural aspects. Coord. Chem. Rev. 2011, 255, 2270–2280. [Google Scholar] [CrossRef]
- Aureliano, M.; Ohlin, C.A. Decavanadate in vitro and in vivo effects: Facts and opinions. J. Inorg. Biochem. 2014, 137, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Aureliano, M.; Crans, D.C. Decavanadate (V10O286−) and oxovanadates: Oxometalates with many biological activities. J. Inorg. Biochem. 2009, 103, 536–546. [Google Scholar] [CrossRef] [PubMed]
- Rehder, D. The future of/for vanadium. Dalton Trans. 2013, 42, 11749–11761. [Google Scholar] [CrossRef] [PubMed]
- Pereira, M.J.; Carvalho, E.; Eriksson, J.W.; Crans, D.C.; Aureliano, M. Effects of decavanadate and insulin enhancing vanadium compounds on glucose uptake in isolated rat adipocytes. J. Inorg. Biochem. 2009, 103, 1687–1692. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, S.; Ozeki, T. Hydrogen-bonded aggregates of protonated decavanadate anions in their tetraalkylammonium salts. J. Chem. Soc. Dalton Trans. 2001, 4, 472–480. [Google Scholar] [CrossRef]
- Arrieta, J.M. Total synthesis of decavanadates of organic bases. X-ray crystal structures of four decavanadates. Polyhedron 1992, 11, 3045–3068. [Google Scholar] [CrossRef]
- Kempf, J.Y.; Rohmer, M.M.; Poblet, J.M.; Bo, C.; Benard, M. Relative basicities of the oxygen sites in [V10O28]6-. An analysis of the ab initio determined distributions of the electrostatic potential and of the Laplacian of charge density. J. Am. Chem. Soc. 1992, 114, 1136–1146. [Google Scholar] [CrossRef]
- Day, V.W.; Klemperer, W.G.; Maltbie, D.J. Where are the protons in H3V10O283-? J. Am. Chem. Soc. 1987, 109, 2991–3002. [Google Scholar] [CrossRef]
- Evans, H.T., Jr. The molecular structure of the isopoly complex ion, decavanadate (V10O286−). Inorg. Chem. 1996, 5, 967–977. [Google Scholar] [CrossRef]
- Bošnjaković-Pavlović, N.; Prevost, J.; Spasojević-de Biré, A. Crystallographic Statistical Study of Decavanadate Anion Based-Structures: Toward a Prediction of Noncovalent Interactions. Cryst. Growth Des. 2011, 11, 3778–3789. [Google Scholar] [CrossRef]
- Chatkon, A.; Chatterjee, P.B.; Sedgwick, M.A.; Haller, K.J.; Crans, D.C. Counterion affects interaction with interfaces: The antidiabetic drugs metformin and decavanadate. Eur. J. Inorg. Chem. 2013, 2013, 1859–1868. [Google Scholar] [CrossRef]
- Kumagai, H.; Arishima, M.; Kitagawa, S.; Ymada, K.; Kawata, S.; Kaizaki, S. New hydrogen bond-supported 3-D molecular assembly from polyoxovanadate and tetramethylbiimidazole. Inorg. Chem. 2002, 41, 1989–1992. [Google Scholar] [CrossRef] [PubMed]
- Correia, I.; Avecilla, F.; Marcão, S.; Pessoa, J.C. Structural studies of decavanadate compounds with organic molecules and inorganic ions in their crystal packing. Inorg. Chim. Acta 2004, 357, 4476–4487. [Google Scholar] [CrossRef]
- Samart, N.; Saeger, J.; Haller, K.J.; Aureliano, M.; Crans, D.C. Interaction of decavanadate with interfaces and biological model membrane systems: Characterization of soft oxometalate systems. J. Mol. Eng. Mater. 2014, 2, 1–21. [Google Scholar] [CrossRef]
- Aureliano, M.; Gândara, R.M. Decavanadate effects in biological systems. J. Inorg. Biochem. 2005, 99, 979–985. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, J.L.F.; da Piedade, M.F.M.; Duarte, M.T. Decavanadates: A building-block for supramolecular assemblies. Inorg. Chim. Acta 2003, 356, 222–242. [Google Scholar] [CrossRef]
- Kasuga, N.C.; Umeda, M.; Kidokoro, H.; Ueda, K.; Hattori, K.; Yamaguchi, K. Four novel solid-state supramolecular assemblies constructed from decavanadate salts and decamethylcucurbit[5]uril. Cryst. Growth Des. 2009, 9, 1494–1498. [Google Scholar] [CrossRef]
- Schulz-Dobrick, M.; Jansen, M. Structure-directing effects in the supramolecular intercluster compound [Au9(PPh3)8]2[V10O28H3]2: Long-range versus short-range bonding interactions. Inorg. Chem. 2007, 46, 4380–4382. [Google Scholar] [CrossRef] [PubMed]
- Román, P.; Aranzabe, A.; Luque, A.; Gutiérrez-Zorrilla, J.M.; Martínez-Ripoll, M. Effects of protonation in decavanadates: Crystal structure of tetrakis (n-hexylammonium) dihydrogendecavanadate (V). J. Chem. Soc. Dalton Trans. 1995, 13, 2225–2231. [Google Scholar] [CrossRef]
- Arrieta, J.M.; Amigo, J.M.; Gili, P. Crystal data for the decavanadates of 2, 4-, 3, 4-, and 3, 5-dimethylpyridine and 2, 4, 6-trimethylpyridine. J. Appl. Cryst. 1981, 14, 472–473. [Google Scholar] [CrossRef]
- Lv, Y.K.; Jiang, Z.G.; Gan, L.H.; Liu, M.X.; Feng, Y.L. Three novel organic-inorganic hybrid materials based on decaoxovanadates obtained from a new liquid phase reaction. CrystEngComm 2012, 14, 314–322. [Google Scholar] [CrossRef]
- Sánchez-Lara, E.; Sánchez-Lombardo, I.; Pérez-Benítez, A.; Mendoza, Á.; Flores-Álamo, M.; Vergara, E.G. A New Dicationic Ring [(Water)6–(Ammonium)2] Acts as a Building Block for a Supramolecular 3D Assembly of Decavanadate Clusters and 4-(N, N-dimethylamino)pyridinium Ions. J. Clust. Sci. 2015, 26, 901–912. [Google Scholar] [CrossRef]
- Nilsson, J.; Nordlander, E.; Behrens, U.; Rehder, D. Hexakis(tetraaquasodium) decavanadate(V) dihydrate. Acta Cryst. 2010, 66, i30–i31. [Google Scholar] [CrossRef] [PubMed]
- Fratzky, D.; Schneider, M.; Rabe, S.; Meisel, M. (NH4)4Na2[V10O28]·10H2O. Acta Cryst. 2010, 56, 740–741. [Google Scholar] [CrossRef]
- Wu, B.; Xu, X.; Chen, K.; Xiao, Z.; Wu, P. Crystal structure of hexakis(4-(dimethylamino)pyridin-1-ium) decavanadate-water (1:16), C42H98N12O44V10. Z. Kristallogr. 2015, 230, 353–355. [Google Scholar] [CrossRef]
- Spek, A.L. Single-crystal structure validation with the program PLATON. J. Appl. Cryst. 2003, 36, 7–13. [Google Scholar] [CrossRef]
- Macrae, C.F.; Bruno, I.J.; Chisholm, J.A.; Edgington, P.R.; McCabe, P.; Pidcock, E.; Wood, P.A. Mercury CSD 2.0–new features for the visualization and investigation of crystal structures. J. Appl. Cryst. 2008, 41, 466–470. [Google Scholar] [CrossRef]
- Adams, S. SOFTBV. Version 0.96. 2004. Available online: http://www.softbv.net/ (accessed on 31 March 2016).
- Sinnokrot, M.O.; Sherrill, C.D. Substituent effects in π-π interactions: Sandwich and T-shaped configurations. J. Am. Chem. Soc. 2004, 126, 7690–7697. [Google Scholar] [CrossRef] [PubMed]
- Bryant, G.L.; King, J.A. Structures of two acylpyridinium salts and one simple pyridinium salt. Acta Cryst. 1992, 48, 2036–2039. [Google Scholar] [CrossRef]
- Chao, M.; Schempp, E.; Rosenstein, D. 4-Dimethylaminopyridine hydrochloride dihydrate. Acta Cryst. 1997, 33, 1820–1823. [Google Scholar] [CrossRef]
- Vembu, N.; Nallu, M.; Garrison, J.; Youngs, W.J. 4-Dimethylaminopyridinium picrate: Supramolecular aggregation through extensive N—H⋯O and C-H⋯O interactions. Acta Cryst. 2003, 59, o913–o916. [Google Scholar] [CrossRef]
- Mustaqim, R.M.; Ali, S.; Razak, I.A.; Fun, H.K.; Goswami, S.; Adak, A. 4-(N, N-Dimethylamino) pyridinium perchlorate. Acta Cryst. 2005, 61, o3733–o3734. [Google Scholar] [CrossRef]
- Groom, C.R.; Allen, F.H. The Cambridge Structural Database in retrospect and prospect. Angew. Chem. Int. Ed. 2014, 53, 662–671. [Google Scholar] [CrossRef] [PubMed]
- Zavalij, P.Y.; Chirayil, T.; Whittingham, M.S. Crystal structure of tetrasodium bis (tetramethylammonium) decavanadate icosahydrate, Na4(N(CH3)4)2(V10O28)(H2O)20. Z. Krist.-New Cryst. 1997, 212, 321–322. [Google Scholar]
- Liu, H.; Wang, J.; Li, Y.; Jian, F. Synthesis and Crystal Structure of the Heteronuclear Decavanadates Complex: [Fe(phen)3]2·[V10O28]·15H2O. J. Chem. Crystallogr. 2011, 41, 1254–1257. [Google Scholar] [CrossRef]
- Etter, M.C. Encoding and decoding hydrogen-bond patterns of organic compounds. Acc. Chem. Res. 1990, 23, 120–126. [Google Scholar] [CrossRef]
- Motherwell, W.S.; Shields, G.P.; Allen, F.H. Automated assignment of graph-set descriptors for crystallographically symmetric molecules. Acta Cryst. 2000, 56, 466–473. [Google Scholar] [CrossRef]
- Gómez-Saiz, P.; García-Tojal, J.; Maestro, M.A.; Arnaiz, F.J.; Rojo, T. Evidence of desulfurization in the oxidative cyclization of thiosemicarbazones. Conversion to 1, 3, 4-oxadiazole derivatives. Inorg. Chem. 2002, 41, 1345–1347. [Google Scholar] [CrossRef] [PubMed]
- Chaplin, M. Water Absoption Spectrum. Available online: http://www1.lsbu.ac.uk/water/water_vibrational_spectrum.html. (accessed on 12 May 2016).
- Ben Nasr, M.; Lefebvre, F.; Ben Nasr, C. Synthesis, Crystal Structure and Infrared Characterization of Bis(4-dimethlyamino-pyridinium) Tetrachlorocuprate. Am. J. Anal. Chem. 2015, 6, 446–456. [Google Scholar] [CrossRef]
- Sundaraganesan, N.; Kalaichelvan, S.; Meganathan, C.; Joshua, B.D.; Cornard, J. FT-IR, FT-Raman spectra and ab initio HF and DFT calculations of 4-N,N′-dimethylamino pyridine. Spectrochim. Acta A 2008, 71, 898–906. [Google Scholar] [CrossRef] [PubMed]
- Koleva, B.B.; Kolev, T.; Seidel, R.W.; Tsanev, T.; Mayer-Figge, H.; Spiteller, M.; Sheldrick, W.S. Spectroscopic and structural elucidation of 4-dimethylaminopyridine and its hydrogensquarate. Spectrochim. Acta A 2008, 71, 695–702. [Google Scholar] [CrossRef] [PubMed]
- Frost, R.L.; Erickson, K.L.; Weier, M.L.; Carmody, O. Raman and infrared spectroscopy of selected vanadates. Spectrochim. Acta A 2005, 61, 829–834. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wery, A.S.; Gutierrez-Zorrila, J.M.; Luque, A.; Roman, P.; Martinez-Ripoll, M. Influence of protonation on crystal packing and thermal behaviour of tert-butylammonium decavanadates. Polyhedron 1996, 15, 4555–4564. [Google Scholar] [CrossRef]
- Omri, I.; Mhiri, T.; Graia, M. Novel decavanadate cluster complex (HImz)12(V10O28)2·3H2O: Synthesis, characterization, crystal structure, optical and thermal properties. J. Mol. Struct. 2015, 1098, 324–331. [Google Scholar] [CrossRef]
- Frost, R.L.; Palmer, S.J. Raman spectroscopic study of pascoite Ca3V10O28·17 H2O. Spectrochim. Acta A 2011, 78, 248–252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Havelková, A.; Tatiersky, J. Preparation, chemical characterization and thermal behaviour of some alkyldiammonium polyoxovanadates (V). Thermochim. Acta 1999, 329, 67–71. [Google Scholar]
- Ulická, Ľ.; Vargová, Č. Thermal analysis of some double decavanadates. Chem. Zvesti 1973, 27, 152–158. Available online: http://www.chempap.org/file_access.php?file=272a152.pdf (accessed on 6 April 2016). [Google Scholar]
- Ulická, Ľ. Thermal decomposition of decavanadates of bivalent metals. J. Therm. Anal. 1980, 18, 127–136. [Google Scholar]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. 2015, 71, 38. [Google Scholar]
- Unpublished results from our current research.
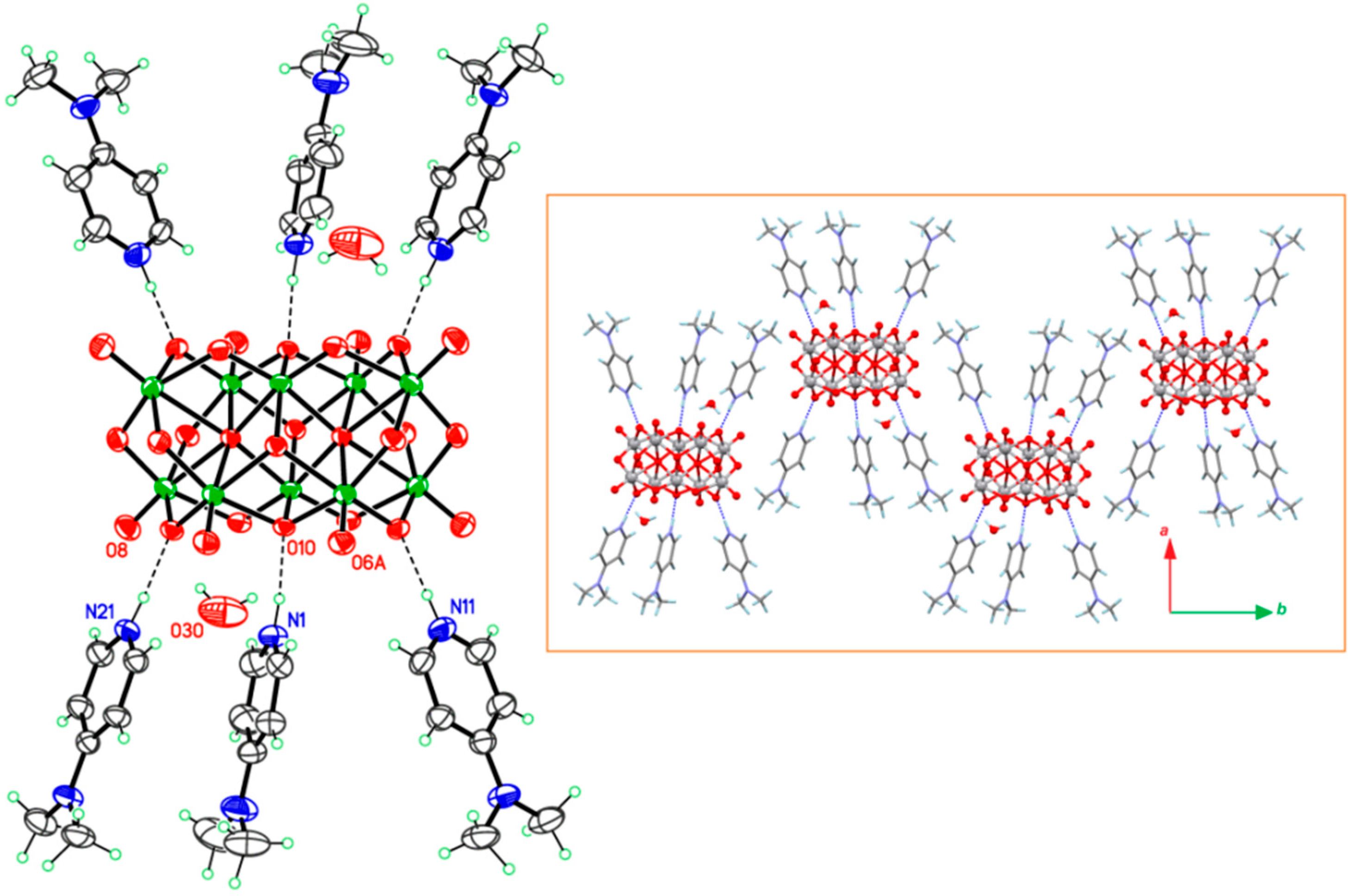
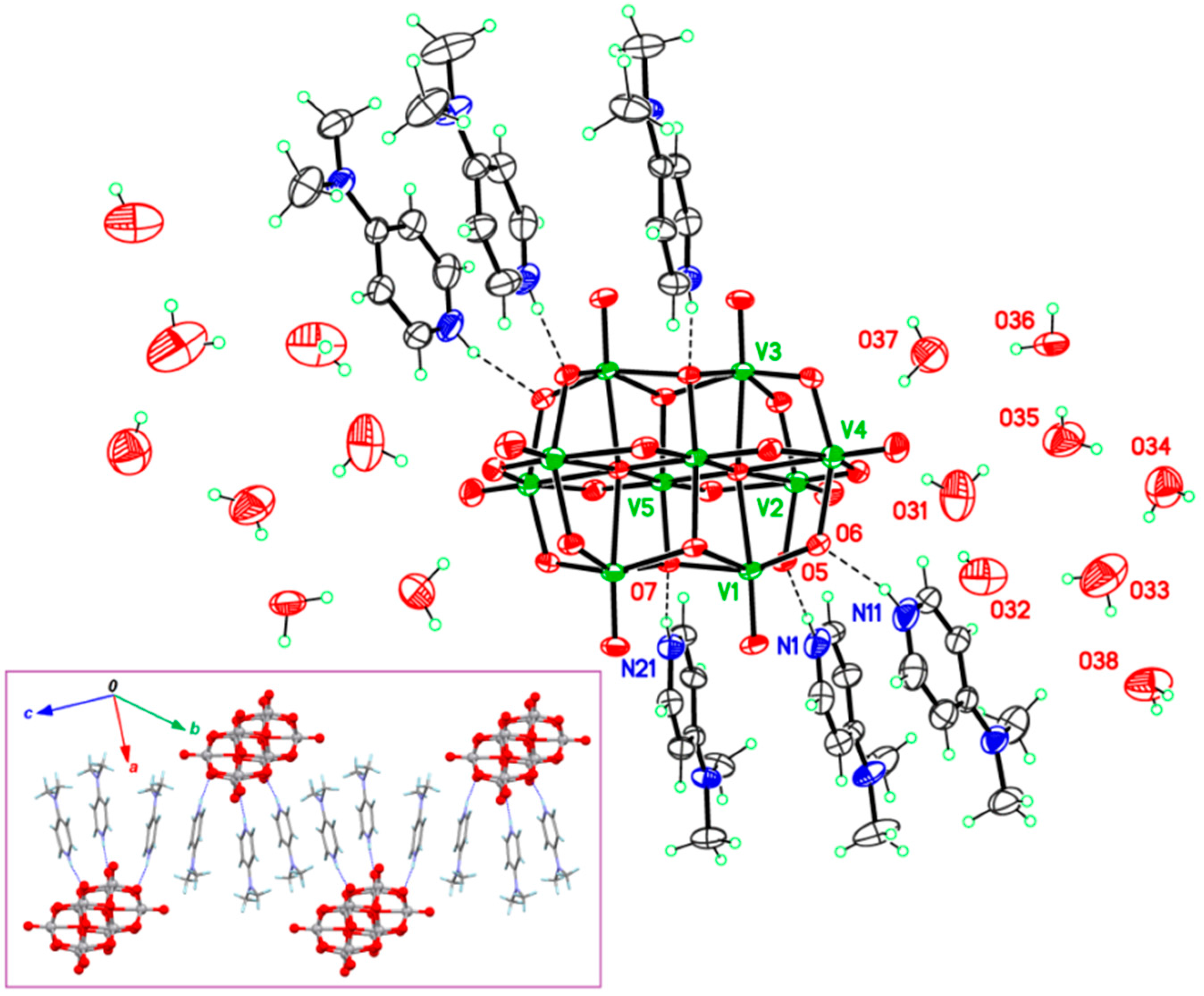


| H bond D–H···A | H···A (Å) | D–H···A (°) | Symmetry for A |
|---|---|---|---|
| Interactions DMAPH/[V10O28] | |||
| N1–H1···O10 | 1.71 (2) | 174 (4) | x, y, z |
| N11–H11···O6 | 1.721 (19) | 179 (4) | 1 − x, 1 − y, 2 − z |
| N21–H21···O8 | 1.754 (19) | 175 (4) | x, y, z |
| H bond D–H…A | H…A (Å) | D–H…A (°) | Symmetry for A |
|---|---|---|---|
| Interactions DMAPH/[V10O28] | |||
| N1–H1∙∙∙O5 | 1.94 (3) | 170 (3) | x, y, z |
| N11–H11∙∙∙O6 | 1.97 (3) | 166 (3) | x, y, z |
| N21–H21∙∙∙O7 | 1.94 (3) | 168 (3) | x, y, z |
| Interactions H2O/H2O building the network | |||
| O31–H31B∙∙∙O35 | 1.98 (3) | 153 (6) | x, y, z |
| O32–H32B∙∙∙O31 | 1.99 (3) | 151 (5) | x, y, z |
| O33–H33A∙∙∙O32 | 1.99 (2) | 173 (6) | x, y, z |
| O33–H33B∙∙∙O38 | 1.92 (2) | 164 (5) | x, y, z |
| O34–H34A∙∙∙O36 | 2.10 (2) | 160 (5) | 1 − x, 3 − y, 1 − z |
| O34–H34B∙∙∙O33 | 1.94 (2) | 168 (5) | x, y, z |
| O35–H35A∙∙∙O36 | 1.959 (18) | 177 (5) | x, y, z |
| O35–H35B∙∙∙O34 | 1.96 (2) | 164 (5) | x, y, z |
| O36–H36B∙∙∙O37 | 1.94 (2) | 166 (3) | x, y, z |
| O38–H38A∙∙∙O32 | 2.19 (2) | 162 (5) | −x, 2 − y, 1 − z |
| Interactions H2O/[V10O28] | |||
| O31–H31A∙∙∙O2 | 2.28 (3) | 141 (4) | x, y, z |
| O32–H32A∙∙∙O13 | 2.11 (2) | 166 (5) | −1 + x, y, z |
| O36–H36A∙∙∙O1 | 1.948 (18) | 178 (3) | x, 1 + y, z |
| O37–H37A∙∙∙O12 | 2.31 (2) | 163 (5) | x, y, z |
| O37–H37B∙∙∙O3 | 2.25 (3) | 144 (4) | 2 − x, 3 − y, 2 − z |
| O38–H38B∙∙∙O6 | 1.96 (2) | 173 (5) | 1 − x, 2 − y, 1 − z |
| Compound 1 | Compound 2 | |
|---|---|---|
| Empirical formula | C42H68N12O29V10 | C42H98N12O44V10 |
| Formula weight | 1714.48 | 1984.72 |
| Crystal system | Monoclinic | Triclinic |
| T [K] | 298 (2) | 283 (2) |
| Wavelength, λ/Å | Cu-,1.5418 | Mo-, 0.71073 |
| Space group | ||
| a [Å] | 11.0212 (2) | 11.2785 (3) |
| b [Å] | 23.845 (4) | 11.7777 (3) |
| c [Å] | 12.20887 (19) | 15.0569 (4) |
| α | 90 | 93.4088 (19) |
| β | 103.3414 (17) | 102.469 (2) |
| γ | 90 | 100.691 (2) |
| V [Å3] | 3122.0 (5) | 1908.65 (9) |
| Z | 2 | 1 |
| Dcalc. [g/cm3] | 1.824 | 1.727 |
| [mm−1] | 12.794 | 1.268 |
| Reflections collected | 42521 | 42872 |
| Independent reflections | 5572 | 9716 |
| Parameters | 445 | 555 |
| Goodness-of-fit on F2 | 1.047 | 1.039 |
| Final R index [I > 2σ(I)] | 0.042 | 0.033 |
| wR2 (all data) | 0.127 | 0.092 |
| Compound 1 | Compound 2 | 4-DMAP | ||||
|---|---|---|---|---|---|---|
| FT-IR a (cm−1) | FT-Raman (cm−1) | FT-IR (cm−1) | FT-Raman (cm−1) | FT-IR (cm−1) | FT-Raman (cm−1) | Vibrational Assignment b |
| 3440 vw | 3416 vbr | (O-H) + O-H···H stretch | ||||
| 3090 w | 3092 w | 3092 vw | CH | |||
| 3041 vw | 3043 vw | 3034 vw | CH | |||
| 2923 vw | 2932 vw | 2919 w | CH in CH3 | |||
| 1640 vs | 1645 vs | 1603 vs | . CC | |||
| 1550 s | 1561 s | 1519 s | CC + CN | |||
| 1443 w | 1444 w | 1445 s | CN | |||
| 1395 w | 1400 w | 1378 w | CH3 umbrella mode + CC | |||
| 1220 s | 1217 s | 1225 w | CH | |||
| 1059 vw | 1062 vw | 1071 w | CH3 | |||
| 1000 vs | 997 vs | 988 vs | 986 vs | VOterminal + Trigonal bending | ||
| 945 vs | 943 s | 948 vs | 947 s | 943 m | 951 s | VOterminal + Ring breathing + methyl rocking |
| 829 s | 837 w | 821 vs | 837 vw | 809 s | 808 w | as (OV2) + CH |
| 719 m | 741 vs | 728 m | 745 vs | 750 m | 752 vs | as (OV2) + CNC + CCC |
| 569 m | 590 w | 566 m | 586 w | s (OV2) | ||
| 515 m | 544 vw | 520 m | 534 w | 538 m | s (OV2) + NCH3 + CCC | |
| 417 w | 413 m | 405 m | CCC | |||
| 322 m | 321 s | Bending of VO3 units VOV bridging bending modes | ||||
| 232 m 216 w 186 m | 230 s 215 m 180 m | 159 w 123 w | V O and lattice modes + CH3 | |||
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez-Lara, E.; Pérez-Benítez, A.; Treviño, S.; Mendoza, A.; Meléndez, F.J.; Sánchez-Mora, E.; Bernès, S.; González-Vergara, E. Synthesis and 3D Network Architecture of 1- and 16-Hydrated Salts of 4-Dimethylaminopyridinium Decavanadate, (DMAPH)6[V10O28]·nH2O. Crystals 2016, 6, 65. https://doi.org/10.3390/cryst6060065
Sánchez-Lara E, Pérez-Benítez A, Treviño S, Mendoza A, Meléndez FJ, Sánchez-Mora E, Bernès S, González-Vergara E. Synthesis and 3D Network Architecture of 1- and 16-Hydrated Salts of 4-Dimethylaminopyridinium Decavanadate, (DMAPH)6[V10O28]·nH2O. Crystals. 2016; 6(6):65. https://doi.org/10.3390/cryst6060065
Chicago/Turabian StyleSánchez-Lara, Eduardo, Aarón Pérez-Benítez, Samuel Treviño, Angel Mendoza, Francisco J. Meléndez, Enrique Sánchez-Mora, Sylvain Bernès, and Enrique González-Vergara. 2016. "Synthesis and 3D Network Architecture of 1- and 16-Hydrated Salts of 4-Dimethylaminopyridinium Decavanadate, (DMAPH)6[V10O28]·nH2O" Crystals 6, no. 6: 65. https://doi.org/10.3390/cryst6060065