Synthesis of Pure Phase NaP2 Zeolite from the Gel of NaY by Conventional and Microwave-Assisted Hydrothermal Methods
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Zeolite Synthesis Gel
2.3. Single Crystallization
2.4. Double Crystallization via MH Method
2.5. Characterization of Solid Products
3. Results and Discussion
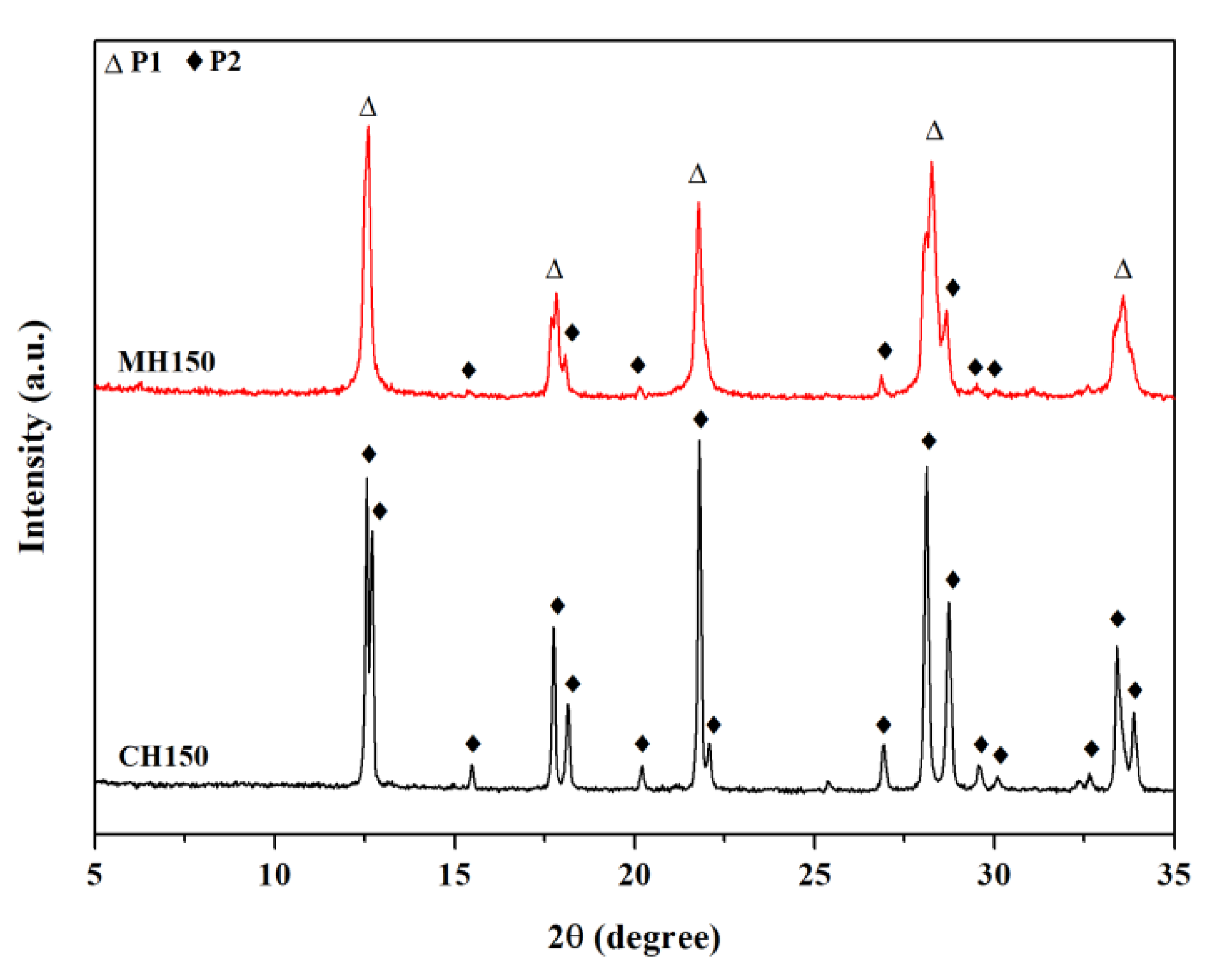
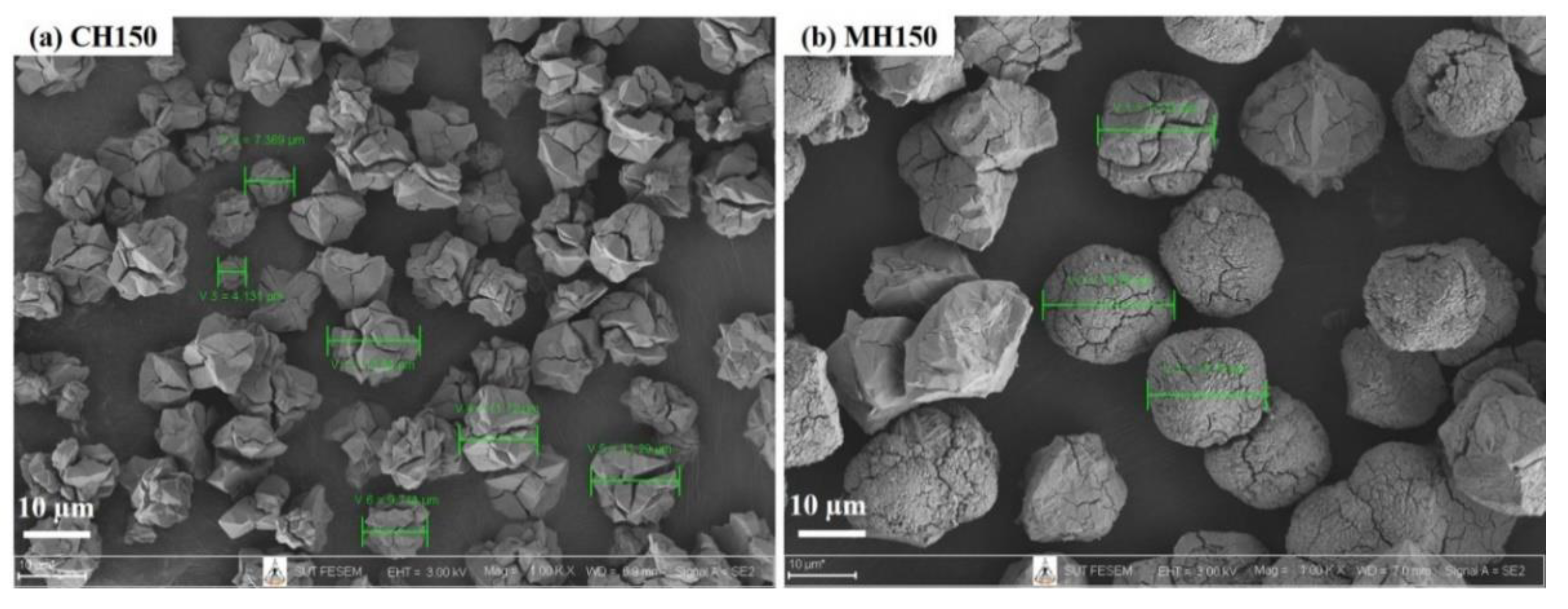
3.1. Comparison of Conventional and Microwave-Assisted Hydrothermal Methods of Single Crystallization
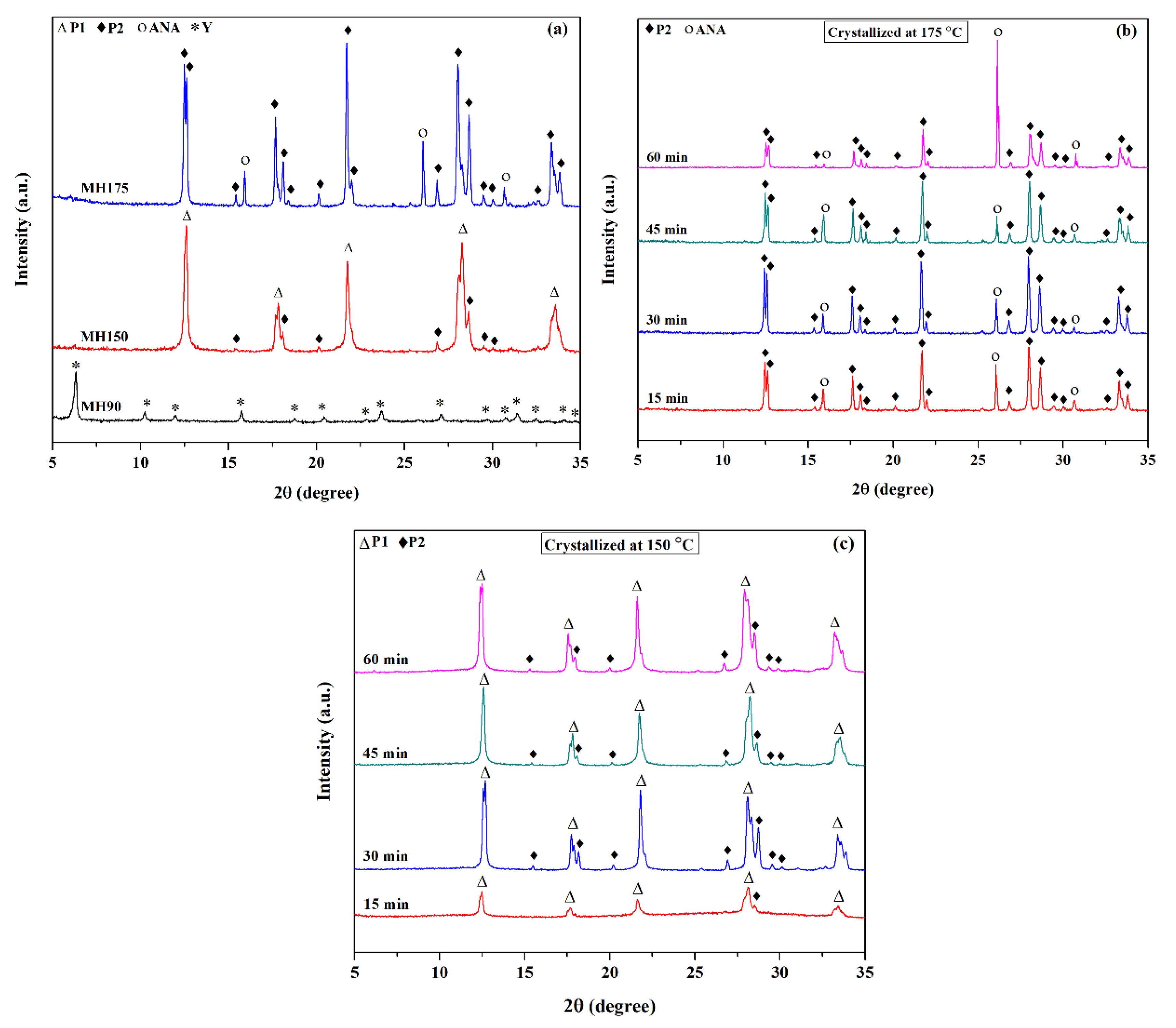
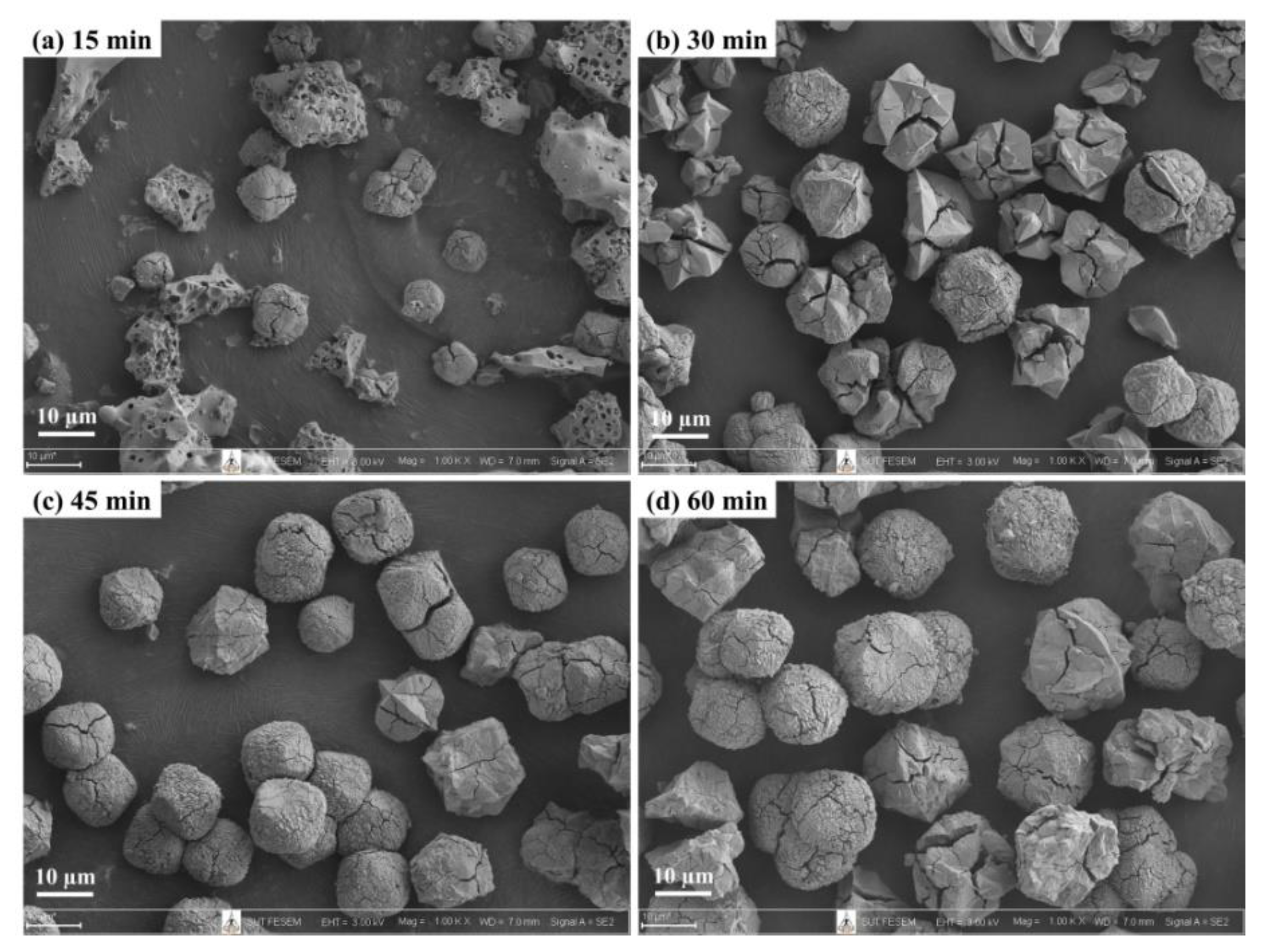
3.2. Effect of Temperature and Time on Single Crystallization by Microwave-Assisted Hydrothermal Methods
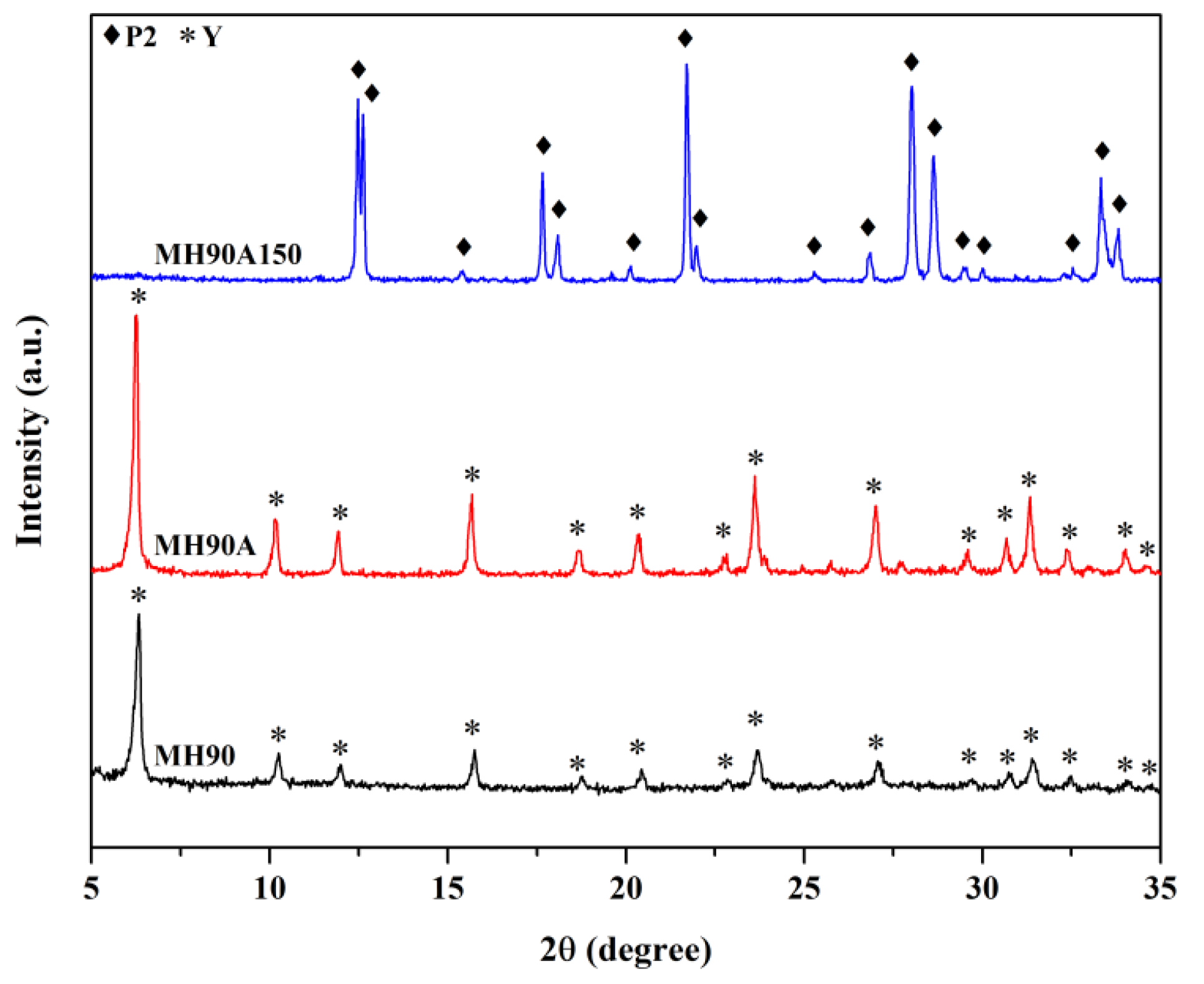
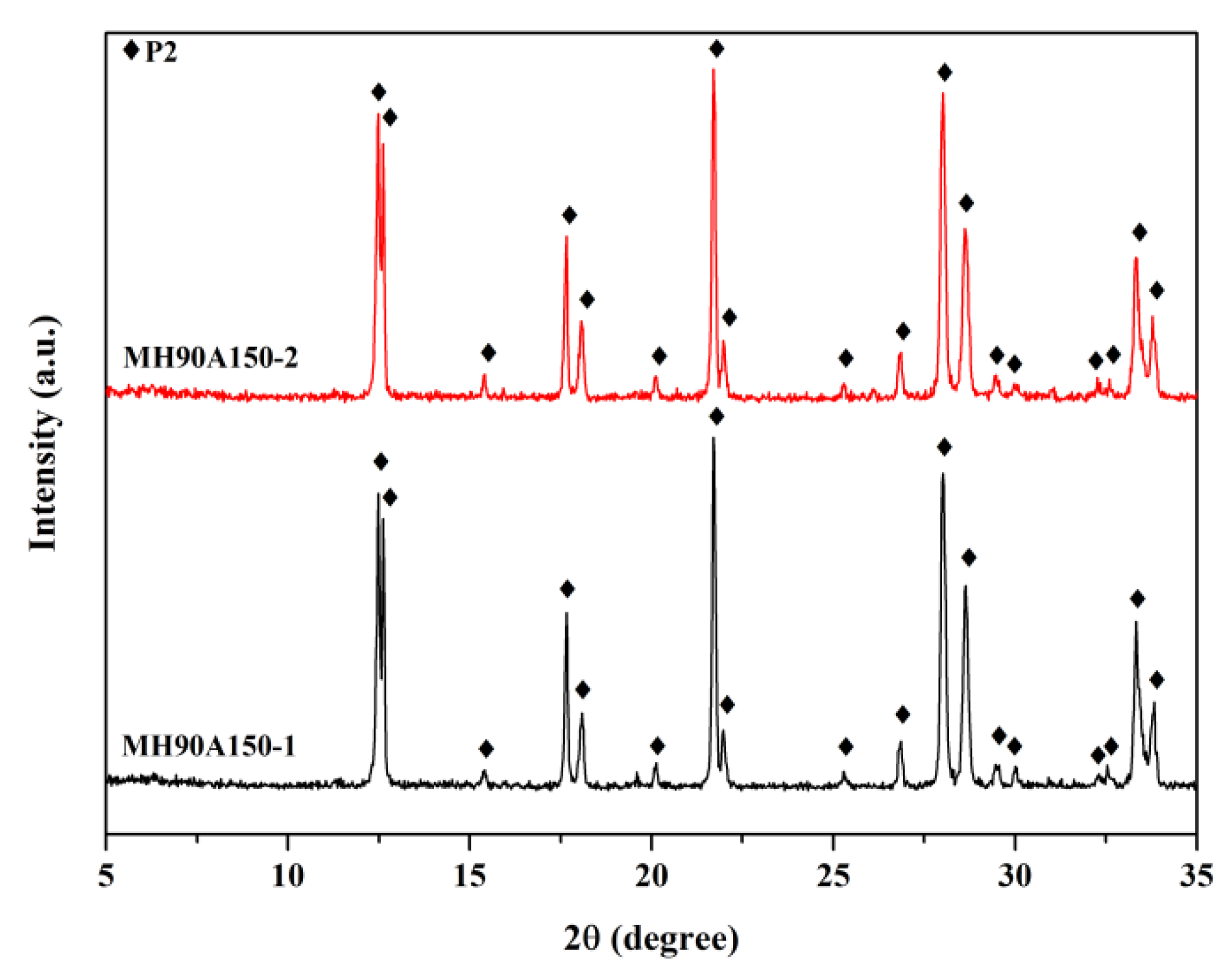
3.3. Effect of Double Crystallization by Microwave-Assisted Hydrothermal Methods
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Baerlocher, C.; McCusker, L.B.; Olson, D.H. GIS—I41/amd. In Atlas of Zeolite Framework, 6th ed.; Baerlocher, C., McCusker, L.B., Olson, D.H., Eds.; Elsevier Science B.V.: Amsterdam, The Netherlands, 2007; pp. 146–147. [Google Scholar] [CrossRef]
- Flanigen, E.M. Chapter 2 Zeolites and molecular sieves: An historical perspective. Stud. Surf. Sci. Catal. 2001, 35, 137. [Google Scholar] [CrossRef]
- Yin, X.; Zhu, G.; Wang, Z.; Yue, N.; Qiu, S. Zeolite P/NaX composite membrane for gas separation. Microporous Mesoporous Mater. 2007, 105, 156–162. [Google Scholar] [CrossRef]
- Dong, J.; Lin, Y. In Situ Synthesis of P-Type Zeolite Membranes on Porous α-Alumina Supports. Ind. Eng. Chem. Res. 1998, 37, 2404–2409. [Google Scholar] [CrossRef]
- Fischer, M.; Bell, R.G. Influence of Zeolite Topology on CO2/N2 Separation Behavior: Force-Field Simulations Using a DFT-Derived Charge Model. J. Phys. Chem. C 2012, 116, 26449–26463. [Google Scholar] [CrossRef]
- Ali, I.O.; El-Sheikh, S.M.; Salama, T.M.; Bakr, M.F.; Fodial, M.H. Controllable synthesis of NaP zeolite and its application in calcium absorption. Sci. China Mater. 2015, 58, 621–633. [Google Scholar] [CrossRef] [Green Version]
- Nery, J.G.; Mascarenhas, Y.P.; Cheetham, A.K. A study of the highly crystalline, low-silica, fully hydrated zeolite P ion exchanged with (Mn2+, Cd2+, Pb2+, Sr2+, Ba2+) cations. Microporous Mesoporous Mater. 2003, 57, 229–248. [Google Scholar] [CrossRef]
- Arrigo, I.; Caprì, M.; Corigliano, F.; Bonavita, A.; Rizzo, G.; Neri, G.; Donato, N. An Exploratory Study on the Potential of Zeolite P-Based Materials for Sensing Applications. In Sensors and Microsystems; Springer: Berlin/Heidelberg, Germany, 2011; Volume 91, pp. 67–71. [Google Scholar] [CrossRef]
- Baerlocher, C.; Meier, W.M. The crystal structure of synthetic zeolite Na-P 1, an isotype of gismondine. Zeitschrift für Kristallographie—Cryst. Mater. 1972, 135, 339–354. [Google Scholar] [CrossRef]
- McCusker, L.; Baerlocher, C.; Nawaz, R. Rietveld refinement of the crystal structure of the new zeolite mineral gobbinsite. Zeitschrift für Kristallographie—Cryst. Mater. 1985, 171, 281–289. [Google Scholar] [CrossRef]
- Oleksiak, M.D.; Ghorbanpour, A.; Conato, M.T.; McGrail, B.P.; Grabow, L.C.; Motkuri, R.K.; Rimer, J.D. Synthesis Strategies for Ultrastable Zeolite GIS Polymorphs as Sorbents for Selective Separations. Chem. A Eur. J. 2016, 22, 15961. [Google Scholar] [CrossRef] [Green Version]
- Rees, L.V.C.; Chandrasekhar, S. Chapter 51—GIS zeolite P Si(54), Al(46). In Verified Syntheses of Zeolitic Materials, 2nd ed.; Robson, H., Lillerud, K.P., Eds.; Elsevier Science: Amsterdam, The Netherlands, 2001; pp. 169–170. [Google Scholar] [CrossRef]
- Albert, B.; Cheetham, A.; Stuart, J.; Adams, C. Investigations on P zeolites: Synthesis, characterisation, and structure of highly crystalline low-silica NaP. Microporous Mesoporous Mater. 1998, 21, 133–142. [Google Scholar] [CrossRef]
- Azizi, S.N.; Tilami, S.E. Recrystallization of Zeolite Y to Analcime and Zeolite P with D-Methionine as Structure-Directing Agent (SDA). Zeitschrift für Anorg. und Allg. Chem. 2009, 635, 2660–2664. [Google Scholar] [CrossRef]
- Yamni, K.; Tijani, N.; Lamsiah, S. Influence of the crystallization temperatureon the synthesis of Y zeolite. IOSR J. Appl. Chem. 2014, 7, 58–61. [Google Scholar] [CrossRef]
- Sathupunya, M.; Gulari, E.; Wongkasemjit, S. ANA and GIS zeolite synthesis directly from alumatrane and silatrane by sol-gel process and microwave technique. J. Eur. Ceram. Soc. 2002, 22, 2305–2314. [Google Scholar] [CrossRef]
- Le, T.; Wang, Q.; Pan, B.; Ravindra, A.; Ju, S.; Peng, J. Process regulation of microwave intensified synthesis of Y-type zeolite. Microporous Mesoporous Mater. 2019, 284, 476–485. [Google Scholar] [CrossRef]
- Yang, G.; Park, S.-J. Conventional and Microwave Hydrothermal Synthesis and Application of Functional Materials: A Review. Materials 2019, 12, 1177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sousa, L.V.; Silva, A.O.S.; Silva, B.J.; Quintela, P.H.; Barbosa, C.B.; Frety, R.; Pacheco, J.G.A. Preparation of zeolite P by desilication and recrystallization of zeolites ZSM-22 and ZSM-35. Mater. Lett. 2018, 217, 259–262. [Google Scholar] [CrossRef]
- Khabuanchalad, S.; Khemthong, P.; Prayoonpokarach, S.; Wittayakun, J. Transformation of zeolite NaY synthesized from rice husk silica to NaP during hydrothermal synthesis. Suranaree J. Sci. Technol. 2008, 15, 225–231. [Google Scholar]
- Ginter, D.M.; Bell, A.T.; Radke, C.J. Chapter 46—FAU Linde type Y Si(71), Al(29). In Verified Syntheses of Zeolitic Materials, 2nd ed.; Robson, H., Lillerud, K.P., Eds.; Elsevier Science: Amsterdam, The Netherlands, 2001; pp. 156–158. [Google Scholar] [CrossRef]
- Bunmai, K.; Osakoo, N.; Deekamwong, K.; Rongchapo, W.; Keawkumay, C.; Chanlek, N.; Prayoonpokarach, S.; Wittayakun, J. Extraction of silica from cogon grass and utilization for synthesis of zeolite NaY by conventional and microwave-assisted hydrothermal methods. J. Taiwan Inst. Chem. Eng. 2018, 83, 152–158. [Google Scholar] [CrossRef]
- Azizi, S.N.; Daghigh, A.A.; Abrishamkar, M. Phase Transformation of Zeolite P to Y and Analcime Zeolites due to Changing the Time and Temperature. J. Spectrosc. 2013, 2013, 1–5. [Google Scholar] [CrossRef]
- Sharma, P.; Song, J.-S.; Han, M.H.; Cho, C.-H. GIS-NaP1 zeolite microspheres as potential water adsorption material: Influence of initial silica concentration on adsorptive and physical/topological properties. Sci. Rep. 2016, 6, 22734. [Google Scholar] [CrossRef]
- Panzarella, B.; Tompsett, G.A.; Yngvesson, K.S.; Conner, W.C. Microwave Synthesis of Zeolites. 2. Effect of Vessel Size, Precursor Volume, and Irradiation Method. J. Phys. Chem. B 2007, 111, 12657–12667. [Google Scholar] [CrossRef]
- Li, Y.; Yang, W. Microwave synthesis of zeolite membranes: A review. J. Membr. Sci. 2008, 316, 3–17. [Google Scholar] [CrossRef]
- Bunmai, K.; Osakoo, N.; Deekamwong, K.; Kosri, C.; Khemthong, P.; Wittayakun, J. Fast synthesis of zeolite NaP by crystallizing the NaY gel under microwave irradiation. Mater. Lett. 2020, 272, 127845. [Google Scholar] [CrossRef]
- Gu, B.; Bai, J.; Yang, W.; Li, C. Synthesis of ANA-zeolite-based Cu nanoparticles composite catalyst and its regularity in styrene oxidation. Microporous Mesoporous Mater. 2019, 274, 318–326. [Google Scholar] [CrossRef]
- Baerlocher, C.; McCusker, L.B.; Olson, D.H. FAU—Fd3¯m. In Atlas of Zeolite Framework, 6th ed.; Baerlocher, C., McCusker, L.B., Olson, D.H., Eds.; Elsevier Science B.V.: Amsterdam, The Netherlands, 2007; pp. 140–141. [Google Scholar] [CrossRef]
- Baerlocher, C.; McCusker, L.B.; Olson, D.H. ANA—Ia3¯d. In Atlas of Zeolite Framework, 6th ed.; Baerlocher, C., McCusker, L.B., Olson, D.H., Eds.; Elsevier Science B.V.: Amsterdam, The Netherlands, 2007; pp. 46–47. [Google Scholar] [CrossRef]
- Maldonado, M.; Oleksiak, M.D.; Chinta, S.; Rimer, J.D. Controlling Crystal Polymorphism in Organic-Free Synthesis of Na-Zeolites. J. Am. Chem. Soc. 2013, 135, 2641–2652. [Google Scholar] [CrossRef]
- Kulawong, S.; Chanlek, N.; Osakoo, N. Facile synthesis of hierarchical structure of NaY zeolite using silica from cogon grass for acid blue 185 removal from water. J. Environ. Chem. Eng. 2020, 8, 104114. [Google Scholar] [CrossRef]
- Shariatinia, Z.; Bagherpour, A. Synthesis of zeolite NaY and its nanocomposites with chitosan as adsorbents for lead(II) removal from aqueous solution. Powder Technol. 2018, 338, 744–763. [Google Scholar] [CrossRef]
- Mozgawa, W.; Król, M.; Barczyk, K. FT-IR studies of zeolites from different structural groups. Chemik 2011, 65, 671–674. [Google Scholar]
- Sharma, P.; Yeo, J.-G.; Han, M.-H.; Cho, C.H. Knobby surfaced, mesoporous, single-phase GIS-NaP1 zeolite microsphere synthesis and characterization for H2 gas adsorption. J. Mater. Chem. A 2013, 1, 2602–2612. [Google Scholar] [CrossRef]
- Chandrasekhar, S.; Pramada, P. Kaolin-based zeolite Y, a precursor for cordierite ceramics. Appl. Clay Sci. 2004, 27, 187–198. [Google Scholar] [CrossRef]
- Flanigen, E.M.; Khatami, H.; Szymanski, H.A. Infrared Structural Studies of Zeolite Frameworks. In Advances in Chemistry; American Chemical Society (ACS): Washington, DC, USA, 1974; Volume 101, pp. 201–229. [Google Scholar]
- Qamar, M.; Baig, I.; Azad, A.-M.; Ahmed, M.; Qamaruddin, M. Synthesis of mesoporous zeolite Y nanocrystals in octahedral motifs mediated by amphiphilic organosilane surfactant. Chem. Eng. J. 2016, 290, 282–289. [Google Scholar] [CrossRef]

| Gel Composition | Main Product | Crystallization Time | Crystallization Temperature (°C) | Method | References |
|---|---|---|---|---|---|
| 2.2 SiO2: Al2O3: 5.28 NaF: 105.6 H2O | P1 | 60 days | 85 | CH 1 | [12] |
| Na8Al8Si8O32 · 15.2 H2O | P2 | 5 days | 100 | CH 1 | [13] |
| 3.75 SiO2: 0.2 Al2O3: 4.3 Na2O: 220 H2O 4 | P1 | 42 h | 100 | CH 1 | [14] |
| 0.0065 NaAlO2: 0.023 Na2Si3O7 (seed) 3 0.04 NaAlO2: 0.147 Na2Si3O7 (feed) | P1 | 24 h | 100 | CH 1 | [15] |
| 1 SiO2: 0.25 Al2O3: 3 Na2O: 410H2O 5,6 | P1 | 3 h | 110 | MH 2 | [16] |
| 17 Na2O: Al2O3: 17 SiO2: 345 H2O (seed) 3 3 Na2O: Al2O3: 8 SiO2: 209 H2O (Overall) | P2 | 2 h | 150 | MH 2 | [17] |
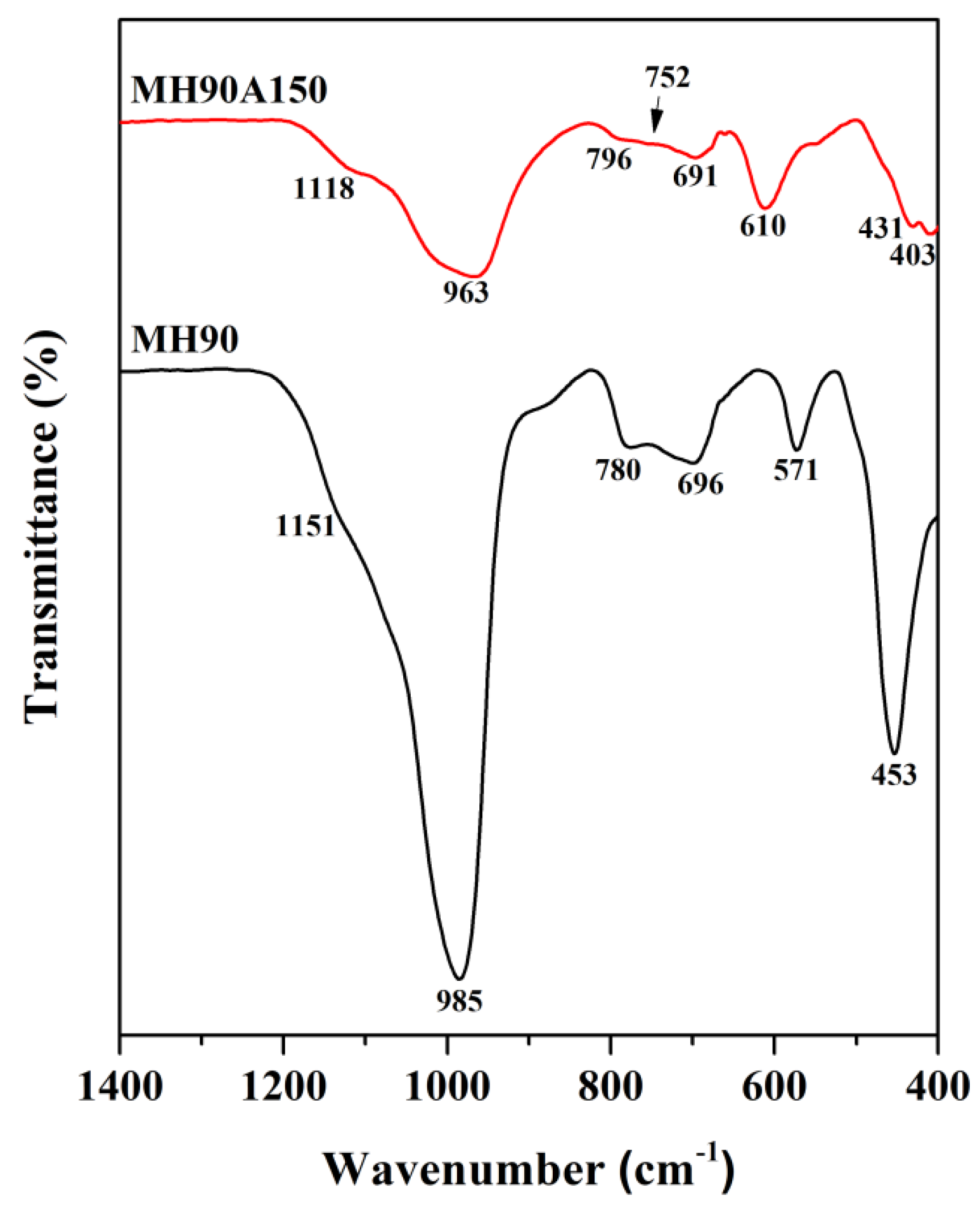
| Wavenumber (cm−1) | Assignment | References |
|---|---|---|
| FAU zeolite | [32,33,34] | |
| 1151 | Asymmetric stretching vibration of external linkage TO4 ( T = Si, Al) | |
| 985 | Asymmetric stretching vibration of internal tetrahedral TO4 | |
| 780 | Symmetric stretching vibration of external linkage TO4 | |
| 696 | Symmetric stretching vibration of internal linkage TO4 | |
| 571 | Vibration of double-6-membered ring (D6R) | |
| 453 | Bending vibration of T–O bond in internal tetrahedron | |
| GIS zeolite | [34,35,36,37] | |
| 1118 | Asymmetric stretching vibration of external linkage TO4 | |
| 963 | Asymmetric stretching vibration of internal tetrahedral TO4 | |
| 796 | Symmetric stretching vibrations of external linkage TO4 | |
| 752 | Vibration of 4-membered ring (4MR) | |
| 691 | Symmetric stretching vibration of internal linkage TO4 | |
| 610 | Double ring vibration in GIS zeolite framework | |
| 431, 403 | Bending vibration of T–O |
| Sample Name | Crystallization Conditions | Phase of Product 4 | ||||
|---|---|---|---|---|---|---|
| Method | Synthesis Process | Temperature (°C) | Time (min) | Major | Minor | |
| CH150 | CH 1 | Single | 150 | 1440 | NaP2 | - |
| MH90 | MH 2 | Single | 90 | 60 | NaY | - |
| MH150 | MH 2 | Single | 150 | 15–60 | NaP1 | NaP2 |
| MH175 | MH 2 | Single | 90 | 15–60 | NaP2 | ANA |
| MH90A | MH 2 | Single | 90 and aged 3 | 60 | NaY | - |
| MH90A150 | MH 2 | Double | 90, 150 | 60, 60 | NaP2 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tayraukham, P.; Jantarit, N.; Osakoo, N.; Wittayakun, J. Synthesis of Pure Phase NaP2 Zeolite from the Gel of NaY by Conventional and Microwave-Assisted Hydrothermal Methods. Crystals 2020, 10, 951. https://doi.org/10.3390/cryst10100951
Tayraukham P, Jantarit N, Osakoo N, Wittayakun J. Synthesis of Pure Phase NaP2 Zeolite from the Gel of NaY by Conventional and Microwave-Assisted Hydrothermal Methods. Crystals. 2020; 10(10):951. https://doi.org/10.3390/cryst10100951
Chicago/Turabian StyleTayraukham, Pimwipa, Nawee Jantarit, Nattawut Osakoo, and Jatuporn Wittayakun. 2020. "Synthesis of Pure Phase NaP2 Zeolite from the Gel of NaY by Conventional and Microwave-Assisted Hydrothermal Methods" Crystals 10, no. 10: 951. https://doi.org/10.3390/cryst10100951





