Structural Variations in Manganese Halide Chain Compounds Mediated by Methylimidazolium Isomers
Abstract
:1. Introduction
2. Experimental Section
2.1. Chemicals
2.2. Synthesis
2.3. Characterisation
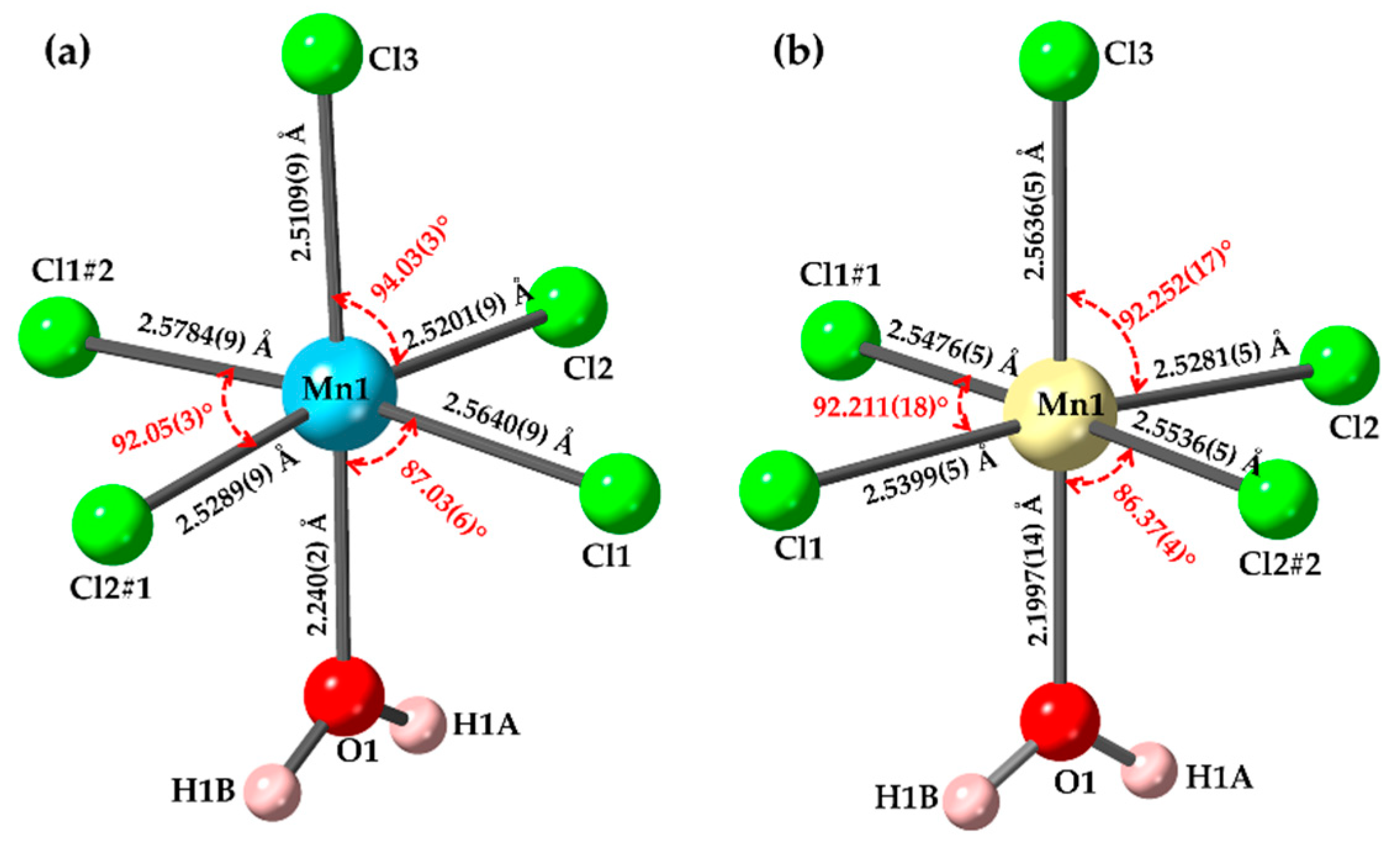
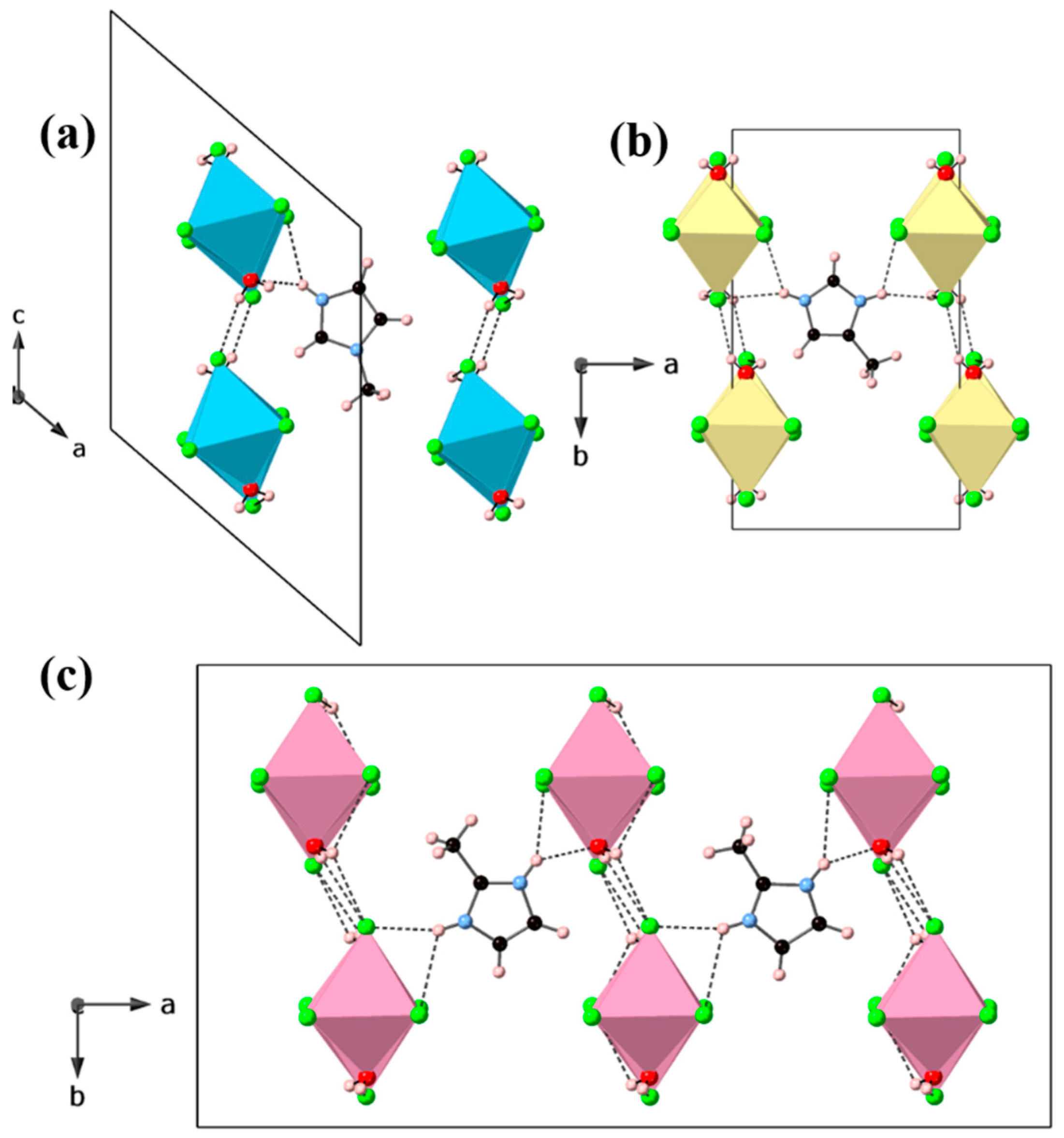
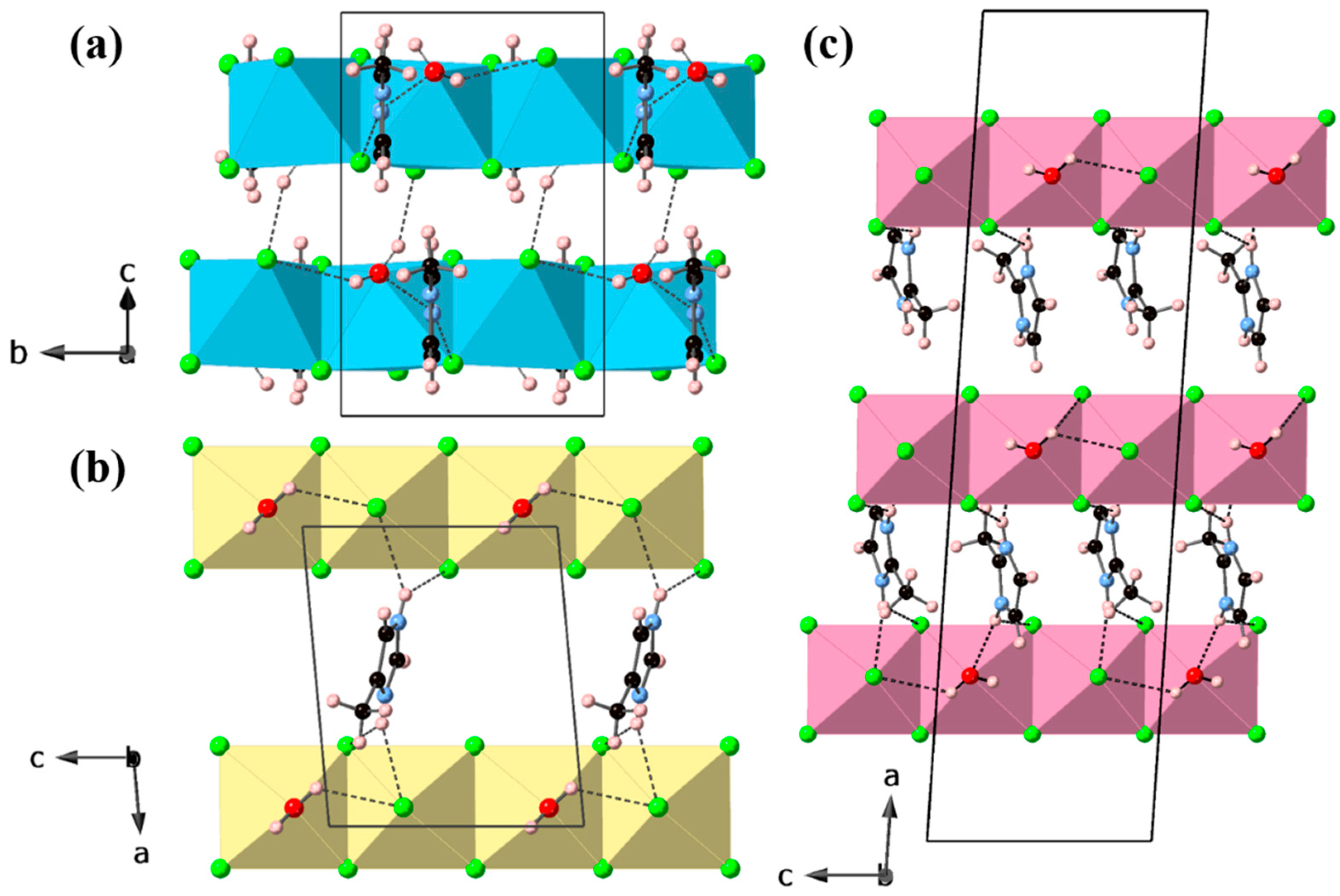
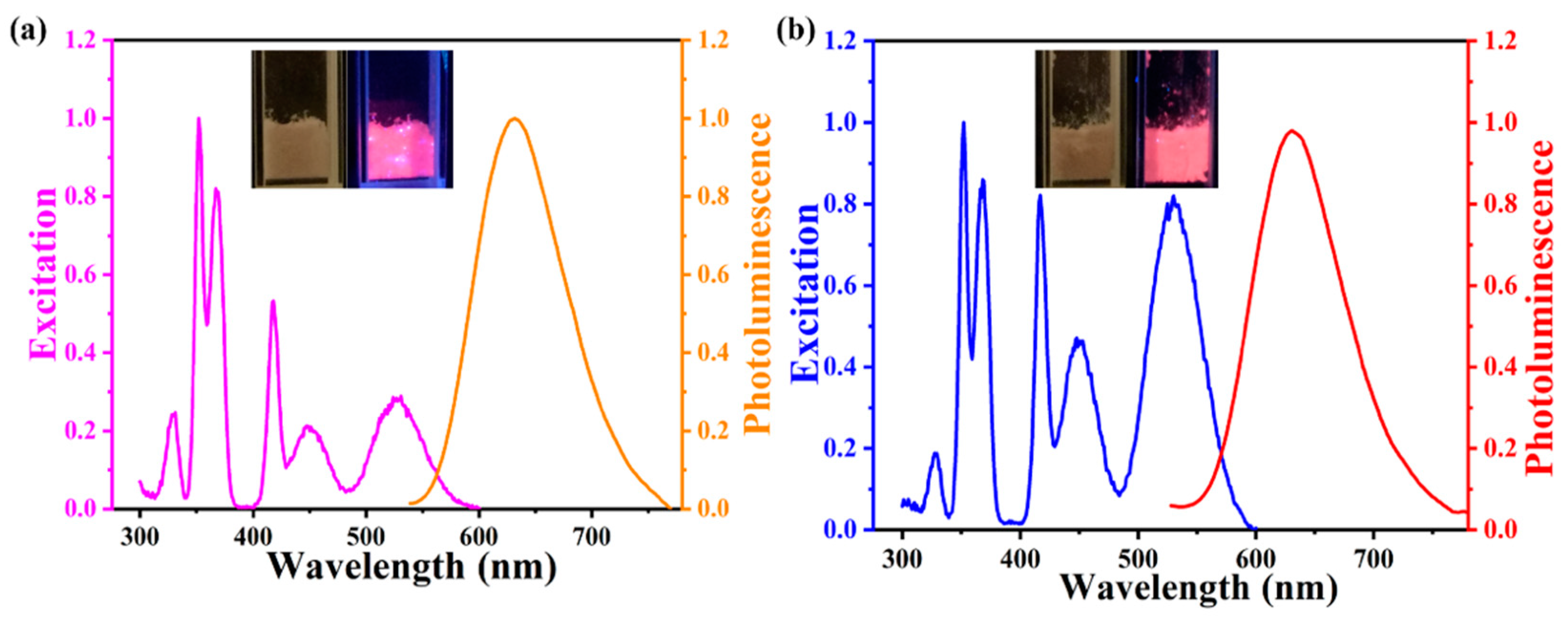
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Protesescu, L.; Yakunin, S.; Bodnarchuk, M.I.; Krieg, F.; Caputo, R.; Hendon, C.H.; Yang, R.X.; Walsh, A.; Kovalenko, M.V. Nanocrystals of cesium lead halide perovskites (CsPbX3, X = Cl, Br, and I): Novel optoelectronic materials showing bright emission with wide color gamut. Nano Lett. 2015, 15, 3692–3696. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, C.L.; Song, Z.N.; Li, C.W.; Zhao, D.W.; Yan, Y.F. Low-Bandgap mixed Tin-Lead perovskites and their applications in all-perovskite tandem solar cells. Adv. Funct. Mater. 2019, 29, 1808801. [Google Scholar] [CrossRef]
- Mao, L.; Ke, W.; Pedesseau, L.; Wu, Y.; Katan, C.; Even, J.; Wasielewski, M.R.; Stoumpos, C.C.; Kanatzidis, M.G. Hybrid Dion–Jacobson 2D lead iodide perovskites. J. Am. Chem. Soc. 2018, 140, 3775–3783. [Google Scholar] [CrossRef] [PubMed]
- Stoumpos, C.C.; Cao, D.H.; Clark, D.J.; Young, J.; Rondinelli, J.M.; Jang, J.I.; Hupp, J.T.; Kanatzidis, M.G. Ruddlesden–Popper hybrid lead iodide perovskite 2D homologous semiconductors. Chem. Mater. 2016, 28, 2852–2867. [Google Scholar] [CrossRef]
- Jacobsson, T.J.; Schwan, L.J.; Ottosson, M.; Hagfeld, A.; Edvinsson, T. Determination of thermal expansion coefficients and locating the temperature-induced phase transition in methylammonium lead perovskites using x-ray diffraction. Inorg. Chem. 2015, 54, 10678–10685. [Google Scholar] [CrossRef] [PubMed]
- Sourisseau, C.; Lucazeau, G. Vibrational study of phase transitions in (NH3(CH2)3NH3)MnCl4. J. Raman Spectrosc. 1979, 8, 311–319. [Google Scholar] [CrossRef]
- Park, S.H.; Oh, I.H.; Park, S.; Park, Y.B.; Kim, J.H.; Huh, Y.D. Canted antiferromagnetism and spin reorientation transition in layered inorganic–organic perovskite (C6H5CH2CH2NH3)2MnCl4. Dalton Trans. 2012, 41, 1237–1242. [Google Scholar] [CrossRef] [PubMed]
- Kojima, N.; Ban, T.; Tsujikawa, I. Magnon sideband of the 4T2g (4D) state in the quasi two-dimensional antiferromagnet (C2H5NH3)2MnCl4. J. Phys. Soc. 1978, 44, 923–929. [Google Scholar] [CrossRef]
- Ye, H.Y.; Zhou, Q.; Niu, X.; Liao, W.Q.; Fu, D.W.; Zhang, Y.; You, Y.M.; Wang, J.; Chen, Z.N.; Xiong, R.G. High-temperature ferroelectricity and photoluminescence in a hybrid organic–inorganic compound:(3-Pyrrolinium)MnCl3. J. Am. Chem. Soc. 2015, 137, 13148–13154. [Google Scholar] [CrossRef]
- Lv, X.H.; Liao, W.Q.; Li, P.F.; Mao, C.Y.; Zhang, Y. Dielectric and photoluminescence properties of a layered perovskite-type organic–inorganic hybrid phase transition compound: NH3(CH2)5NH3MnCl4. J. Mater. Chem. C 2016, 4, 1881–1885. [Google Scholar] [CrossRef]
- Kay, H.F.; Bailey, P.C. Structure and properties of CaTiO3. Acta Crystallogr. 1957, 10, 219–226. [Google Scholar] [CrossRef]
- Saparov, B.; Mitzi, D.B. Organic–inorganic perovskites: Structural versatility for functional materials design. Chem. Rev. 2016, 116, 4558–4596. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Zhang, D.D.; Yang, P.D. Ruddlesden–Popper phase in two-dimensional inorganic halide perovskites: A plausible model and the supporting observations. Nano Lett. 2017, 17, 5489–5494. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Amstel, W.D.; De Jongh, L.J. Magnetic measurements on (CH3NH3)2MnCl4, a quasi two-dimensional Heisenberg antiferromagnet. Solid State Commun. 1972, 11, 1423–1429. [Google Scholar] [CrossRef]
- Arend, H.; Tichy, K.; Baberschke, K.; Rys, F. Chloride perovskite layer compounds of [NH3(CH2)nNH3] MnCl4 formula. Solid State Commun. 1976, 18, 999–1003. [Google Scholar] [CrossRef]
- Li, T.; Clulow, R.; Bradford, A.J.; Lee, S.L.; Slawin, A.M.Z.; Lightfoot, P. A hybrid fluoride layered perovskite, (enH2)MnF4. Dalton Trans. 2019, 48, 4784–4787. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Liao, W.Q.; Fu, D.W.; Ye, H.Y.; Chen, Z.N.; Xiong, R.G. Highly efficient red-light emission in an organic–inorganic hybrid ferroelectric:(pyrrolidinium) MnCl3. J. Am. Chem. Soc. 2015, 137, 4928–4931. [Google Scholar] [CrossRef]
- Ai, Y.; Chen, X.G.; Shi, P.P.; Tang, Y.Y.; Li, P.F.; Liao, W.Q.; Xiong, R.G. Fluorine Substitution Induced High T c of Enantiomeric Perovskite Ferroelectrics:(R)-and (S)-3-(Fluoropyrrolidinium)MnCl3. J. Am. Chem. Soc. 2019, 141, 4474–4479. [Google Scholar] [CrossRef]
- Hachuła, B.; Pędras, M.; Nowak, M.; Kusz, J.; Pentak, D.; Borek, J. The crystal structure and spectroscopic properties of catena-(2-methylimidazolium bis (μ2-chloro)-aqua-chloromanganese (II)). J. Serbian Chem. Soc. 2011, 76, 235–247. [Google Scholar] [CrossRef]
- Guo, Q.; Zhang, W.Y.; Chen, C.; Ye, Q.; Fu, D.W. Red-light emission and dielectric reversible duple opto-electronic switches in a hybrid multifunctional material:(2-methylimidazolium)MnCl3(H2O). J. Mater. Chem. C 2017, 5, 5458–5464. [Google Scholar] [CrossRef]
- Su, C.W.; Wu, C.P.; Chen, J.D.; Liou, L.S.; Wang, J.C. Synthesis and structural characterization of two chain complexes of Mn (II) containing 2-aminopyridinium. Inorg. Chem. Commun. 2002, 5, 215–219. [Google Scholar] [CrossRef]
- Adams, C.J.; Kurawa, M.A.; Orpen, A.G. Coordination chemistry in the solid state: Reactivity of manganese and cadmium chlorides with imidazole and pyrazole and their hydrochlorides. Inorg. Chem. 2010, 49, 10475–10485. [Google Scholar] [CrossRef]
- Li, C.Y.; Bai, X.W.; Guo, Y.C.; Zou, B.S. Tunable emission properties of manganese chloride small single crystals by pyridine incorporation. ACS Omega 2019, 4, 8039–8045. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bai, X.; Zhong, H.Z.; Chen, B.K.; Chen, C.; Han, J.B.; Zeng, R.S.; Zou, B.S. Pyridine-modulated Mn ion emission properties of C10H12N2MnBr4 and C5H6NMnBr3 single crystals. J. Phys. Chem. C 2018, 122, 3130–3137. [Google Scholar] [CrossRef]
- Chen, S.Q.; Gao, J.M.; Chang, J.Y.; Zhang, Y.; Feng, L. Organic-inorganic manganese (II) halide hybrids based paper sensor for the fluorometric determination of pesticide ferbam. Sens. Actuators B Chem. 2019, 297, 126701. [Google Scholar] [CrossRef]
- Jiang, C.; Zhong, N.; Luo, C.; Lin, H.; Zhang, Y.; Peng, H.; Duan, C.G. (Diisopropylammonium)2 MnBr4: A multifunctional ferroelectric with efficient green-emission and excellent gas sensing properties. Chem. Commun. 2017, 53, 5954–5957. [Google Scholar] [CrossRef]
- Guo, Y.Y.; Yang, L.J.; Lightfoot, P. Three new lead iodide chain compounds, APbI3, templated by molecular cations. Crystals 2019, 9, 616. [Google Scholar] [CrossRef] [Green Version]
- Guo, Y.Y.; Lightfoot, P. Structural diversity of lead halide chain compounds, APbX3, templated by isomeric molecular cations. Dalton Trans. 2020, 49, 12767–12775. [Google Scholar] [CrossRef]
- Rigaku. CrystalClear; Rigaku Corporation: Tokyo, Japan, 2014. [Google Scholar]
- Rigaku Oxford Diffraction. CrysAlisPro v1.171.40.40a.; Rigaku Corporation: Oxford, UK, 2015. [Google Scholar]
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. A Found. Crystallogr. 2015, 71, 3–8. [Google Scholar] [CrossRef] [Green Version]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Farrugia, L.J. WinGX and ORTEP for Windows: An update. J. Appl. Cryst. 2012, 45, 849–854. [Google Scholar] [CrossRef]
- Palmer, D.C. CrystalMaker; Agilent Technologies Ltd.: Yarnton, Oxfordshire, UK, 2014. [Google Scholar]
- Lufaso, M.W.; Woodward, P.M. Jahn-Teller distortions, cation ordering and octahedral tilting in perovskites. Acta Crystallogr. Sect. B Struct. Sci. 2004, 60, 10–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robinson, K.; Gibbs, G.V.; Ribbe, P.H. Quadratic Elongation: A Quantitative Measure of Distortion in Coordination Polyhedra. Science 1971, 172, 567–570. [Google Scholar] [CrossRef] [PubMed]
- Brese, N.E.; O’Keeffe, M. Bond-valence parameters for solids. Acta Cryst. B 1991, 47, 192–197. [Google Scholar] [CrossRef]
- Sen, A.; Swain, D.; Row, T.N.G.; Sundaresan, A. Unprecedented 30 K hysteresis across switchable dielectric and magnetic properties in a bright luminescent organic–inorganic halide (CH6N3)2MnCl4. J. Mater. Chem. C. 2019, 7, 4838–4845. [Google Scholar] [CrossRef] [Green Version]
- Nataf, L.; Rodríguez, F.; Valiente, R. Spectroscopic and luminescence properties of (CH3)4NMnCl3: A sensitive Mn2+-based pressure gauge. High Press Res. 2009, 29, 653–659. [Google Scholar] [CrossRef]
- Orgel, L.E. Phosphorescence of solids containing the manganous or ferric ions. J. Chem. Phys. 1955, 23, 1958. [Google Scholar] [CrossRef]
- Qin, Y.Y.; She, P.F.; Huang, X.M.; Huang, W.; Zhao, Q. Luminescent manganese (II) complexes: Synthesis, properties and optoelectronic applications. Coord. Chem. Rev. 2020, 416, 213331. [Google Scholar] [CrossRef]
- Barreda-Argueso, J.A.; Nataf, L.; Rodriguez-Lazcano, Y.; Aguado, F.; Gonzalez, J.; Valiente, R.; Rodriguez, F.; Wilhelm, H.; Jephcoat, A.P. Bulk and molecular compressibilities of organic–inorganic hybrids [(CH3)4N]2MnX4 (X = Cl, Br); role of intermolecular interactions. Inorg. Chem. 2014, 53, 10708–10715. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, Q.; Zheng, F.K.; Liu, Z.F.; Wang, S.H.; Wu, A.Q.; Guo, G.C. Intense photo-and tribo-luminescence of three tetrahedral manganese (II) dihalides with chelating bidentate phosphine oxide ligand. Dalton Trans. 2015, 44, 3289–3294. [Google Scholar] [CrossRef]
- Xu, L.J.; Lin, X.S.; He, Q.Q.; Worku, M.; Ma, B.W. Highly efficient eco-friendly X-ray scintillators based on an organic manganese halide. Nat. Commun. 2020, 11, 1–7. [Google Scholar] [CrossRef] [PubMed]
| Compound | [1MiH]MnCl3(H2O) | [4MiH]MnCl3(H2O) |
|---|---|---|
| Formula | C4N2H7MnCl3(H2O) | C4N2H7MnCl3(H2O) |
| Formula weight | 262.42 | 262.42 |
| Crystal system | Monoclinic | Monoclinic |
| Space group | P21/c | P21/c |
| a/Å | 11.6861 (6) | 8.6344 (6) |
| b/Å | 7.2891 (4) | 15.1238 (6) |
| c/Å | 14.7870 (9) | 7.3164 (3) |
| β/° | 130.932 (3) | 95.069(5) |
| V/Å3 | 951.59 (10) | 951.68 (9) |
| Z | 4 | 4 |
| Measured ref | 11543 | 9328 |
| Independent ref | 3115 [R(int) = 0.053] | 2565 [R(int) = 0.0315] |
| GOOF | 1.059 | 1.028 |
| Final R indices (I > 2σ(I)) | R1 = 0.0513 wR2 = 0.0953 | R1 = 0.0318 wR2 = 0.0651 |
| Compound | Δd (×104) | σ2 | ∑v (Mn) | ∑v (Cl1) | ∑v (Cl2) | ∑v (Cl3) | ∑v (O1) |
|---|---|---|---|---|---|---|---|
| [1MiH]MnCl3(H2O) | 20.92 | 12.24 | 1.93 | 0.61 | 0.69 | 0.36 | 0.27 |
| [4MiH]MnCl3(H2O) | 27.15 | 6.52 | 1.93 | 0.66 | 0.66 | 0.31 | 0.30 |
| [2MiH]MnCl3(H2O) | 21.46 | 16.53 | 1.98 | 0.61 | 0.74 | 0.36 | 0.27 |
| (2-aminopyridinium)MnCl3(H2O) | 33.85 | 9.22 | 1.93 | 0.64 | 0.60 | 0.36 | 0.32 |
| (pyrazolium)MnCl3(H2O) | 24.72 | 7.22 | 1.99 | 0.72 | 0.63 | 0.34 | 0.29 |
| (pyridinium)MnCl3(H2O) | 22.77 | 10.69 | 1.95 | 0.60 | 0.70 | 0.37 | 0.27 |
| Compound | D-H…A | d(D-H) | d(H…A) | d(D…A) | ∠(DHA) |
|---|---|---|---|---|---|
| [1MiH]MnCl3(H2O) | N2-H2…Cl1#1 | 0.88 | 2.54 | 3.256(3) | 139.1 |
| N2-H2…O1 | 0.88 | 2.38 | 3.018(4) | 129.9 | |
| O1-H1B…Cl3#2 | 0.95 | 2.56 | 3.171(2) | 122.6 | |
| O1-H1A…Cl3#3 | 0.95 | 2.32 | 3.201(2) | 154.0 | |
| [4MiH]MnCl3(H2O) | O1-H1A…Cl3#3 | 0.95 | 2.41 | 3.227(2) | 144.0 |
| O1-H1B…Cl3#2 | 0.95 | 2.54 | 3.177(2) | 124.2 | |
| N1-H1…Cl1#1 | 0.88 | 2.82 | 3.363(2) | 121.8 | |
| N1-H1…Cl3 | 0.88 | 2.47 | 3.263(2) | 149.9 | |
| N2-H2…Cl2#4 | 0.88 | 2.65 | 3.279(2) | 129.1 | |
| N2-H2…Cl3#5 | 0.88 | 2.63 | 3.349(2) | 140.0 | |
| [2MiH]MnCl3(H2O) | N1-H1B···O2 | 0.88 | 2.25 | 2.959(11) | 138 |
| N1-H1B···Cl5 | 0.88 | 2.66 | 3.362(9) | 137 | |
| N2-H2B···Cl8 | 0.88 | 2.30 | 3.125(9) | 157 | |
| N2-H2B···Cl9 | 0.88 | 2.98 | 3.496(8) | 119 | |
| N3-H3A···O3#7 | 0.88 | 2.25 | 2.831(10) | 137 | |
| N3-H3A···Cl9#7 | 0.88 | 2.80 | 3.417(8) | 143 | |
| N4-H4D···Cl4 | 0.88 | 2.36 | 3.168(8) | 154 | |
| N4-H4D···Cl5 | 0.88 | 2.99 | 3.518(9) | 120 | |
| O1—H1C···Cl1#1 | 0.85 | 2.67 | 3.180(6) | 128 | |
| O1—H1D···Cl8 | 0.85 | 2.70 | 3.152(6) | 122 | |
| O2—H2C···Cl4#1 | 0.85 | 2.60 | 3.174(7) | 126 | |
| O2—H2D···Cl4#5 | 0.85 | 2.59 | 3.143(6) | 124 | |
| O3—H3B···Cl8#2 | 0.85 | 2.64 | 3.160(7) | 129 | |
| O3—H3C···Cl1#6 | 0.85 | 2.73 | 3.185(6) | 122 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, C.; Cordes, D.B.; Slawin, A.M.Z.; Lightfoot, P. Structural Variations in Manganese Halide Chain Compounds Mediated by Methylimidazolium Isomers. Crystals 2020, 10, 930. https://doi.org/10.3390/cryst10100930
Han C, Cordes DB, Slawin AMZ, Lightfoot P. Structural Variations in Manganese Halide Chain Compounds Mediated by Methylimidazolium Isomers. Crystals. 2020; 10(10):930. https://doi.org/10.3390/cryst10100930
Chicago/Turabian StyleHan, Ceng, David B. Cordes, Alexandra M. Z. Slawin, and Philip Lightfoot. 2020. "Structural Variations in Manganese Halide Chain Compounds Mediated by Methylimidazolium Isomers" Crystals 10, no. 10: 930. https://doi.org/10.3390/cryst10100930






