The Effect of Equal-Channel Angular Pressing on Microstructure, Mechanical Properties, and Biodegradation Behavior of Magnesium Alloyed with Silver and Gadolinium
Abstract
:1. Introduction
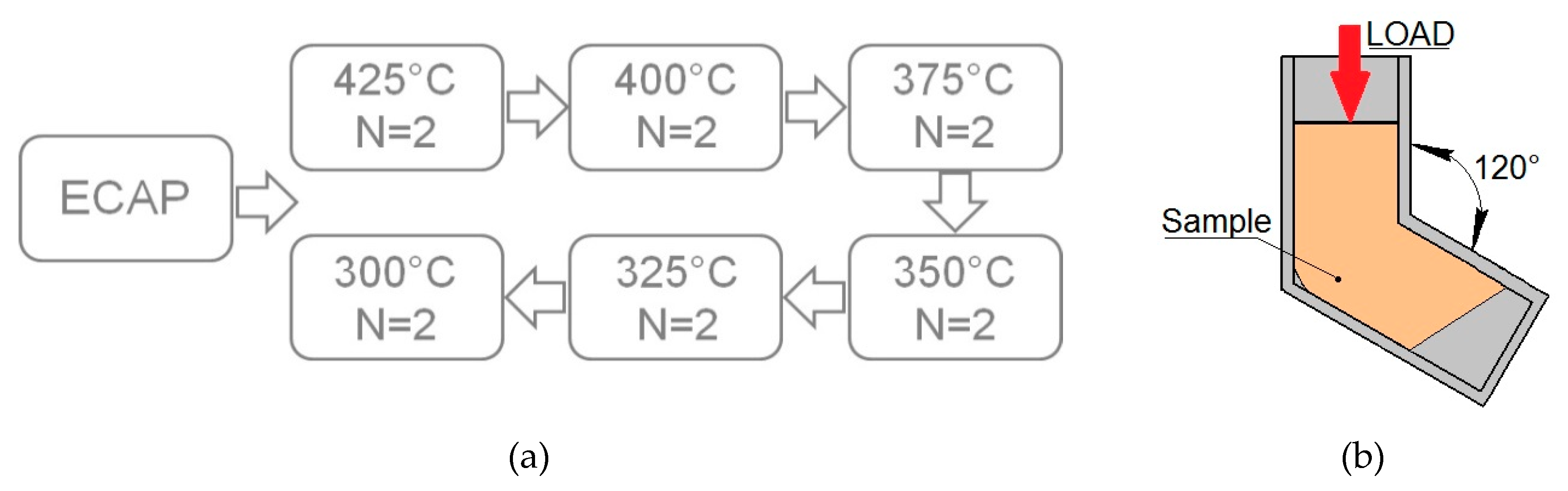
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
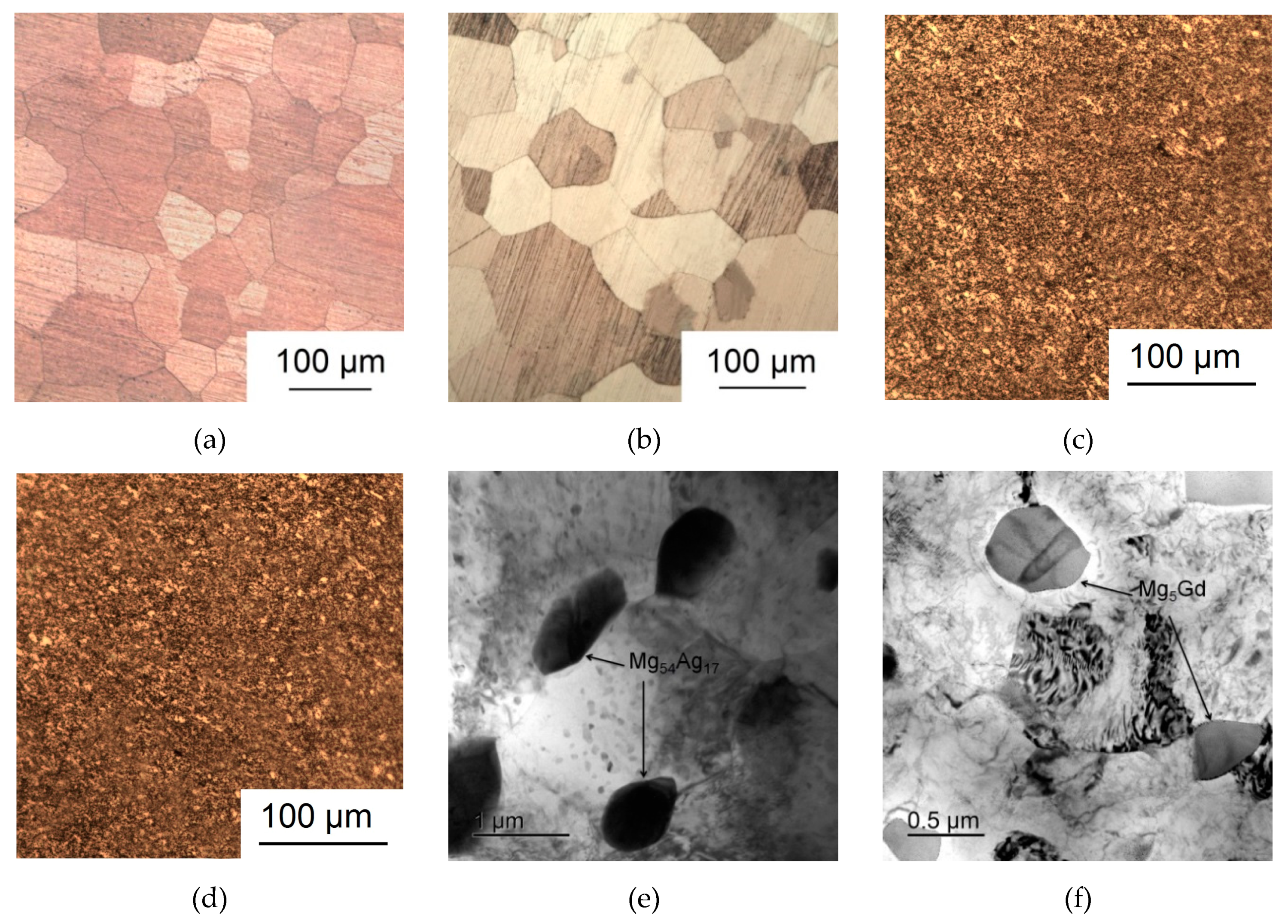
- Processing by ECAP was shown to cause a decrease in the average grain size down to 2–3 μm for the Mg-6.0%Ag alloy and 1–2 μm for the Mg-10.0%Gd alloy. In addition, ECAP-induced precipitation of particles of the Mg54Ag17 and Mg5Gd phases with a size of 500–600 nm and 400–500 nm and a volume fraction of ~9 and ~18.6%, respectively, was observed.
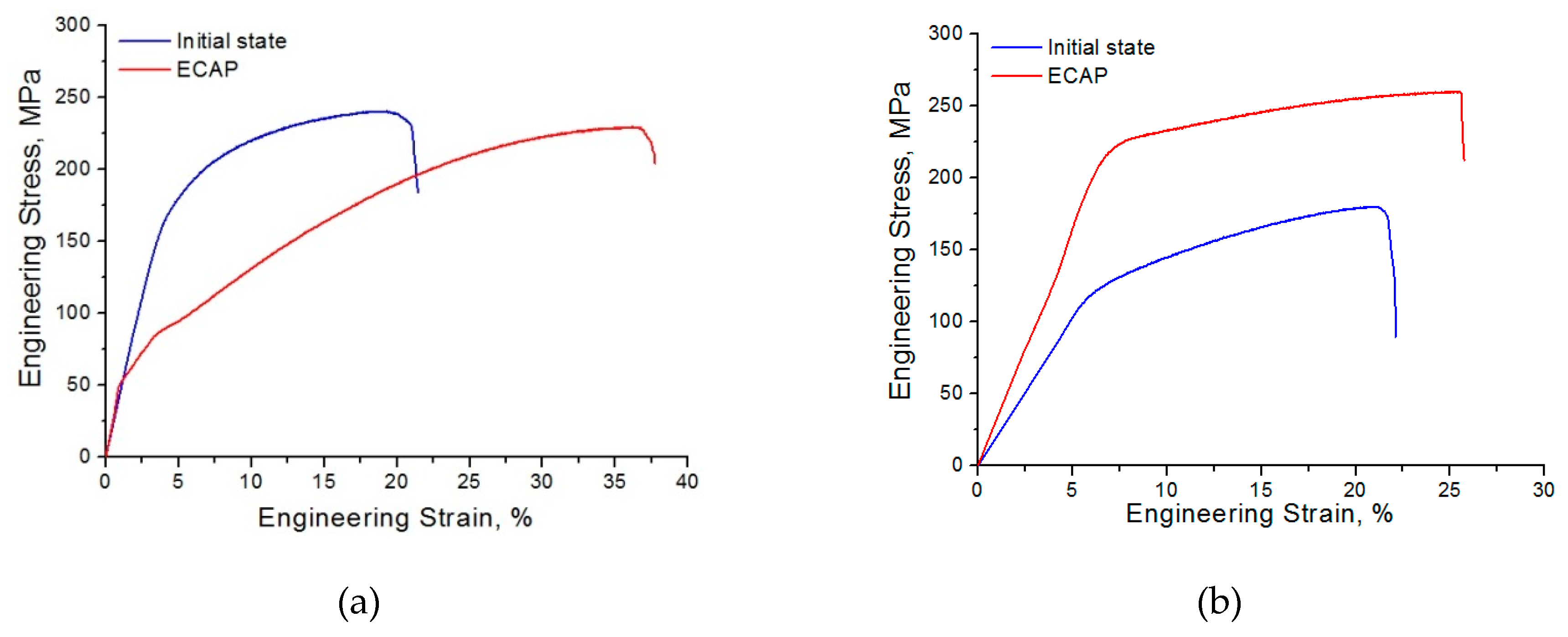
- ECAP was also found to lead to an increase in both strength and ductility of the Mg-10.0%Gd alloy. In the case of the Mg-6.0%Ag alloy, a decrease in the UTS and especially in the YS was observed. This was accompanied with an almost twofold increase in tensile ductility as represented by the strain to failure: from 16.0 ± 0.3% in the initial state to 30.6 ± 3.0% after ECAP.
- An increase in the ductility of the Mg-6.0%Ag alloy combined with a decrease in the strength characteristics is associated with the formation of a sharp inclined basal texture and the predominant slip of dislocations on the basal planes. The improvement in the ductility of the Mg-10.0%Gd alloy achieved by ECAP is associated with an increase in the probability of dislocation slip on prismatic planes, with a retained high probability of slip on the basal planes.
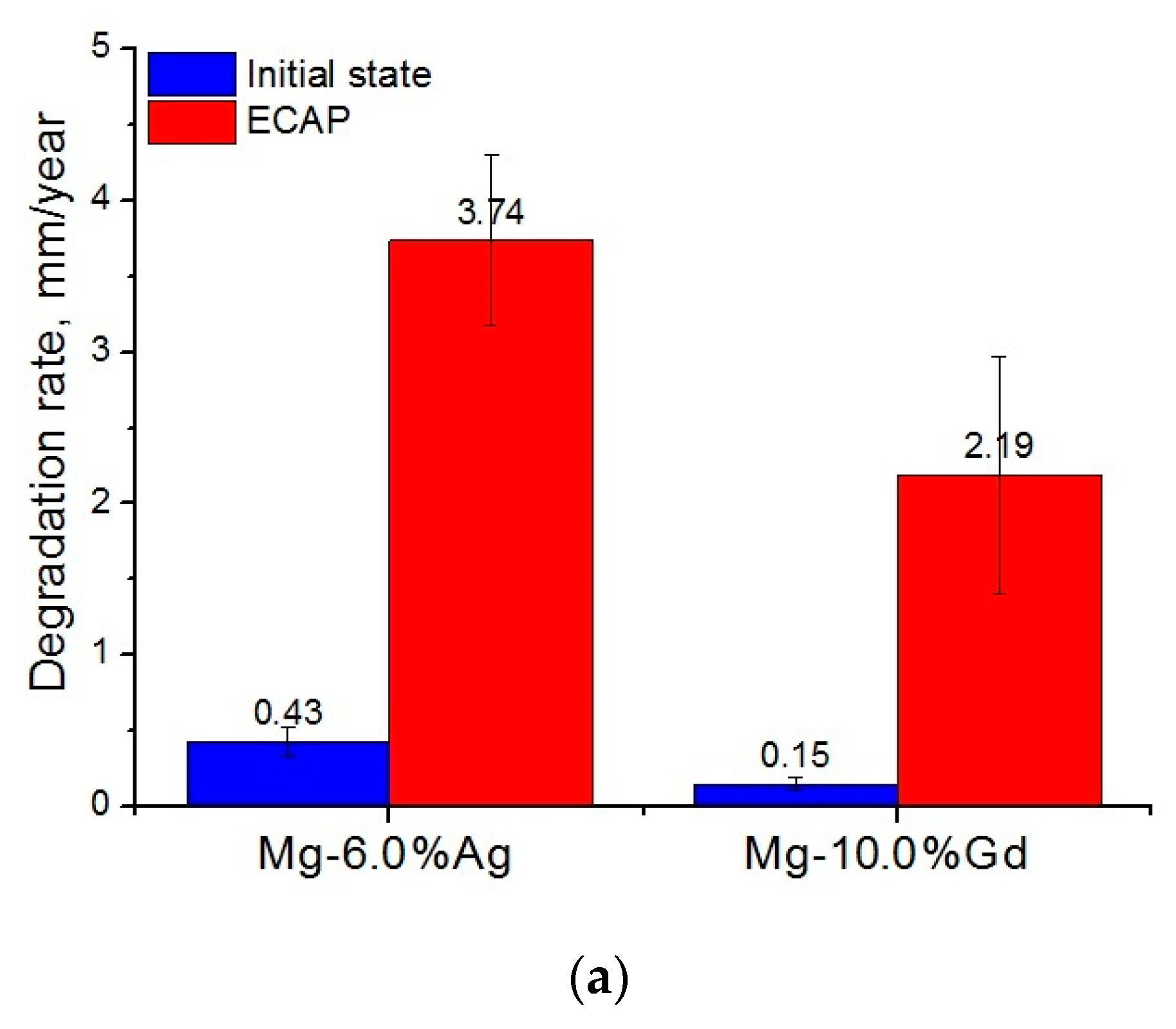
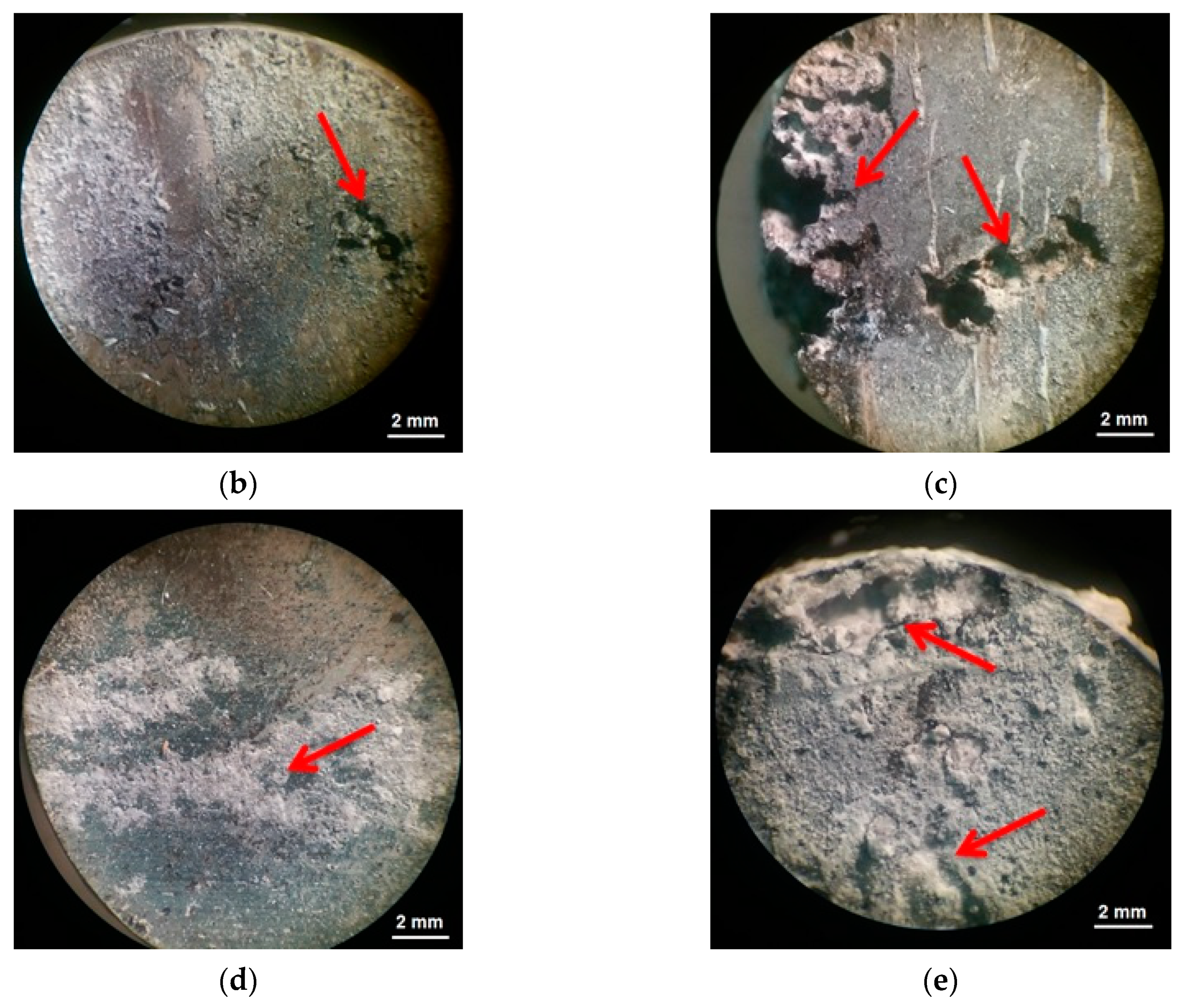
- For both alloys studied, ECAP led to a significant increase in the biodegradation rate and to a pronounced pitting. In the case of the Mg-6.0%Ag alloy, the degradation rate increased from 0.43 ± 0.10 mm/year in the initial state to 3.74 ± 0.56 mm/year after ECAP. For the Mg-10.0%Gd alloy, the degradation rate after ECAP increased to 2.19 ± 0.78 mm/year compared to 0.15 ± 0.04 mm/year in the initial state. The observed rise of the degradation rate after ECAP is associated with an increase in the dislocation density after deformation and the occurrence of micro galvanic corrosion due to the precipitation of particles of the Mg54Ag17 and Mg5Gd phases.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Han, H.-S.; Loffredo, S.; Jun, I.; Edwards, J.; Kim, Y.-C.; Seok, H.-K.; Witte, F.; Mantovani, D.; Glyn-Jones, S. Current status and outlook on the clinical translation of biodegradable metals. Mater. Today 2019, 23, 57–71. [Google Scholar] [CrossRef]
- Zheng, Y.; Gu, X.; Witte, F. Biodegradable metals. Mater. Sci. Eng. R Rep. 2014, 77, 1–34. [Google Scholar] [CrossRef]
- Parfenov, E.; Kulyasova, O.; Mukaeva, V.; Mingo, B.; Farrakhov, R.; Cherneikina, Y.; Yerokhin, A.; Zheng, Y.; Valiev, R. Influence of ultra-fine grain structure on corrosion behaviour of biodegradable Mg-1Ca alloy. Corr. Sci. 2020, 163, 108303. [Google Scholar] [CrossRef]
- Kiani, F.; Wen, C.; Li, Y. Prospects and strategies for magnesium alloys as biodegradable implants from crystalline to bulk metallic glasses and composites—A review. Acta Biomater. 2020, 103, 1–23. [Google Scholar] [CrossRef]
- Costantino, M.; Schuster, A.; Helmholz, H.; Meyer-Rachner, A.; Willumeit-Römer, R.; Luthringer-Feyerabend, B.J. Inflammatory response to magnesium-based biodegradable implant materials. Acta Biomater. 2020, 101, 598–608. [Google Scholar] [CrossRef]
- Merson, D.L.; Brilevesky, A.; Myagkikh, P.; Tarkova, A.; Prokhorikhin, A.; Kretov, E.; Frolova, T.S.; Vinogradov, A. The Functional Properties of Mg–Zn–X Biodegradable Magnesium Alloys. Materials 2020, 13, 544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bazhenov, V.E.; Koltygin, A.; Komissarov, A.; Li, A.; Bautin, V.; Khasenova, R.; Anishchenko, A.; Seferyan, A.; Komissarova, J.; Estrin, Y. Gallium-containing magnesium alloy for potential use as temporary implants in osteosynthesis. J. Magnes. Alloy. 2020, 8, 352–363. [Google Scholar] [CrossRef]
- Zhao, W.; Wang, J.; Weiyang, J.; Qiao, B.; Wang, Y.; Li, Y.; Jiang, D.-M. A novel biodegradable Mg-1Zn-0.5Sn alloy: Mechanical properties, corrosion behavior, biocompatibility, and antibacterial activity. J. Magnes. Alloy. 2020, 8, 374–386. [Google Scholar] [CrossRef]
- Jiang, W.; Wang, J.; Liu, Q.; Zhao, W.; Jiang, D.; Guo, S. Low hydrogen release behavior and antibacterial property of Mg-4Zn-xSn alloys. Mater. Lett. 2019, 241, 88–91. [Google Scholar] [CrossRef]
- Gao, Z.; Song, M.; Liu, R.-L.; Shen, Y.; Ward, L.; Cole, I.; Chen, X.; Liu, X. Improving in vitro and in vivo antibacterial functionality of Mg alloys through micro-alloying with Sr and Ga. Mater. Sci. Eng. C 2019, 104, 109926. [Google Scholar] [CrossRef]
- Tie, D.; Feyerabend, F.; Müller, W.D.; Schade, R.; Liefeith, K.; Kainer, K.; Willumeit, R. Antibacterial biodegradable Mg-Ag alloys. Eur. Cells Mater. 2013, 25, 284–298. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Zhu, S.; Wang, L.; Chang, L.; Hou, Y.; Guan, S. Fabrication and characterization of biodegradable Mg-Zn-Y-Nd-Ag alloy: Microstructure, mechanical properties, corrosion behavior and antibacterial activities. Bioact. Mater. 2018, 3, 225–235. [Google Scholar] [CrossRef] [PubMed]
- Shuai, C.; Zhou, Y.; Yang, Y.; Gao, C.; Peng, S.; Wang, G. Ag-Introduced Antibacterial Ability and Corrosion Resistance for Bio-Mg Alloys. BioMed Res. Int. 2018, 2018, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Liu, H.; Tang, Y.; Xu, X.; Long, H.; Yan, Y.; Luo, Z.; Zhang, Y.; Yu, K.; Zhu, Y. A Potential Biodegradable Mg-Y-Ag Implant with Strengthened Antimicrobial Properties in Orthopedic Applications. Metals 2018, 8, 948. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Zhang, X.; Li, J.; Liu, C.; Guan, S. Investigation of Mg–Zn–Y–Nd alloy for potential application of biodegradable esophageal stent material. Bioact. Mater. 2020, 5, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Wang, W.; Zhu, Y.; Lu, Y.; Wan, P.; Yang, K.; Zhang, Y.; Mao, C. Molecular and cellular mechanisms for zoledronic acid-loaded magnesium-strontium alloys to inhibit giant cell tumors of bone. Acta Biomater. 2018, 77, 365–379. [Google Scholar] [CrossRef]
- Dai, Y.; Tang, Y.; Xu, X.; Luo, Z.; Zhang, Y.; Li, Z.; Lin, Z.; Zhao, S.; Zeng, M.; Sun, B.; et al. Evaluation of the mechanisms and effects of Mg–Ag–Y alloy on the tumor growth and metastasis of the MG63 osteosarcoma cell line. J. Biomed. Mater. Res. Part B Appl. Biomater. 2019, 107, 2537–2548. [Google Scholar] [CrossRef]
- Wu, Y.; He, G.; Zhang, Y.; Liu, Y.; Li, M.; Wang, X.; Li, N.; Li, K.; Zheng, G.; Zheng, Y.; et al. Unique antitumor property of the Mg-Ca-Sr alloys with addition of Zn. Sci. Rep. 2016, 6, 21736. [Google Scholar] [CrossRef] [Green Version]
- Shuai, C.; Liu, L.; Shuai, C.; Gao, C.; Zhao, M.-C.; Yi, L.; Peng, S. Lanthanum-Containing Magnesium Alloy with Antitumor Function Based on Increased Reactive Oxygen Species. Appl. Sci. 2018, 8, 2109. [Google Scholar] [CrossRef] [Green Version]
- Anisimova, N.; Kiselevskiy, M.; Martynenko, N.; Straumal, B.; Willumeit-Römer, R.; Dobatkin, S.; Estrin, Y. Cytotoxicity of biodegradable magnesium alloy WE43 to tumor cells in vitro: Bioresorbable implants with antitumor activity? J. Biomed. Mater. Res. Part B Appl. Biomater. 2019, 108, 167–173. [Google Scholar] [CrossRef]
- Estrin, Y.; Martynenko, N.; Anisimova, N.; Temralieva, D.; Kiselevskiy, M.; Serebryany, V.; Raab, G.; Straumal, B.; Wiese, B.; Willumeit-Römer, R.; et al. The Effect of Equal-Channel Angular Pressing on the Microstructure, the Mechanical and Corrosion Properties and the Anti-Tumor Activity of Magnesium Alloyed with Silver. Materials 2019, 12, 3832. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valiev, R.; Langdon, T.G. Principles of equal-channel angular pressing as a processing tool for grain refinement. Prog. Mater. Sci. 2006, 51, 881–981. [Google Scholar] [CrossRef]
- Muñoz, J.A.; Higuera, O.F.; Cabrera-Marrero, J.-M. Microstructural and mechanical study in the plastic zone of ARMCO iron processed by ECAP. Mater. Sci. Eng. A 2017, 697, 24–36. [Google Scholar] [CrossRef] [Green Version]
- Bochvar, N.; Rybalchenko, O.V.; Shangina, D.; Dobatkin, S. Effect of equal-channel angular pressing on the precipitation kinetics in Cu-Cr-Hf alloys. Mater. Sci. Eng. A 2019, 757, 84–87. [Google Scholar] [CrossRef]
- Rybal’Chenko, O.; Dobatkin, S.; Kaputkina, L.; Raab, G.; Krasilnikov, N. Strength of ultrafine-grained corrosion-resistant steels after severe plastic deformation. Mater. Sci. Eng. A 2004, 387–389. [Google Scholar] [CrossRef]
- Bryła, K. Microstructure and mechanical characterisation of ECAP-ed ZE41A alloy. Mater. Sci. Eng. A 2020, 772, 138750. [Google Scholar] [CrossRef]
- Li, W.; Shen, Y.; Shen, J.; Shen, D.; Liu, X.; Zheng, Y.; Yeung, K.W.; Guan, S.; Kulyasova, O.B.; Valiev, R.Z. In vitro and in vivo studies on pure Mg, Mg–1Ca and Mg–2Sr alloys processed by equal channel angular pressing. Nano Mater. Sci. 2020, 2, 96–108. [Google Scholar] [CrossRef]
- Martynenko, N.; Lukyanova, E.; Anisimova, N.; Kiselevskiy, M.; Serebryany, V.; Yurchenko, N.; Raab, G.; Birbilis, N.; Salishchev, G.; Dobatkin, S.; et al. Improving the property profile of a bioresorbable Mg-Y-Nd-Zr alloy by deformation treatments. Materialia 2020, 13, 100841. [Google Scholar] [CrossRef]
- Minárik, P.; Veselý, J.; Král, R.; Bohlen, J.; KUBÁSEK, J.; Janeček, M.; Stráská, J. Exceptional mechanical properties of ultra-fine grain Mg-4Y-3RE alloy processed by ECAP. Mater. Sci. Eng. A 2017, 708, 193–198. [Google Scholar] [CrossRef]
- Bryła, K.; Horky, J.; Krystian, M.; Lityńska-Dobrzyńska, L.; Mingler, B. Microstructure, mechanical properties, and degradation of Mg-Ag alloy after equal-channel angular pressing. Mater. Sci. Eng. C 2020, 109, 110543. [Google Scholar] [CrossRef]
- Alizadeh, R.; Ngan, A.H.W.; Pereira, P.H.R.; Huang, Y.; Langdon, T.G.; Mahmudi, R. Microstructure, Texture, and Superplasticity of a Fine-Grained Mg-Gd-Zr Alloy Processed by Equal-Channel Angular Pressing. Met. Mater. Trans. A 2016, 47, 6056–6069. [Google Scholar] [CrossRef] [Green Version]
- Fu, Y.; Sun, J.; Yang, Z.; Xu, B.; Han, J.; Chen, Y.; Jiang, J.; Ma, A. Aging behavior of a fine-grained Mg-10.6Gd-2Ag alloy processed by ECAP. Mater. Charact. 2020, 165, 110398. [Google Scholar] [CrossRef]
- Sun, J.; Xu, B.; Yang, Z.; Zhou, H.; Han, J.; Wu, Y.; Song, D.; Yuan, Y.; Zhuo, X.; Liu, H.; et al. Achieving excellent ductility in high-strength Mg-10.6Gd-2 Ag alloy via equal channel angular pressing. J. Alloy. Compd. 2020, 817, 152688. [Google Scholar] [CrossRef]
- Serebryany, V.N.; Kurtasov, S.F.; Savyolova, T. Pole Figure Measurement Plan Influence on Accuracy ODF Coefficients Determined by Modified Harmonic Method. Mater. Sci. Forum 2005, 495, 1693. [Google Scholar] [CrossRef]
- Savyolova, T.; Kourtasov, S. ODF Restoration by Orientations Grid. Mater. Sci. Forum 2005, 495, 301–306. [Google Scholar] [CrossRef]
- Serebryany, V.N.; Rokhlin, L.L.; Monina, A.N. Texture and anisotropy of mechanical properties of the magnesium alloy of Mg-Y-Gd-Zr system. Inorg. Mater. Appl. Res. 2014, 5, 116–123. [Google Scholar] [CrossRef]
- Fornasini, M.L.; Manfrinetti, P.; Gschneidner, K.A. GdMg5: A complex structure with a large cubic cell. Acta Crystallogr. Sect. C Cryst. Struct. Commun. 1986, 42, 138–141. [Google Scholar] [CrossRef]
- Campos, M.D.R.S.; Blawert, C.; Mendis, C.L.; Mohedano, M.; Zimmermann, T.; Proefrock, D.; Zheludkevich, M.L.; Kainer, K.U. Effect of Heat Treatment on the Corrosion Behavior of Mg-10Gd Alloy in 0.5% NaCl Solution. Front. Mater. 2020, 7. [Google Scholar] [CrossRef] [Green Version]
- Persson, K. Materials Data on Mg54Ag17 (SG:71) by Materials Project; Lawrence Berkeley National Lab. (LBNL): Berkeley, CA, USA, 2020. [Google Scholar] [CrossRef]
- Kraus, T.; Fischerauer, S.F.; Hänzi, A.C.; Uggowitzer, P.J.; Löffler, J.F.; Weinberg, A. Magnesium alloys for temporary implants in osteosynthesis: In vivo studies of their degradation and interaction with bone. Acta Biomater. 2012, 8, 1230–1238. [Google Scholar] [CrossRef]
- Martynenko, N.; Lukyanova, E.; Serebryany, V.; Prosvirnin, D.; Terentiev, V.; Raab, G.; Dobatkin, S.; Estrin, Y. Effect of equal channel angular pressing on structure, texture, mechanical and in-service properties of a biodegradable magnesium alloy. Mater. Lett. 2019, 238, 218–221. [Google Scholar] [CrossRef]
- Suh, J.; Victoria-Hernández, J.; Letzig, D.; Golle, R.; Volk, W. Effect of processing route on texture and cold formability of AZ31 Mg alloy sheets processed by ECAP. Mater. Sci. Eng. A 2016, 669, 159–170. [Google Scholar] [CrossRef]
- Akbaripanah, F.; Fereshteh-Saniee, F.; Mahmudi, R.; Kim, H. Microstructural homogeneity, texture, tensile and shear behavior of AM60 magnesium alloy produced by extrusion and equal channel angular pressing. Mater. Des. 2013, 43, 31–39. [Google Scholar] [CrossRef]
- Martynenko, N.; Lukyanova, E.A.; Serebryany, V.N.; Gorshenkov, M.; Shchetinin, I.; Raab, G.; Dobatkin, S.V.; Estrin, Y. Increasing strength and ductility of magnesium alloy WE43 by equal-channel angular pressing. Mater. Sci. Eng. A 2018, 712, 625–629. [Google Scholar] [CrossRef]
- Dobatkin, S.; Galkin, S.; Estrin, Y.; Serebryany, V.; Diez, M.; Martynenko, N.; Lukyanova, E.; Perezhogin, V. Grain refinement, texture, and mechanical properties of a magnesium alloy after radial-shear rolling. J. Alloy. Compd. 2019, 774, 969–979. [Google Scholar] [CrossRef]
- Nie, J. Precipitation and Hardening in Magnesium Alloys. Met. Mater. Trans. A 2012, 43, 3891–3939. [Google Scholar] [CrossRef] [Green Version]
- Vostrý, P.; Smola, B.; Stulíková, I.; Von Buch, F.; Mordike, B.L. Microstructure Evolution in Isochronally Heat Treated Mg–Gd Alloys. Phys. Status Solidi A 1999, 175, 491–500. [Google Scholar] [CrossRef]
- Gao, X.; He, S.; Zeng, X.; Fu, P.; Ding, W.; Nie, J. Microstructure evolution in a Mg–15Gd–0.5Zr (wt.%) alloy during isothermal aging at 250 °C. Mater. Sci. Eng. A 2006, 431, 322–327. [Google Scholar] [CrossRef]
- Shi, Z.-Z.; Zhang, W.-Z. Enhanced age-hardening response and microstructure study of an Ag-modified Mg–Sn–Zn based alloy. Philos. Mag. Lett. 2013, 93, 473–480. [Google Scholar] [CrossRef]
- Kim, H.S.; Kim, W.J. Annealing effects on the corrosion resistance of ultrafine-grained pure titanium. Corr. Sci. 2014, 89, 331–337. [Google Scholar] [CrossRef]
- Song, D.; Ma, A.; Jiang, J.; Lin, P.; Yang, D.; Fan, J. Corrosion behavior of equal-channel-angular-pressed pure magnesium in NaCl aqueous solution. Corr. Sci. 2010, 52, 481–490. [Google Scholar] [CrossRef]
- Niu, H.-Y.; Deng, K.-K.; Nie, K.-B.; Wang, C.-J.; Liang, W.; Wu, Y. Degradation behavior of Mg-4Zn-2Ni alloy with high strength and high degradation rate. Mater. Chem. Phys. 2020, 249, 123131. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, R.; Peng, C.; Cai, Z. Microstructure and corrosion behavior of as-extruded Mg-xLi-3Al-2Zn-0.2Zr alloys (x = 5, 8, 11 wt.%). Corr. Sci. 2020, 167, 108487. [Google Scholar] [CrossRef]
- Birbilis, N.; Ralston, K.; Virtanen, S.; Fraser, H.L.; Davies, C.H.J. Grain character influences on corrosion of ECAPed pure magnesium. Corr. Eng. Sci. Technol. 2010, 45, 224–230. [Google Scholar] [CrossRef]
- Ralston, K.; Birbilis, N. Effect of Grain Size on Corrosion: A Review. Corrosion 2010, 66, 075005. [Google Scholar] [CrossRef]
- Dobatkin, S.V.; Lukyanova, E.A.; Martynenko, N.; Anisimova, N.; Kiselevskiy, M.V.; Gorshenkov, M.V.; Yurchenko, N.Y.; I Raab, G.; Yusupov, V.S.; Birbilis, N.; et al. Strength, corrosion resistance, and biocompatibility of ultrafine-grained Mg alloys after different modes of severe plastic deformation. IOP Conf. Ser. Mater. Sci. Eng. 2017, 194, 12004. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Wu, L.; Liu, H.; Huang, G.; Jiang, B.; Atrens, A.; Pan, F. Microstructure and corrosion behavior of Mg-Sc binary alloys in 3.5 wt.% NaCl solution. Corr. Sci. 2020, 174, 108831. [Google Scholar] [CrossRef]
- Liu, Y.; Cheng, W.-L.; Liu, Y.-H.; Niu, X.-F.; Wang, H.-X.; Wang, L.-F.; Cui, Z.-Q. Effect of alloyed Ca on the microstructure and corrosion behavior of extruded Mg-Bi-Al-based alloys. Mater. Charact. 2020, 163, 110292. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Y.; Wang, P.; Zhang, D.; Yu, B.; Xu, Z.; Jiang, H. Effect of LPSO phases and aged-precipitations on corrosion behavior of as-forged Mg–6Gd–2Y–1Zn–0.3Zr alloy. J. Mater. Res. Technol. 2020, 9, 7087–7099. [Google Scholar] [CrossRef]
- Ding, Z.-Y.; Cui, L.-Y.; Zeng, R.-C.; Zhao, Y.-B.; Guan, S.-K.; Xu, D.K.; Lin, C.-G. Exfoliation corrosion of extruded Mg-Li-Ca alloy. J. Mater. Sci. Technol. 2018, 34, 1550–1557. [Google Scholar] [CrossRef]
- Esmaily, M.; Svensson, J.; Fajardo, S.; Birbilis, N.; Frankel, G.; Virtanen, S.; Arrabal, R.; Thomas, S.; Johansson, L. Fundamentals and advances in magnesium alloy corrosion. Prog. Mater. Sci. 2017, 89, 92–193. [Google Scholar] [CrossRef]
- Dong, J.-H.; Tan, L.; Ren, Y.; Yang, K. Effect of Microstructure on Corrosion Behavior of Mg–Sr Alloy in Hank’s Solution. Acta Metall. Sin. 2018, 32, 305–320. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Li, J.; Lai, H.; Xu, Y. Effect of Homogenization on Microstructure Characteristics, Corrosion and Biocompatibility of Mg-Zn-Mn-xCa Alloys. Materials 2018, 11, 227. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| Processing | YS, MPa | UTS, MPa | El, % | Grain Size, μm | |
|---|---|---|---|---|---|
| Mg-6.0%Ag | Initial state | 162 ± 3 | 239 ± 1 | 16.0 ± 0.3 | 70.9 ± 4.0 |
| ECAP | 44 ± 6 | 224 ± 5 | 30.6 ± 3.0 | 2–3 | |
| Mg-10.0%Gd | Initial state | 123 ± 7 | 185 ± 4 | 13.2 ± 1.7 | 70.8 ± 4.0 |
| ECAP | 211 ± 1 | 258 ± 2 | 18.0 ± 3.6 | 1–2 | |
| State of the Alloys | Basal {0001}<1120> | Prismatic {1010}<1120> | Pyramidal <c+a> | Twinning | ||
|---|---|---|---|---|---|---|
| Mg-6.0%Ag | CS | Initial state | 7.5 | 3.6 | 4.9 | 4.7 |
| ECAP | 4.0 | 5.1 | 5.3 | 5.9 | ||
| LS | Initial state | 4.7 | 5.1 | 4.4 | 4.4 | |
| ECAP | 4.3 | 5.1 | 5.1 | 5.5 | ||
| Mg-10.0%Gd | CS | Initial state | 4.7 | 5.7 | 5.1 | 5.3 |
| ECAP | 4.9 | 5.3 | 5.9 | 6.6 | ||
| LS | Initial state | 4.5 | 4.7 | 4.9 | 5.0 | |
| ECAP | 4.5 | 5.1 | 5.0 | 5.2 | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Straumal, B.; Martynenko, N.; Temralieva, D.; Serebryany, V.; Tabachkova, N.; Shchetinin, I.; Anisimova, N.; Kiselevskiy, M.; Kolyanova, A.; Raab, G.; et al. The Effect of Equal-Channel Angular Pressing on Microstructure, Mechanical Properties, and Biodegradation Behavior of Magnesium Alloyed with Silver and Gadolinium. Crystals 2020, 10, 918. https://doi.org/10.3390/cryst10100918
Straumal B, Martynenko N, Temralieva D, Serebryany V, Tabachkova N, Shchetinin I, Anisimova N, Kiselevskiy M, Kolyanova A, Raab G, et al. The Effect of Equal-Channel Angular Pressing on Microstructure, Mechanical Properties, and Biodegradation Behavior of Magnesium Alloyed with Silver and Gadolinium. Crystals. 2020; 10(10):918. https://doi.org/10.3390/cryst10100918
Chicago/Turabian StyleStraumal, Boris, Natalia Martynenko, Diana Temralieva, Vladimir Serebryany, Natalia Tabachkova, Igor Shchetinin, Natalia Anisimova, Mikhail Kiselevskiy, Alexandra Kolyanova, Georgy Raab, and et al. 2020. "The Effect of Equal-Channel Angular Pressing on Microstructure, Mechanical Properties, and Biodegradation Behavior of Magnesium Alloyed with Silver and Gadolinium" Crystals 10, no. 10: 918. https://doi.org/10.3390/cryst10100918









