High-Pressure Structural Behavior and Equation of State of Kagome Staircase Compound, Ni3V2O8
Abstract
:1. Introduction
2. Materials and Methods
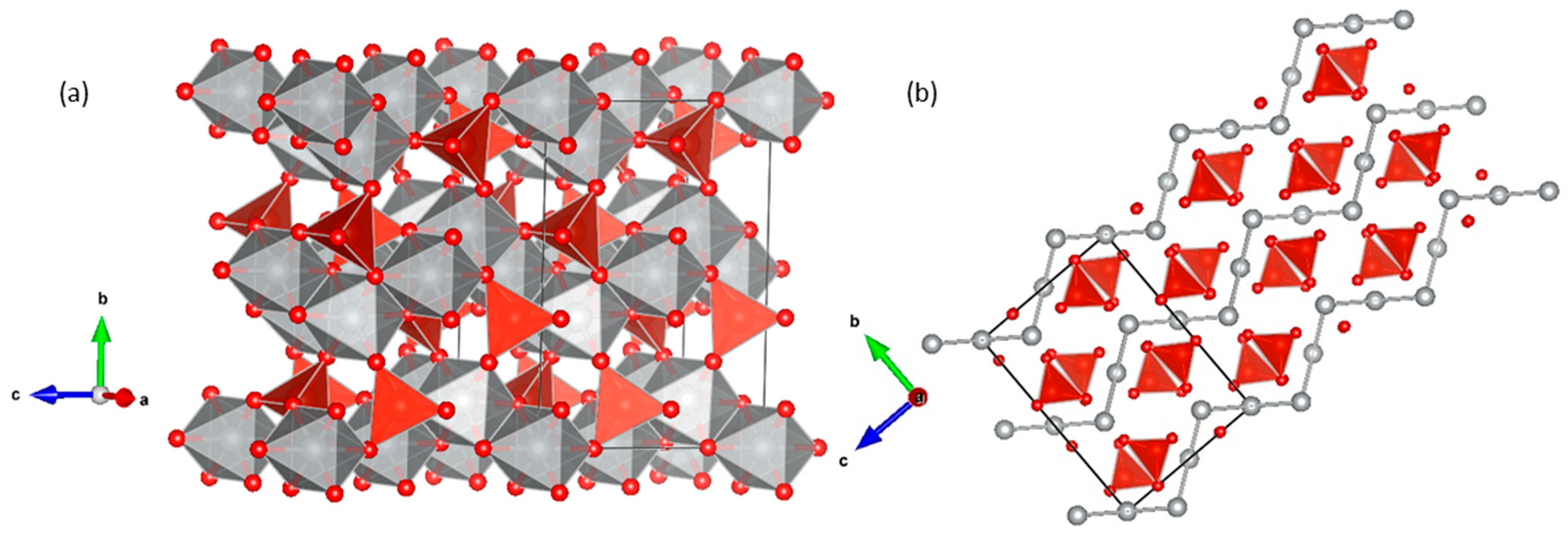
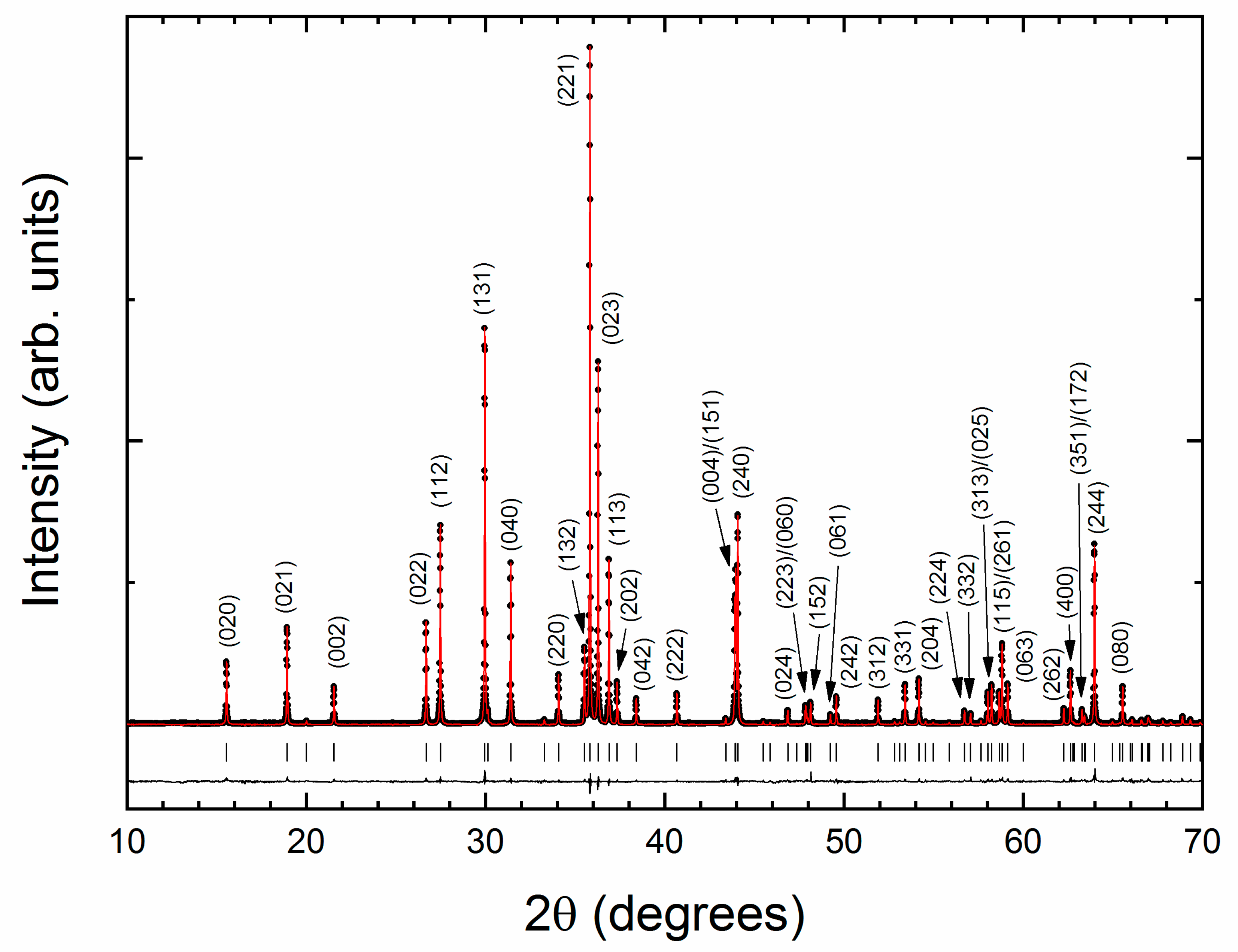
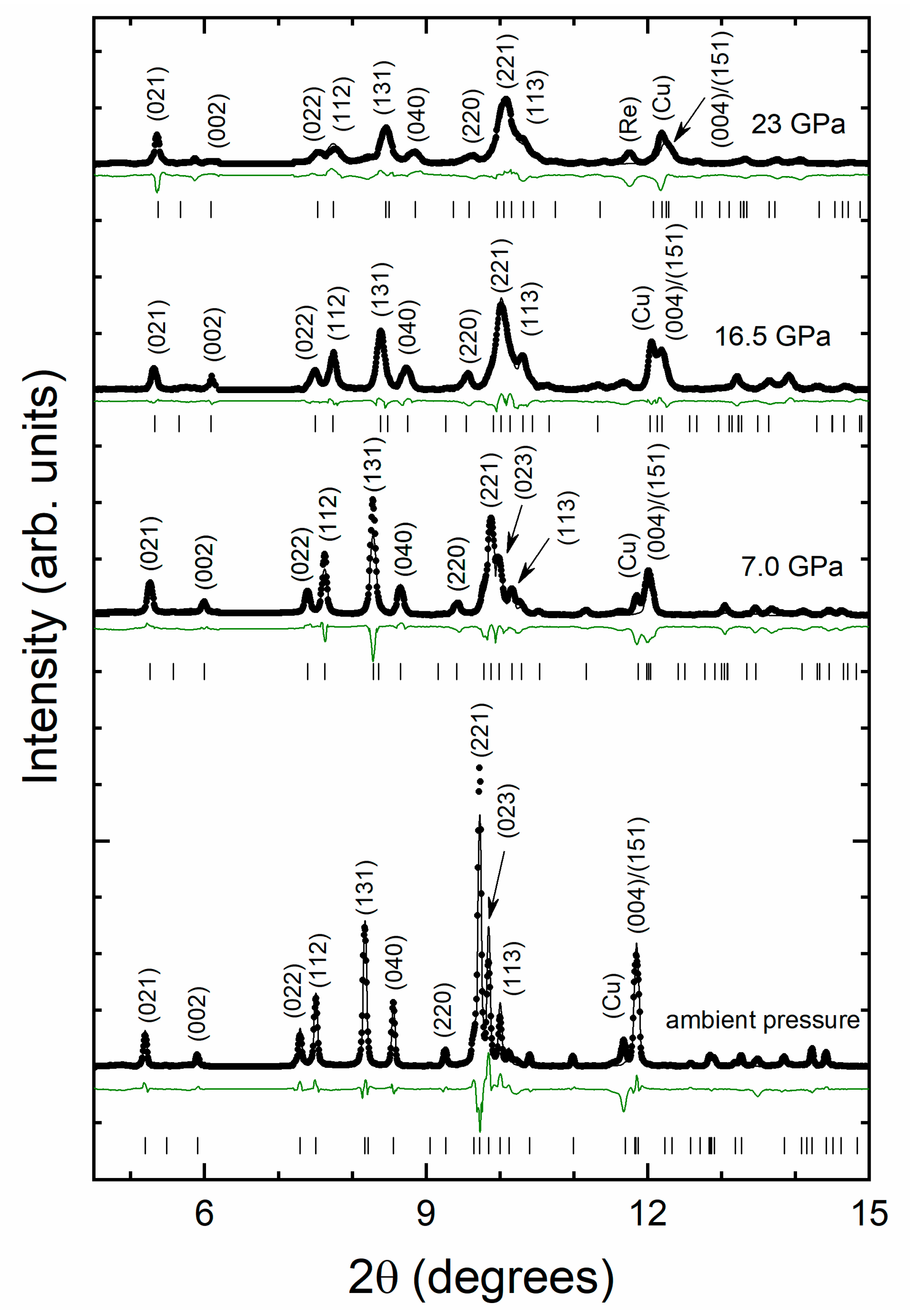
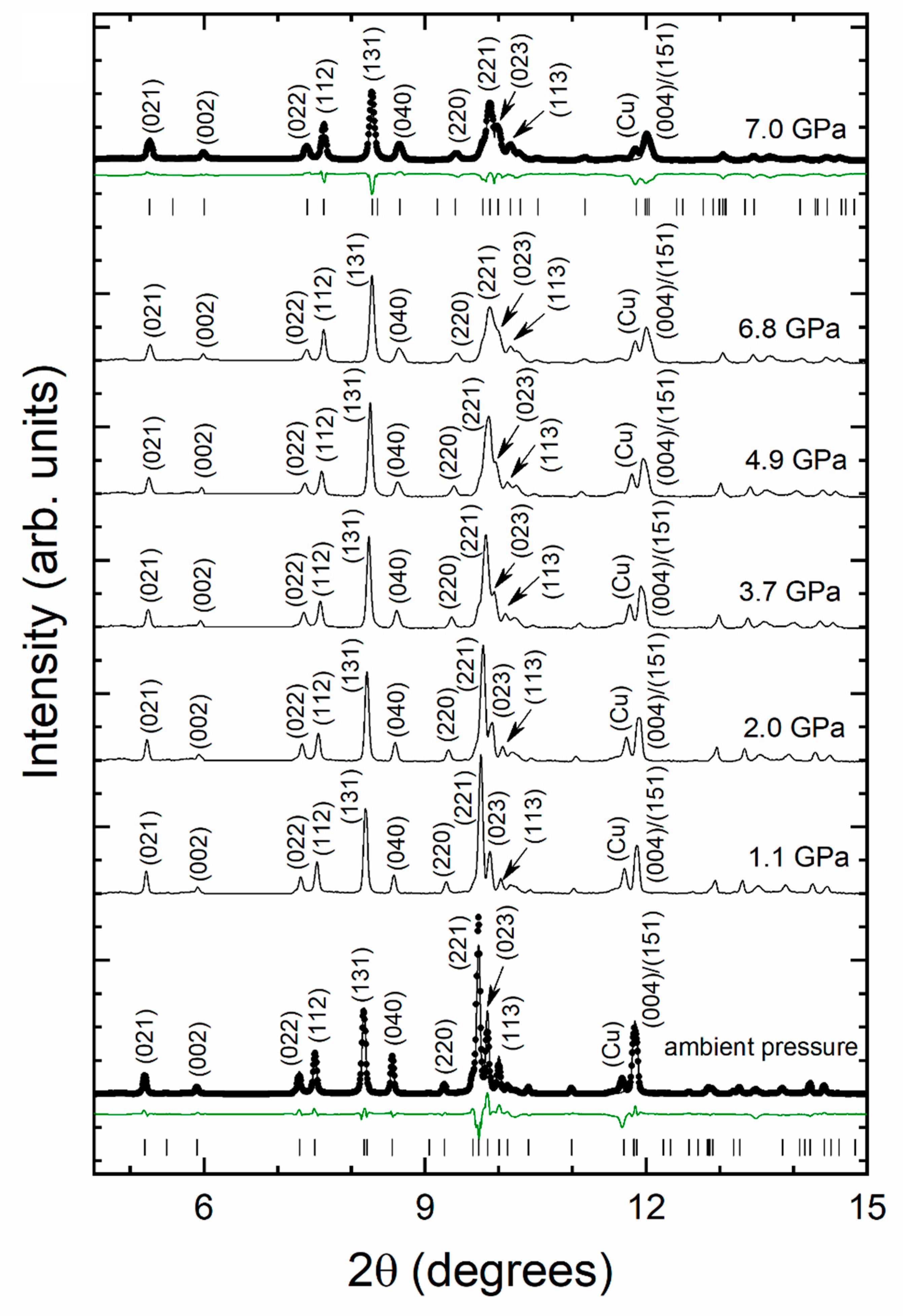
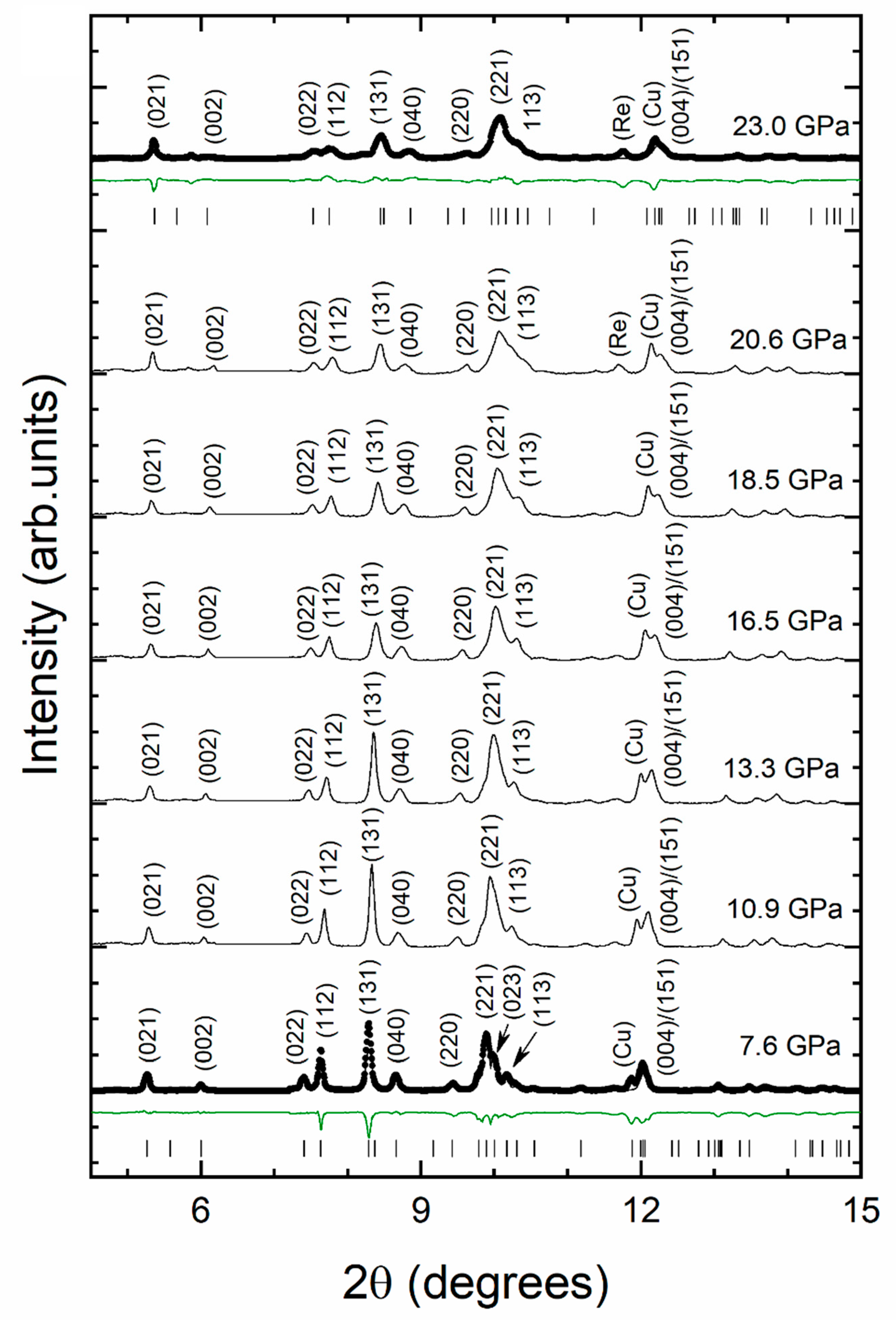
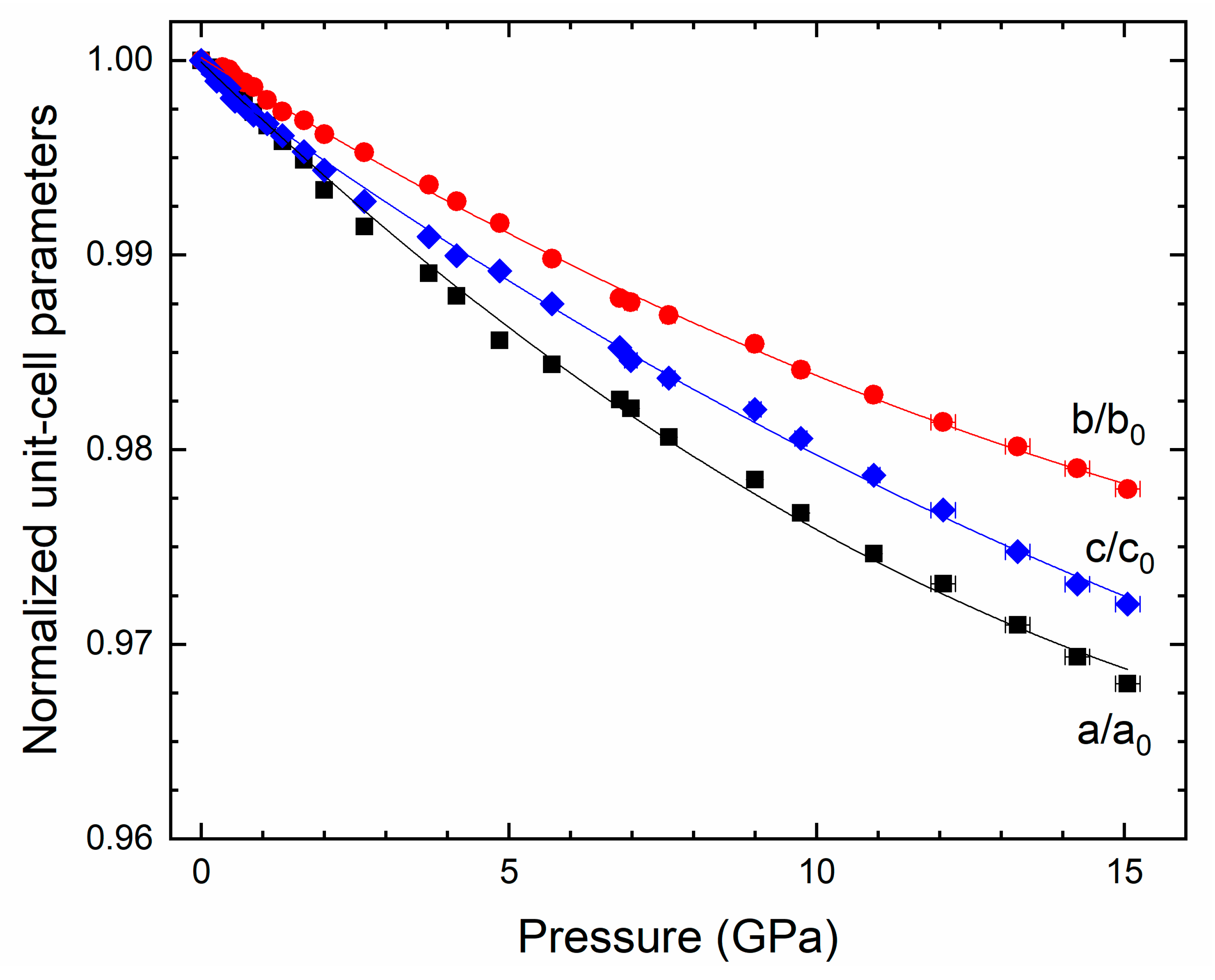
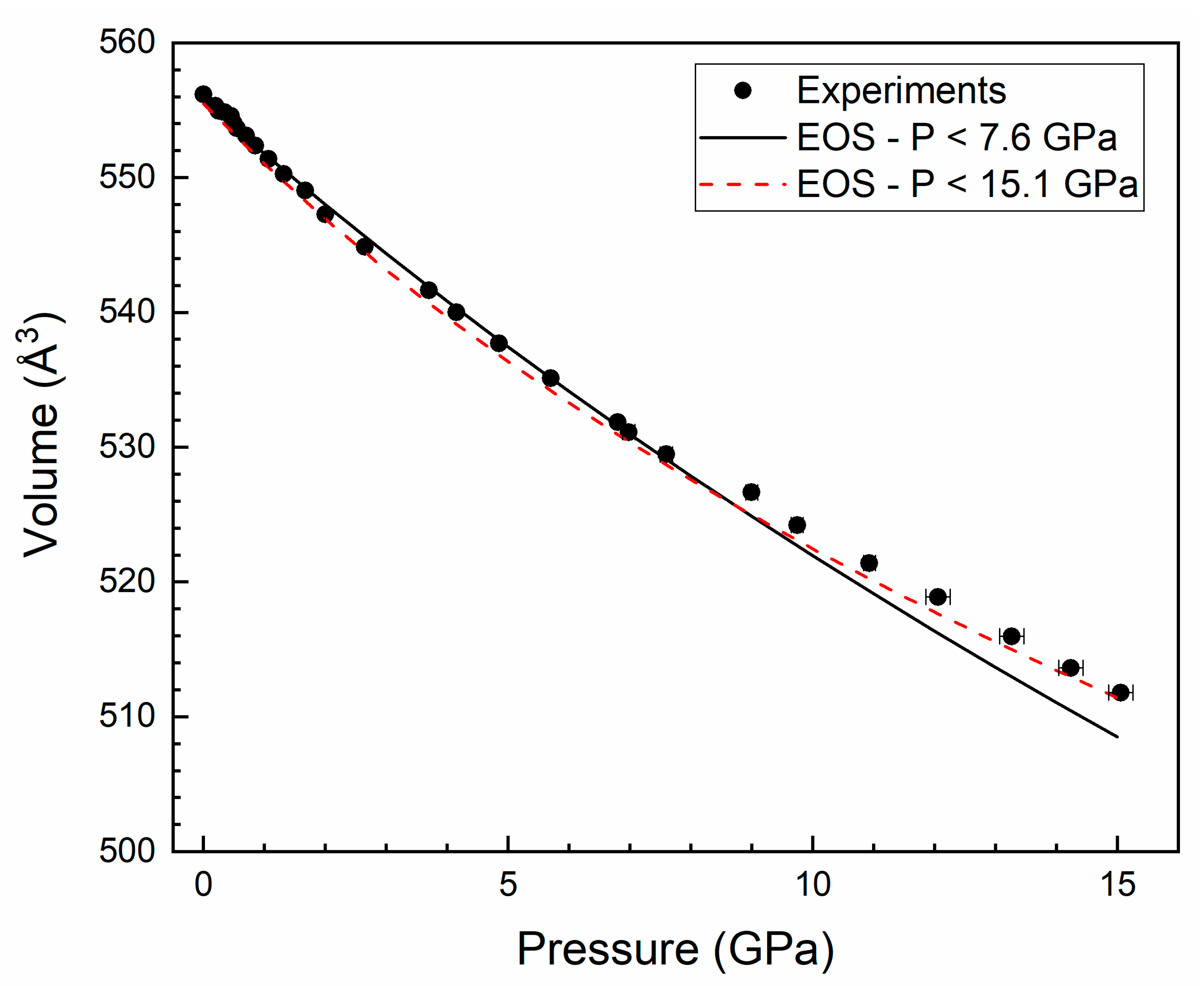
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Vijayakumar, S.; Lee, S.-H.; Ryu, K.-S. Synthesis of Zn3V2O8 nanoplatelets for lithium-ion battery and supercapacitor applications. RSC Adv. 2015, 5, 91822–91828. [Google Scholar] [CrossRef]
- Cabrera, I.; Kenzelmann, M.; Lawes, G.; Chen, Y.; Chen, W.; Erwin, R.; Gentile, T.R.; Leao, J.B.; Lynn, J.W.; Rogado, N.; et al. Coupled Magnetic and Ferroelectric Domains in Multiferroic Ni3V2O8. Phys. Rev. Lett. 2009, 103, 087201. [Google Scholar] [CrossRef] [Green Version]
- Bîrdeanu, M.-I.; Vaida, M.; Ursu, D.; Fagadar-Cosma, E. Obtaining and characterization of Zn3V2O8 and Mg3V2O8 pseudo binary oxide nanomaterials by hydrothermal method. In HIGH ENERGY GAMMA-RAY ASTRONOMY: 6th International Meeting on High Energy Gamma-Ray Astronomy; AIP Publishing LLC: Melville, NY, USA, 2017; p. 030006. [Google Scholar] [CrossRef] [Green Version]
- Mazloom, F.; Masjedi-Arani, M.; Salavati-Niasari, M. Novel size-controlled fabrication of pure Zn3V2O8 nanostructures via a simple precipitation approach. J. Mater. Sci. Mater. Electron. 2015, 27, 1974–1982. [Google Scholar] [CrossRef]
- Qian, T.; Fan, B.; Wang, H.; Zhu, S. Structure and luminescence properties of Zn3V2O8 yellow phosphor for white light emitting diodes. Chem. Phys. Lett. 2019, 715, 34–39. [Google Scholar] [CrossRef]
- Liu, Y.; Li, Q.; Ma, K.; Yang, G.; Wang, C.; Qian, L. Graphene Oxide Wrapped CuV2O6 Nanobelts as High-Capacity and Long-Life Cathode Materials of Aqueous Zinc-Ion Batteries. ACS Nano 2019, 13, 12081–12089. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Anichtchenko, D.; Santamaria-Perez, D.; Marqueño, T.; Pellicer-Porres, J.; Ruiz-Fuertes, J.; Ribes, R.; Ibañez, J.; Achary, S.N.; Popescu, C.; Errandonea, D. Comparative study of the high-pressure behavior of ZnV2O6, Zn2V2O7, and Zn3V2O8. J. Alloy. Compd. 2020, 837, 155505. [Google Scholar] [CrossRef]
- Rogado, N.; Lawes, G.; Huse, D.; Ramirez, A.; Cava, R. The Kagomé-staircase lattice: Magnetic ordering in Ni3V2O8 and Co3V2O8. Solid State Commun. 2002, 124, 229–233. [Google Scholar] [CrossRef] [Green Version]
- Chaudhury, R.P.; Yen, F.; Cruz, C.D.; Lorenz, B.; Wang, Y.Q.; Sun, Y.Y.; Chu, C.W. Pressure-temperature phase diagram of multiferroic Ni3V2O8. Phys. Rev. B 2007, 75, 012407. [Google Scholar] [CrossRef] [Green Version]
- Errandonea, D. High pressure crystal structures of orthovanadates and their properties. J. Appl. Phys. 2020, 128, 040903. [Google Scholar] [CrossRef]
- Errandonea, D.; Garg, A.B. Recent progress on the characterization of the high-pressure behaviour of AVO4 orthovanadates. Prog. Mater. Sci. 2018, 97, 123–169. [Google Scholar] [CrossRef] [Green Version]
- Bandiello, E.; Sánchez-Martín, J.; Errandonea, D.; Bettinelli, M. Pressure Effects on the Optical Properties of NdVO4. Crystals 2019, 9, 237. [Google Scholar] [CrossRef] [Green Version]
- Dewaele, A.; Loubeyre, P.; Mezouar, M. Equations of state of six metals above 94GPa. Phys. Rev. B 2004, 70, 094112. [Google Scholar] [CrossRef] [Green Version]
- Klotz, S.; Chervin, J.-C.; Munsch, P.; Le Marchand, G. Hydrostatic limits of 11 pressure transmitting media. J. Phys. D Appl. Phys. 2009, 42, 075413. [Google Scholar] [CrossRef]
- Errandonea, D.; Meng, Y.; Somayazulu, M.; Hausermann, D. Pressure-induced alpha-to-omega transition in titanium metal: A systematic study of the effects of uniaxial stress. Physica B 2005, 355, 116–125. [Google Scholar] [CrossRef] [Green Version]
- Prescher, C.; Prakapenka, V.B. DIOPTAS: A program for reduction of two-dimensional X-ray diffraction data and data exploration. High. Press. Res. 2015, 35, 223–230. [Google Scholar] [CrossRef]
- Sauerbrei, E.E.; Faggiani, R.; Calvo, C. Refinement of the crystal structure of Co3V2O8 and Ni3V2O8. Acta Crystallogr. Sect. B Struct. Crystallogr. Cryst. Chem. 1973, 29, 2304–2306. [Google Scholar] [CrossRef]
- Errandonea, D.; Muñoz, A.; Gonzalez-Platas, J. Comment on “High-pressure x-ray diffraction study of YBO3/Eu3+, GdBO3, and EuBO3: Pressure-induced amorphization in GdBO3”. J. Appl. Phys. 2014, 115, 216101. [Google Scholar] [CrossRef]
- Errandonea, D.; Ruiz-Fuertes, J. A Brief Review of the Effects of Pressure on Wolframite-Type Oxides. Crystals 2018, 8, 71. [Google Scholar] [CrossRef] [Green Version]
- Laverock, J.; Piper, L.F.J.; Preston, A.R.H.; Chen, B.; McNulty, J.; Smith, K.E.; Kittiwatanakul, S.; Lu, J.W.; Wolf, S.A.; Glans, P.-A.; et al. Strain dependence of bonding and hybridization across the metal-insulator transition of VO2. Phys. Rev. B 2012, 85, 081104. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Qiu, X.; Fang, D. Mechanical Properties of two novel planar lattice structures. Int. J. Solids Struct. 2008, 45, 3751–3768. [Google Scholar] [CrossRef] [Green Version]
- Turnbull, R.; Errandonea, D.; Cuenca-Gotor, V.P.; Sans, J.Á.; Gomis, O.; Gonzalez, A.; Rodríguez-Hernandez, P.; Popescu, C.; Bettinelli, M.; Mishra, K.K.; et al. Experimental and theoretical study of dense YBO3 and the influence of non-hydrostaticity. J. Alloy. Compd. 2021, 850, 156562. [Google Scholar] [CrossRef]
- Birch, F. Finite elastic strain of cubic crystals. Phys. Rev. 1947, 71, 809–824. [Google Scholar] [CrossRef]
- Gonzalez-Platas, J.; Alvaro, M.; Nestola, F.; Angel, R.J. EosFit7-GUI: A new GUI tool for equation of state calculations, analyses, and teaching. J. Appl. Crystallogr. 2016, 49, 1377–1382. [Google Scholar] [CrossRef]
- Koc, H.; Palaz, S.; Mamedov, A.M.; Ozbay, E. Electronic and elastic properties of the multiferroic crystals with the Kagome type lattices -Mn3V2O8 and Ni3V2O8: First principle calculations. Ferroelectrics 2019, 544, 11–19. [Google Scholar] [CrossRef] [Green Version]
- Jezierski, A.; Kaczkowski, J. Electronic structure and thermodynamic properties of Cu3V2O8 compound. Phase Transit. 2015, 88, 1–9. [Google Scholar] [CrossRef]
- Errandonea, D.; Manjón, F.J. Pressure effects on the structural and electronic properties of ABX4 scintillating crystals. Prog. Mater. Sci. 2008, 53, 711–773. [Google Scholar] [CrossRef]
- Errandonea, D.; Muñoz, A.; Rodríguez-Hernández, P.; Gomis, O.; Achary, S.N.; Popescu, C.; Patwe, S.J.; Tyagi, A.K. High-Pressure Crystal Structure, Lattice Vibrations, and Band Structure of BiSbO4. Inorg. Chem. 2016, 55, 4958–4969. [Google Scholar]
- Zhang, G.-X.; Reilly, A.M.; Tkatchenko, A.; Scheffler, M. Performance of various density-functional approximations for cohesive properties of 64 bulk solids. New J. Phys. 2018, 20, 063020. [Google Scholar] [CrossRef]
- Barbier, J.; Frampton, C. Structures of orthorhombic and monoclinic Ni3(AsO4)2. Acta Crystallogr. Sect. B Struct. Sci. 1991, 47, 457–462. [Google Scholar] [CrossRef]
- Nord, A.G.; Werner, P.-E. Cation distribution studies of three (Ni, Mg) orthovanadates. Zeitschrift für Kristallographie - Crystalline Materials 1991, 194, 49. [Google Scholar] [CrossRef]
- Wang, P.; Yang, H.; Wang, D.; Chen, Y.; Dai, W.L.; Zhao, X.; Yang, J.; Wang, X. Activation of kagome lattice-structured Cu3V2O7(OH)2·2H2O volborthite via hydrothermal crystallization for boosting visible light-driven water oxidation. Phys. Chem. Chem. Phys. 2018, 20, 24561–24569. [Google Scholar] [CrossRef] [PubMed]
| Atom | Wyckoff Position | x | y | z |
|---|---|---|---|---|
| Ni1 | 4a | 0 | 0 | 0 |
| Ni2 | 8e | 0.25 | 0.1319(4) | 0.25 |
| V | 8f | 0 | 0.3766(5) | 0.1191(5) |
| O1 | 8f | 0 | 0.2491(9) | 0.2302(9) |
| O2 | 8f | 0 | 0.0011(9) | 0.2445(9) |
| O3 | 16g | 0.2663(9) | 0.1193(9) | 0.0009(9) |
| a = 2.7(1) 10−3 GPa−1 | V0 = 555. 7(2) Å3 | V0 = 555.5(2) Å3 |
| b = 1.79(6) 10−3 GPa−1 | K0 = 139(3) GPa | K0 = 118(1) GPa |
| c = 2.33(5) 10−3 GPa−1 | K0′ = 4.4(3) | K0′ = 11.1(9) |
| Compound | V0 (Å3) | Experimental K0 (GPa) | Empirical K0 (GPa) | DFT K0 (GPa) |
|---|---|---|---|---|
| Ni3V2O8 | 555.5(3) | 139(3) a | 140 c | |
| Ni3V2O8 | 562.64 | 130.4–131.2 b | ||
| Mg3V2O8 | 576.92 | 132 c | ||
| Co3V2O8 | 578.96 | 131 c | ||
| Zn3V2O8 | 585.1(1) | 120(2) d | 128 c | |
| Cu3V2O8 | 586.95 | 127 c | 95–110 e | |
| Mn3V2O8 | 622.1 | 115 c | 89.7–93 b |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Diaz-Anichtchenko, D.; Turnbull, R.; Bandiello, E.; Anzellini, S.; Errandonea, D. High-Pressure Structural Behavior and Equation of State of Kagome Staircase Compound, Ni3V2O8. Crystals 2020, 10, 910. https://doi.org/10.3390/cryst10100910
Diaz-Anichtchenko D, Turnbull R, Bandiello E, Anzellini S, Errandonea D. High-Pressure Structural Behavior and Equation of State of Kagome Staircase Compound, Ni3V2O8. Crystals. 2020; 10(10):910. https://doi.org/10.3390/cryst10100910
Chicago/Turabian StyleDiaz-Anichtchenko, Daniel, Robin Turnbull, Enrico Bandiello, Simone Anzellini, and Daniel Errandonea. 2020. "High-Pressure Structural Behavior and Equation of State of Kagome Staircase Compound, Ni3V2O8" Crystals 10, no. 10: 910. https://doi.org/10.3390/cryst10100910





