Promotional Effect of Cerium and/or Zirconium Doping on Cu/ZSM-5 Catalysts for Selective Catalytic Reduction of NO by NH3
Abstract
:1. Introduction
2. Results and Discussion
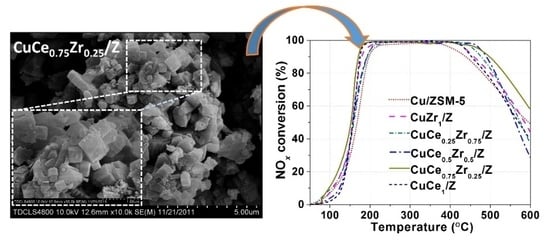
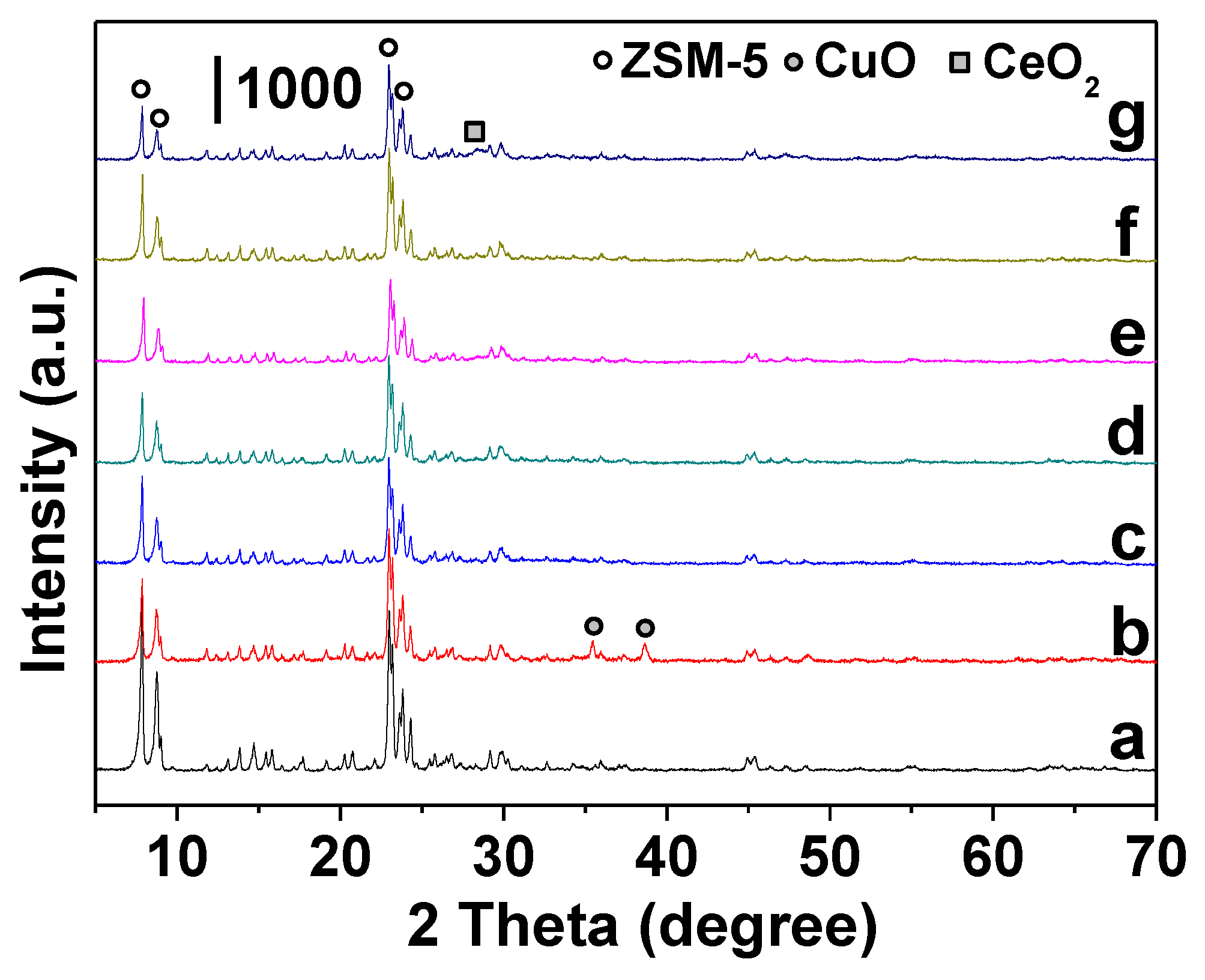
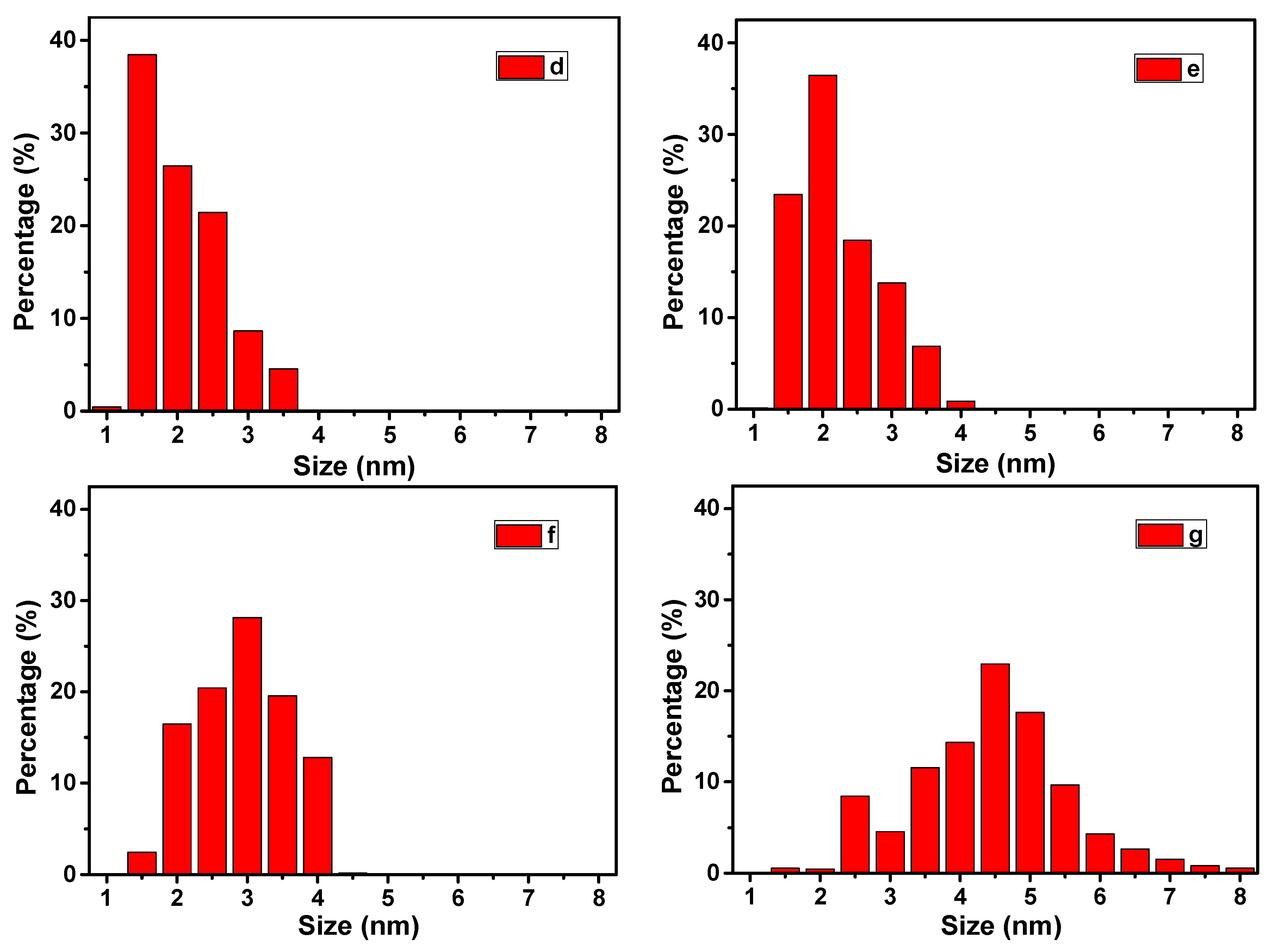
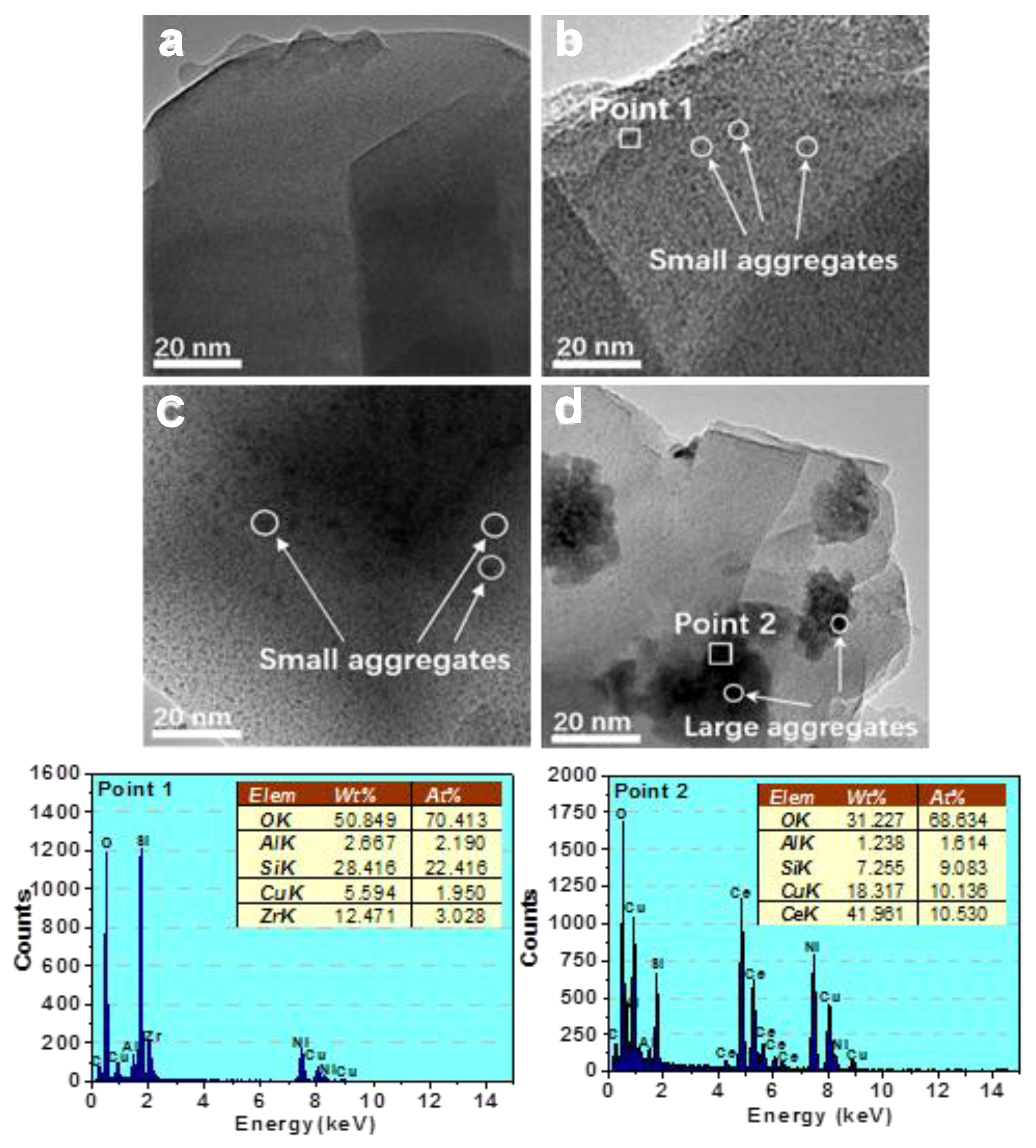
2.1. Structure and Morphology
2.2. XPS Analysis
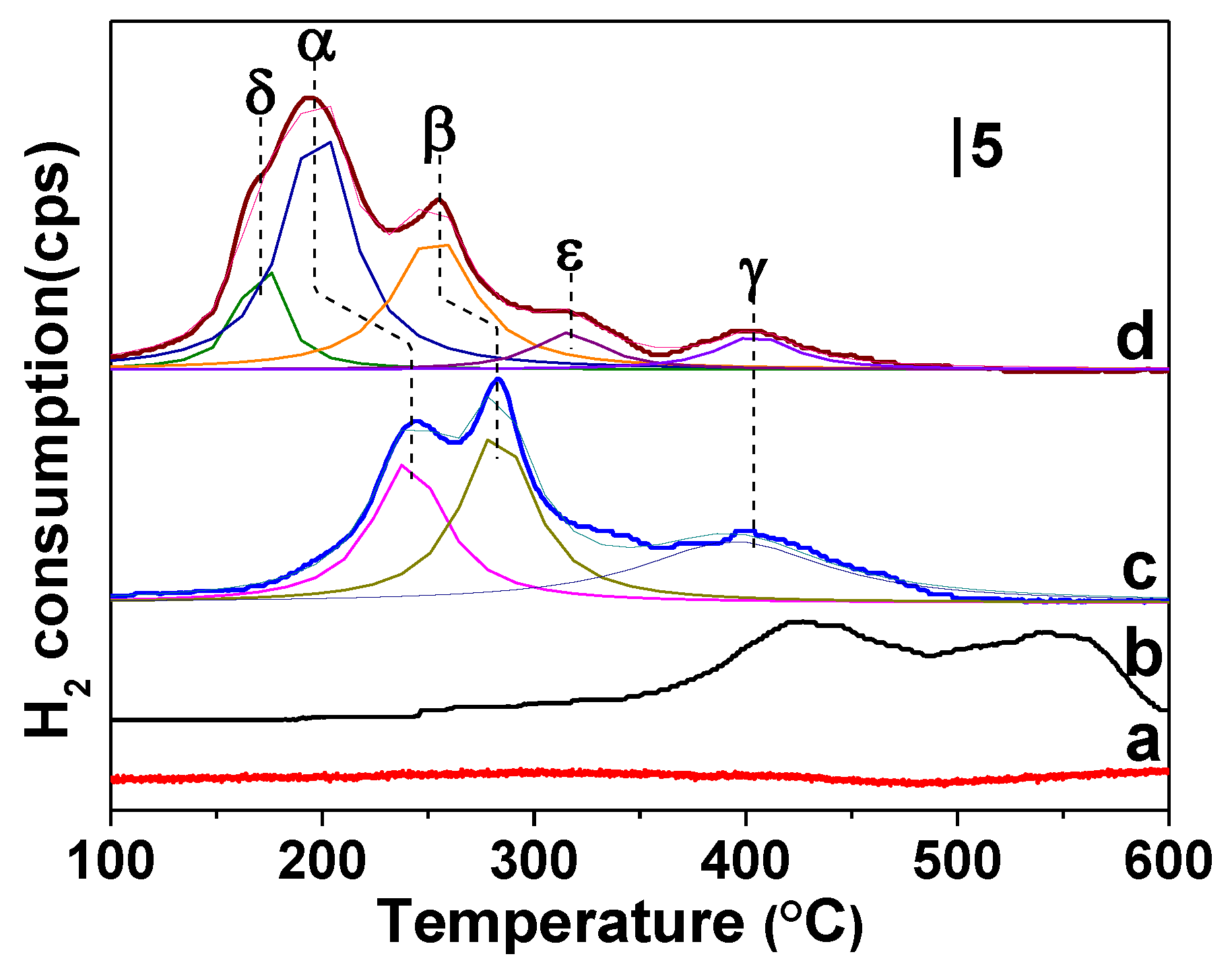
2.3. H2-TPR
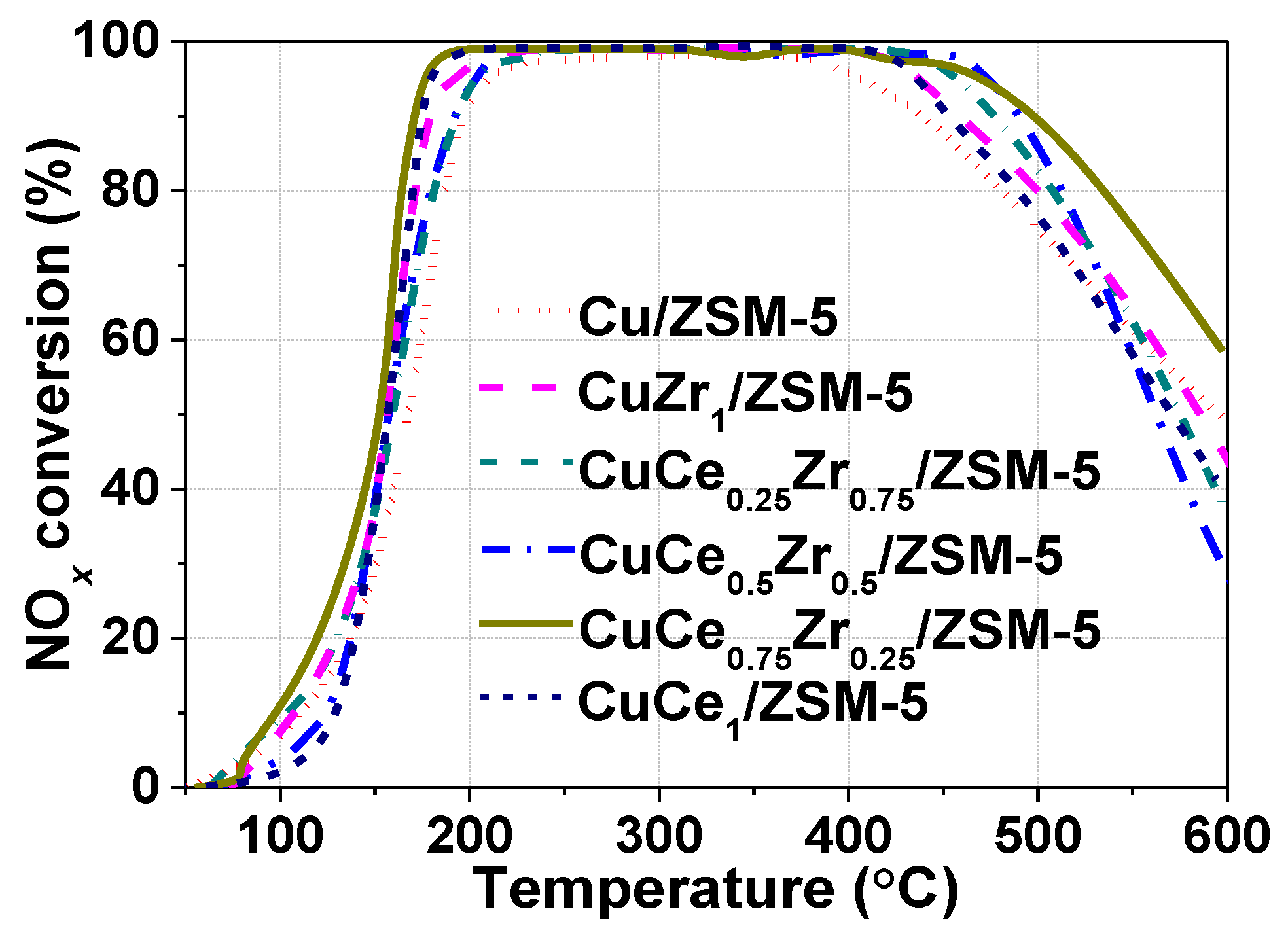
2.4. Catalytic Activity Test
- (1)
- The active copper species are well dispersed on the surface of the catalysts. The XRD and TEM results show that the introduction of zirconium promotes surface copper enrichment and prevents copper crystallization.
- (2)
- The addition of cerium results in high Cu+/Cu2+ ratios, as evidenced from the XPS results. In the SCR reaction, NO preferentially chemisorbs on Cu+, and the formation of intermediate Cu+–NO adsorbed sites is crucial for the activation of the NO molecule [26,27]. Thus, a high percentage of Cu+ species favors catalytic NO reduction.
- (3)
- Partial substitution of cerium and zirconium ions by copper ions contributes to the formation of copper/cerium or copper/zirconium composite oxides. As shown in the H2-TPR experiment, these composite oxides greatly increase the reactive lattice oxygen content and reducibility, and thus improve the low-temperature activity [11].
3. Experimental
3.1. Synthesis of Catalysts
3.2. Characterization
3.3. Catalytic Activity
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Landi, G.; Lisi, L.; Pirone, R.; Russo, G.; Tortorelli, M. Effect of water on NO adsorption over Cu-ZSM-5 based catalysts. Catal. Today 2012, 191, 138–141. [Google Scholar] [CrossRef]
- Ji, Y.; Yang, H.; Yan, W. Strategies to enhance the catalytic performance of ZSM-5 zeolite in hydrocarbon cracking: A review. Catalysts 2017, 7, 367. [Google Scholar] [CrossRef]
- Wang, T.; Wan, Z.; Yang, X.; Zhang, X.; Niu, X.; Sun, B. Promotional effect of iron modification on the catalytic properties of Mn-Fe/ZSM-5 catalysts in the Fast SCR reaction. Fuel Process. Technol. 2018, 169, 112–121. [Google Scholar] [CrossRef]
- Xu, W.J.; Zhang, G.X.; Chen, H.W.; Zhang, G.M.; Han, Y.; Chang, Y.C.; Gong, P. Mn/beta and Mn/ZSM-5 for the low-temperature selective catalytic reduction of NO with ammonia: Effect of manganese precursors. Chin. J. Catal. 2018, 39, 118–127. [Google Scholar] [CrossRef]
- De Lucas, A.; Valverde, J.L.; Dorado, F.; Romero, A.; Asencio, I. Influence of the ion exchanged metal (Cu, Co, Ni and Mn) on the selective catalytic reduction of NOx over mordenite and ZSM-5. J. Mol. Catal. 2005, 225, 47–58. [Google Scholar] [CrossRef]
- Ma, T.; Imai, H.; Yamawaki, M.; Terasaka, K.; Li, X. Selective synthesis of gasoline-ranged hydrocarbons from syngas over hybrid catalyst consisting of metal-loaded ZSM-5 coupled with copper-zinc oxide. Catalysts 2014, 4, 116–128. [Google Scholar] [CrossRef]
- Abu-Zied, B.M.; Schwieger, W.; Unger, A. Nitrous oxide decomposition over transition metal exchanged ZSM-5 zeolites prepared by the solid-state ion-exchange method. Appl. Catal. B 2008, 84, 277–288. [Google Scholar] [CrossRef]
- Chen, P.; Simböck, J.; Schönebaum, S.; Rauch, D.; Simons, T.; Palkovits, R.; Simon, U. Monitoring NH3 storage and conversion in Cu-ZSM-5 and Cu-SAPO-34 catalysts for NH3-SCR by simultaneous impedance and DRIFT spectroscopy. Sens. Actuators B 2016, 236, 1075–1082. [Google Scholar] [CrossRef]
- Jiang, L.; Zhu, H.; Razzaq, R.; Zhu, M.; Li, C.; Li, Z. Effect of zirconium addition on the structure and properties of CuO/CeO2 catalysts for high-temperature water-gas shift in an IGCC system. Int. J. Hydrogen Energy 2012, 37, 15914–15924. [Google Scholar] [CrossRef]
- Seo, C.K.; Choi, B.; Kim, H.; Lee, C.H.; Lee, C.B. Effect of ZrO2 addition on de-NOx performance of Cu-ZSM-5 for SCR catalyst. Chem. Eng. J. 2012, 191, 331–340. [Google Scholar] [CrossRef]
- Zhang, R.; Teoh, W.Y.; Amal, R.; Chen, B.; Kaliaguine, S. Catalytic reduction of NO by CO over Cu/CexZr1−xO2 prepared by flame synthesis. J. Catal. 2010, 272, 210–219. [Google Scholar] [CrossRef]
- Fu, M.; Yue, X.; Ye, D.; Ouyang, J.; Huang, B.; Wu, J.; Liang, H. Soot oxidation via CuO doped CeO2 catalysts prepared using coprecipitation and citrate acid complex-combustion synthesis. Catal. Today 2010, 153, 125–132. [Google Scholar] [CrossRef]
- Łamacz, A.; Krztoń, A.; Djéga-Mariadassou, G. Catalytic decomposition of nitrogen oxides from coal combustion flue gases on CeZrO2 supported Cu catalysts. Catal. Today 2011, 176, 126–130. [Google Scholar] [CrossRef]
- Si, R.; Zhang, Y.W.; Li, S.J.; Lin, B.X.; Yan, C.H. Urea-Based Hydrothermally derived homogeneous nanostructured Ce1-xZrxO2 (x = 0−0.8) solid solutions: A strong correlation between oxygen storage capacity and lattice strain. J. Phys. Chem. B 2004, 108, 12481–12488. [Google Scholar] [CrossRef]
- Qi, G.; Yang, R.T. Selective catalytic oxidation (SCO) of ammonia to nitrogen over Fe/ZSM-5 catalysts. Appl. Catal. A 2005, 287, 25–33. [Google Scholar] [CrossRef]
- Ohtsuka, H.; Tabata, T.; Okada, O.; Sabatino, L.M.; Bellussi, G. A study on selective reduction of NOx by propane on Co-Beta. Catal. Lett. 1997, 44, 265–270. [Google Scholar] [CrossRef]
- Oliveira, M.L.M.; Silva, C.M.; Moreno-Tost, R.; Farias, T.L.; Jiménez-López, A.; Rodríguez-Castellón, E. A study of copper-exchanged mordenite natural and ZSM-5 zeolites as SCR-NOx catalysts for diesel road vehicles: Simulation by neural networks approach. Appl. Catal. B 2009, 88, 420–429. [Google Scholar] [CrossRef]
- Jones, S.D.; Neal, L.M.; Everett, M.L.; Hoflund, G.B.; Hagelin-Weaver, H.E. Characterization of ZrO2-promoted Cu/ZnO/nano-Al2O3 methanol steam reforming catalysts. Appl. Surf. Sci. 2010, 256, 7345–7353. [Google Scholar] [CrossRef]
- Correa, C.M.; Castrillón, F.C. Supported bimetallic Pd-Co catalysts: characterization and catalytic activity. J. Mol. Catal. A Chem. 2005, 228, 267–273. [Google Scholar] [CrossRef]
- Wu, Z.; Jin, R.; Liu, Y.; Wang, H. Ceria modified MnOx/TiO2 as a superior catalyst for NO reduction with NH3 at low-temperature. Catal. Commun. 2008, 9, 2217–2220. [Google Scholar] [CrossRef]
- Hwang, I.C.; Kim, D.H.; Woo, S.I. Role of oxygen on NOx SCR catalyzed over Cu/ZSM-5 studied by FTIR, TPD, XPS and micropulse reaction. Catal. Today 1998, 44, 47–55. [Google Scholar] [CrossRef]
- Liu, L.; Yao, Z.; Liu, B.; Dong, L. Correlation of structural characteristics with catalytic performance of CuO/CexZr1−xO2 catalysts for NO reduction by CO. J. Catal. 2010, 275, 45–60. [Google Scholar] [CrossRef]
- Wang, L.C.; Liu, Q.; Chen, M.; Liu, Y.M.; Cao, Y.; He, H.Y.; Fan, K.N. Structural evolution and catalytic properties of nanostructured Cu/ZrO2 catalysts prepared by oxalate gel-coprecipitation technique. J. Phys. Chem. C 2007, 111, 16549–16557. [Google Scholar] [CrossRef]
- Nelson, A.E.; Schulz, K.H. Surface chemistry and microstructural analysis of CexZr1−xO2−y model catalyst surfaces. Appl. Surf. Sci. 2003, 210, 206–221. [Google Scholar] [CrossRef]
- Deo, G.; Wachs, I.E. Reactivity of supported vanadium oxide catalysts: The partial oxidation of methanol. J. Catal. 1994, 146, 323–334. [Google Scholar] [CrossRef]
- Ansell, G.; Diwell, A.; Golunski, S.; Hayes, J.; Rajaram, R.; Truex, T.; Walker, A. Mechanism of the lean NOx reaction over Cu/ZSM-5. Appl. Catal. B. 1993, 2, 81–100. [Google Scholar] [CrossRef]
- Curtin, T.; Grange, P.; Delmon, B. The effect of pretreatments on different copper exchanged ZSM-5 for the decomposition of NO. Catal. Today 1997, 36, 57–64. [Google Scholar] [CrossRef]
- Liu, Q.; Liu, Z.; Li, C. Adsorption and activation of NH3 during selective catalytic reduction of NO by NH3. Chin. J. Catal. 2006, 27, 636–646. [Google Scholar] [CrossRef]
- Koebel, M.; Elsener, M.; Madia, G. Reaction pathways in the selective catalytic reduction process with NO and NO2 at low temperatures. Ind. Eng. Chem. Res. 2001, 40, 52–59. [Google Scholar] [CrossRef]
- Sirdeshpande, A.R.; Lighty, J.S. Kinetics of the selective catalytic reduction of NO with NH3 over CuO/γ-Al2O3. Ind. Eng. Chem. Res. 2000, 39, 1781–1787. [Google Scholar] [CrossRef]


| Sample | Cu+/Cu2+ | Ce3+/Ce4+ | Cu/Si (Atomic) | Ce/Si (Atomic) | Zr/Si (Atomic) | |||
|---|---|---|---|---|---|---|---|---|
| AAS | XPS | AAS | XPS | AAS | XPS | |||
| CuZr1/ZSM-5 | 0.64 | / | 0.025 | 0.118 | 0 | 0 | 0.026 | 0.804 |
| CuCe0.25Zr0.75/ZSM-5 | 0.498 | 0.187 | 0.026 | 0.116 | 0.005 | 0.015 | 0.018 | 0.723 |
| CuCe0.5Zr0.5/ZSM-5 | 0.35 | 0.198 | 0.025 | 0.119 | 0.013 | 0.029 | 0.014 | 0.643 |
| CuCe0.75Zr0.25/ZSM-5 | 0.672 | 0.298 | 0.024 | 0.117 | 0.020 | 0.034 | 0.007 | 0.312 |
| CuCe1/ZSM-5 | 0.71 | 0.247 | 0.026 | 0.116 | 0.025 | 0.066 | 0 | 0 |
| Sample | δ Peak | α Peak | β Peak | ε Peak | γ Peak | Total H2 Consumption (μmol/gcat) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| T (°C) | H2 Consumption (μmol/gcat) | T (°C) | H2 Consumption (μmol/gcat) | T (°C) | H2 Consumption (μmol/gcat) | T (°C) | H2 Consumption (μmol/gcat) | T (°C) | H2 Consumption (μmol/gcat) | ||
| Cu/ZSM-5 | / | / | 236 | 56.4 | 278 | 68.23 | / | / | 393 | 63.96 | 188.59 |
| CuZr1/ZSM-5 | 171 | 15.49 | 198 | 45.06 | 261 | 76.22 | 342 | 25.12 | 398 | 28.53 | 190.42 |
| CuCe0.25Zr0.75/ZSM-5 | 175 | 16.92 | 209 | 66.36 | 259 | 68.49 | 332 | 17.51 | 407 | 23.54 | 192.82 |
| CuCe0.5Zr0.5/ZSM-5 | 178 | 22.49 | 213 | 91.39 | 263 | 61.40 | 338 | 3.24 | 399 | 18.66 | 197.18 |
| CuCe0.75Z0.25/ZSM-5 | 170 | 24.97 | 192 | 91.07 | 255 | 58.10 | 315 | 14.16 | 402 | 14.09 | 202.39 |
| CuCe1/ZSM-5 | 164 | 18.55 | 182 | 67.15 | 260 | 82.83 | 322 | 10.37 | 405 | 23.33 | 202.23 |
| Sample | Element Composition Measured by AAS (wt. %) | |||
|---|---|---|---|---|
| Cu | Ce | Zr | Si | |
| CuZr1/ZSM-5 | 3.0 | 0 | 4.4 | 52.4 |
| CuCe0.25Zr0.75/ZSM-5 | 3.1 | 1.3 | 3.1 | 52.2 |
| CuCe0.5Zr0.5/ZSM-5 | 2.9 | 3.4 | 2.3 | 52.3 |
| CuCe0.75Zr0.25/ZSM-5 | 2.8 | 5.2 | 1.2 | 52.4 |
| CuCe1/ZSM-5 | 3.1 | 6.5 | 0 | 52.2 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Song, C.; Lv, G.; Fan, C.; Li, X. Promotional Effect of Cerium and/or Zirconium Doping on Cu/ZSM-5 Catalysts for Selective Catalytic Reduction of NO by NH3. Catalysts 2018, 8, 306. https://doi.org/10.3390/catal8080306
Liu Y, Song C, Lv G, Fan C, Li X. Promotional Effect of Cerium and/or Zirconium Doping on Cu/ZSM-5 Catalysts for Selective Catalytic Reduction of NO by NH3. Catalysts. 2018; 8(8):306. https://doi.org/10.3390/catal8080306
Chicago/Turabian StyleLiu, Ye, Chonglin Song, Gang Lv, Chenyang Fan, and Xiaodong Li. 2018. "Promotional Effect of Cerium and/or Zirconium Doping on Cu/ZSM-5 Catalysts for Selective Catalytic Reduction of NO by NH3" Catalysts 8, no. 8: 306. https://doi.org/10.3390/catal8080306