Adsorption and Catalytic Decomposition Mechanism of C6F12O on Cu Surfaces: A Density Functional Theory Study
Abstract
1. Introduction
2. Results
2.1. Adsorption
2.1.1. Adsorption of C6F12O and Products on Cu (1 0 0) Surface
2.1.2. Adsorption of C6F12O and Products on Cu (1 1 0) Surface
2.1.3. Adsorption of C6F12O and Products on Cu (1 1 1) Surface
2.2. Co-Adsorption
2.3. Electronic Properties of C6F12O on Cu (1 0 0), Cu (1 1 0), and Cu (1 1 1) Surfaces
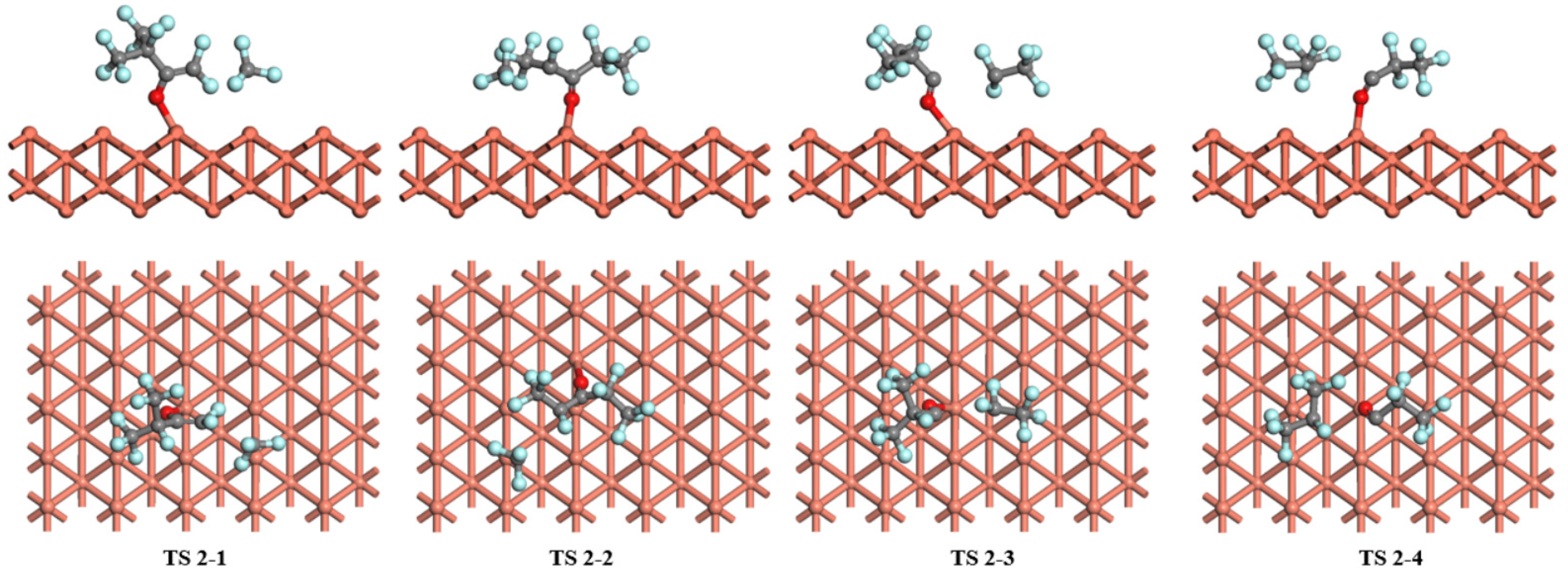
2.4. Decomposition of C6F12O on Cu (1 0 0), Cu (1 1 0), and Cu (1 1 1) Surfaces
3. Methods
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xing, H.; Lu, S.; Yang, H.; Zhang, H. Review on Research Progress of C6F12O as a Fire Extinguishing Agent. Fire 2022, 5, 50. [Google Scholar] [CrossRef]
- Krebs, R.; Owens, J.; Luckarift, H. Formation and detection of hydrogen fluoride gas during fire fighting scenarios. Fire Saf. J. 2022, 127, 103489. [Google Scholar] [CrossRef]
- Al-Sayyab, A.K.S.; Navarro-Esbri, J.; Mota-Babiloni, A. Energy, exergy, and environmental (3E) analysis of a compound ejector-heat pump with low GWP refrigerants for simultaneous data center cooling and district heating. Int. J. Refrig. 2022, 133, 61–72. [Google Scholar] [CrossRef]
- Cui, H.; Yan, C.; Jia, P.F.; Cao, W. Adsorption and sensing behaviors of SF(6)decomposed species on Ni-doped C3N monolayer: A first-principles study. Appl. Surf. Sci. 2020, 512. [Google Scholar] [CrossRef]
- Abas, N.; Kalair, A.R.; Khan, N.; Haider, A.; Saleem, Z.; Saleem, M.S. Natural and synthetic refrigerants, global warming: A review. Renew. Sustain. Energy Rev. 2018, 90, 557–569. [Google Scholar] [CrossRef]
- Kieffel, Y.; Irwin, T.; Ponchon, P.; Owens, J. Green Gas to Replace SF6 in Electrical Grids. IEEE Power Energy Mag. 2016, 14, 32–39. [Google Scholar] [CrossRef]
- Andersen, S.O.; Sherman, N.J.; Carvalho, S.; Gonzalez, M. The global search and commercialization of alternatives and substitutes for ozone-depleting substances. Comptes Rendus Geosci. 2018, 350, 410–424. [Google Scholar] [CrossRef]
- Burkholder, J.B.; Cox, R.A.; Ravishankara, A.R. Atmospheric degradation of ozone depleting substances, their substitutes, and related species. Chem. Rev. 2015, 115, 3704–3759. [Google Scholar] [CrossRef]
- Kyoto Protocol. UNFCCC Website. 1997, pp. 230–240. Available online: http://unfccc.int/kyoto_protocol/items/2830.php (accessed on 1 January 2011).
- Heath, E.A. Amendment to the Montreal protocol on substances that deplete the ozone layer (Kigali amendment). Int. Leg. Mater. 2017, 56, 193–205. [Google Scholar] [CrossRef]
- Rabie, M.; Franck, C.M. Assessment of Eco-friendly Gases for Electrical Insulation to Replace the Most Potent Industrial Greenhouse Gas SF6. Environ. Sci. Technol. 2018, 52, 369–380. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, X.; Tian, S.; Xiao, S.; Li, Y.; Chen, D. Insight into the decomposition mechanism of C6F12O-CO2 gas mixture. Chem. Eng. J. 2019, 360, 929–940. [Google Scholar] [CrossRef]
- Zhang, X.; Lan, J.; Tian, S.; Rao, X.; Li, X.; Yuan, Z.; Jin, X.; Gao, S.; Zhang, X. Study of compatibility between eco-friendly insulating medium C6F12O and sealing material EPDM. J. Mol. Struct. 2021, 1244, 130949. [Google Scholar] [CrossRef]
- Sikora, M.; Bohdal, T. Heat and flow investigation of NOVEC649 refrigerant condensation in pipe minichannels. Energy 2020, 209, 118447. [Google Scholar] [CrossRef]
- Fu, B.-R.; Lin, W.-J. Supercritical heat transfer of NOVEC 649 refrigerant in horizontal minichannels. Int. Commun. Heat Mass Transf. 2020, 117, 104740. [Google Scholar] [CrossRef]
- Xing, H.; Cheng, Y.; Lu, S.; Tao, N.; Zhang, H. A reactive molecular dynamics study of the pyrolysis mechanism of C6F12O. Mol. Phys. 2021, 119, e1976425. [Google Scholar] [CrossRef]
- Zeng, F.P.; Lei, Z.C.; Miao, Y.L.; Yao, Q.; Tang, J. Reaction Thermodynamics of Overthermal Decomposition of C6F12O. In Proceedings of the 21st International Symposium on High Voltage Engineering, Budapest, Hungary, 26–30 August 2019; Németh, B., Ed.; Springer International Publishing: Cham, Switzerland, 2020; pp. 43–51. [Google Scholar]
- Jackson, D.A.; Young, C.J.; Hurley, M.D.; Wallington, T.J.; Mabury, S.A. Atmospheric degradation of perfluoro-2-methyl-3-pentanone: Photolysis, hydrolysis and hydration. Environ. Sci. Technol. 2011, 45, 8030–8036. [Google Scholar] [CrossRef]
- Diaz-de-Mera, Y.; Aranda, A.; Notario, A.; Rodriguez, A.; Rodriguez, D.; Bravo, I. Photolysis study of fluorinated ketones under natural sunlight conditions. Phys. Chem. Chem. Phys. 2015, 17, 22991–22998. [Google Scholar] [CrossRef]
- Zhang, X.X.; Tian, S.S.; Xiao, S.; Deng, Z.T.; Li, Y.; Tang, J. Insulation Strength and Decomposition Characteristics of a C6F12O and N-2 Gas Mixture. Energies 2017, 10, 1170. [Google Scholar] [CrossRef]
- Xing, H.; Lu, S.; Tao, J.; Zhou, Y.; Zhao, J.; Zhang, H. Insight into C6F12O fire suppression mechanism on coaxial n-heptane flame: Combined experimental and ReaxFF molecular dynamics simulation. Process Saf. Environ. Prot. 2025, 200, 107383. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, X.; Tian, S.; Xiao, S.; Chen, Q.; Chen, D.; Cui, Z.; Tang, J. Insight Into the Compatibility Between C6F12O and Metal Materials: Experiment and Theory. IEEE Access 2018, 6, 58154–58160. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, X.; Chen, D.; Li, Y.; Zhang, J.; Cui, Z.; Xiao, S.; Tang, J. Theoretical study on the interaction between C5-PFK and Al (1 1 1), Ag (1 1 1): A comparative study. Appl. Surf. Sci. 2019, 464, 586–596. [Google Scholar] [CrossRef]
- Zhang, B.; Zhang, Z.; Xiong, J.; Yang, T.; Li, X.; Chen, L.; Li, C.; Deng, Y. Thermal and electrical decomposition products of C5F10O and their compatibility with Cu (1 1 1) and Al (1 1 1) surfaces. Appl. Surf. Sci. 2020, 513, 145882. [Google Scholar] [CrossRef]
- Cui, Z.; Zhang, X.; Li, Y.; Chen, D. Adsorption and decomposition of SF6 molecule on α-Al2O3 (0 0 0 1) surface: A DFT study. Adsorption 2019, 25, 1625–1632. [Google Scholar] [CrossRef]
- Huo, E.; Liu, C.; Xu, X.; Li, Q.; Dang, C. Dissociation mechanisms of HFO-1336mzz(Z) on Cu(1 1 1), Cu(1 1 0) and Cu(1 0 0) surfaces: A density functional theory study. Appl. Surf. Sci. 2018, 443, 389–400. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, Y.; Li, Y.; Li, Y.; Ye, F.; Tian, S.; Chen, D.; Xiao, S.; Tang, J. Thermal compatibility properties of C6F12O-air gas mixture with metal materials. AIP Adv. 2019, 9, 125024. [Google Scholar] [CrossRef]
- Jiang, Z.; Qin, P.; Fang, T. Decomposition mechanism of formic acid on Cu (111) surface: A theoretical study. Appl. Surf. Sci. 2017, 396, 857–864. [Google Scholar] [CrossRef]
- Zhang, M.; Yao, R.; Jiang, H.; Li, G.; Chen, Y. Insights into the mechanism of acetic acid hydrogenation to ethanol on Cu(111) surface. Appl. Surf. Sci. 2017, 412, 342–349. [Google Scholar] [CrossRef]
- Wu, Z.; Zhang, M.; Jiang, H.; Zhong, C.-J.; Chen, Y.; Wang, L. Competitive C–C and C–H bond scission in the ethanol oxidation reaction on Cu(100) and the effect of an alkaline environment. Phys. Chem. Chem. Phys. 2017, 19, 15444–15453. [Google Scholar] [CrossRef]
- Wu, H.; Xia, Y.; Zhang, C.; Xie, S.; Wu, S.; Cui, H. Adsorptions of C5F10O decomposed compounds on the Cu-decorated NiS2 monolayer: A first-principles theory. Mol. Phys. 2023, 121, e2163715. [Google Scholar] [CrossRef]
- Peng, G.; Rao, X.; Li, D.; Liu, B.; Wu, Y. Theoretical study on the compatibility of C4F7N decomposition products with metal oxides: First-principles. J. Fluor. Chem. 2025, 283–284, 110417. [Google Scholar] [CrossRef]
- Jiang, Z.; Qin, P.; Fang, T. Mechanism of ammonia decomposition on clean and oxygen-covered Cu (111) surface: A DFT study. Chem. Phys. 2014, 445, 59–67. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [PubMed]
- Delley, B. From molecules to solids with the DMol3 approach. J. Chem. Phys. 2000, 113, 7756–7764. [Google Scholar] [CrossRef]
- Delley, B. An all—electron numerical method for solving the local density functional for polyatomic molecules. J. Chem. Phys. 1990, 92, 508–517. [Google Scholar] [CrossRef]
- Delley, B. Hardness conserving semilocal pseudopotentials. Phys. Rev. B 2002, 66, 155125. [Google Scholar] [CrossRef]
- Grimme, S. Semiempirical GGA-type density functional constructed with a long-range dispersion correction. J. Comput. Chem. 2006, 27, 1787–1799. [Google Scholar] [CrossRef]
- McNellis, E.R.; Meyer, J.; Reuter, K. Azobenzene at coinage metal surfaces: Role of dispersive van der Waals interactions. Phys. Rev. B 2009, 80, 205414. [Google Scholar] [CrossRef]
- Monkhorst, H.J.; Pack, J.D. Special points for Brillouin-zone integrations. Phys. Rev. B 1976, 13, 5188–5192. [Google Scholar] [CrossRef]
- Rumble, J. (Ed.) CRC Handbook of Chemistry and Physics, 102nd ed.; CRC Press: Boca Raton, FL, USA, 2021. [Google Scholar]
- Halgren, T.A.; Lipscomb, W.N. The synchronous-transit method for determining reaction pathways and locating molecular transition states. Chem. Phys. Lett. 1977, 49, 225–232. [Google Scholar] [CrossRef]
- Xu, H.; Miao, B.; Zhang, M.; Chen, Y.; Wang, L. Mechanism of C–C and C–H bond cleavage in ethanol oxidation reaction on Cu2O(111): A DFT-D and DFT+U study. Phys. Chem. Chem. Phys. 2017, 19, 26210–26220. [Google Scholar] [CrossRef]




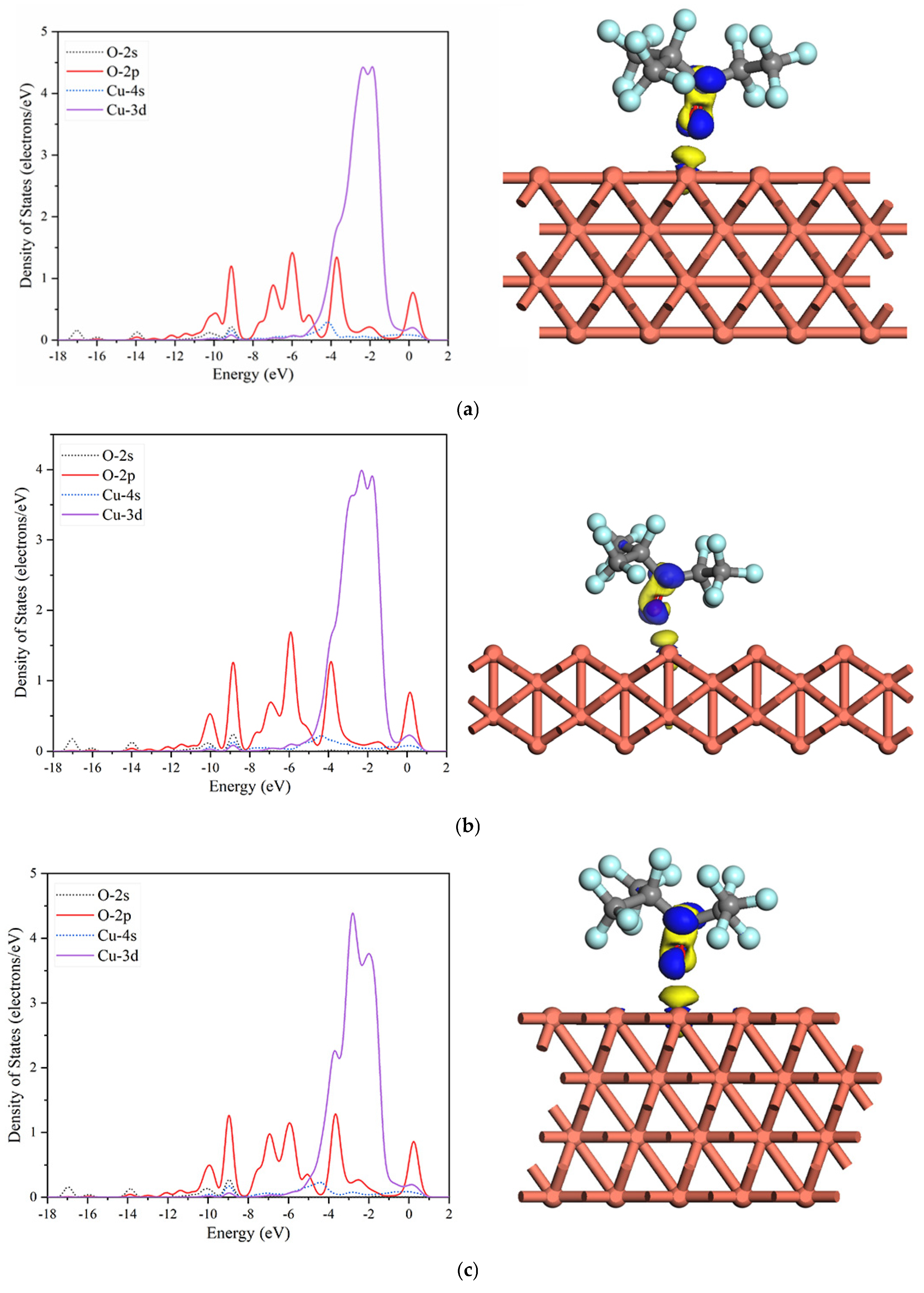



| Specie | Adsorption Site | Configuration | Bond Length/Å | Eads/eV |
|---|---|---|---|---|
| C6F12O | Top | O-bound | 2.005 (O-Cu) | 1.02 |
| C5F9O | Top | C-bound, O-bound | 2.040 (C-Cu), 2.102 (O-Cu) | 2.07 |
| C5F9O-(2) | Top | C-bound, O-bound | 2.093 (C-Cu), 2.132 (O-Cu) | 2.55 |
| C4F7O | Hole | C=O-bound | 2.030 (C-Cu1), 2.022 (C-Cu2), 2.131 (O-Cu3), 2.111 (O-Cu4) | 2.62 |
| C3F5O | Hole | C=O-bound | 2.020 (C-Cu1), 2.026 (C-Cu2), 2.123 (O-Cu3), 2.109 (O-Cu4) | 2.52 |
| C3F7 | Top | C-bound | 2.051 (C-Cu) | 2.38 |
| C2F5 | Top | C-bound | 1.996 (C-Cu) | 2.23 |
| CF3 | Top | C-bound | 1.977 (C-Cu) | 3.19 |
| Specie | Adsorption Site | Configuration | Bond Length/Å | Eads/eV |
|---|---|---|---|---|
| C6F12O | Top | O-bound | 1.967 (O-Cu) | 1.21 |
| C5F9O | Top | C-bound, O-bound | 2.012 (C-Cu), 2.072 (O-Cu) | 2.26 |
| C5F9O-(2) | Top | C-bound, O-bound | 2.068 (C-Cu), 2.073 (O-Cu) | 2.76 |
| C4F7O | Long Bridge | C=O-bound | 1.919 (C-Cu), 2.060 (O-Cu) | 2.81 |
| C3F5O | Long Bridge | C=O-bound | 1.925 (C-Cu), 2.067 (O-Cu) | 2.67 |
| C3F7 | Top | C-bound | 2.032 (C-Cu) | 2.59 |
| C2F5 | Top | C-bound | 1.979 (C-Cu) | 2.35 |
| CF3 | Top | C-bound | 1.967 (C-Cu) | 3.32 |
| Specie | Adsorption Site | Configuration | Bond Length/Å | Eads/eV |
|---|---|---|---|---|
| C6F12O | Top | O-bound | 2.090 (O-Cu) | 0.93 |
| C5F9O | Top | C-bound, O-bound | 2.045 (C-Cu), 2.136 (O-Cu) | 1.77 |
| C5F9O-(2) | Top | C-bound, O-bound | 2.090 (C-Cu), 2.129 (O-Cu) | 2.23 |
| C4F7O | Bridge | C=O-bound | 1.919 (C-Cu), 2.191 (O-Cu) | 2.35 |
| C3F5O | Bridge | C=O-bound | 1.925 (C-Cu), 2.181 (O-Cu) | 2.21 |
| C3F7 | Top | C-bound | 2.080 (C-Cu) | 2.15 |
| C2F5 | Top | C-bound | 2.013 (C-Cu) | 2.06 |
| CF3 | Top | C-bound | 1.993 (C-Cu) | 3.06 |
| Surface | Species | Adsorption Site | Eco-ads/eV | Esum/eV |
|---|---|---|---|---|
| Cu (1 0 0) | C5F9O + CF3 | Top + Top | 5.17 | 5.26 |
| C5F9O-(2) + CF3 | Top, Bridge + Top | 5.65 | 5.74 | |
| C4F7O + C2F5 | Hole + Top | 4.84 | 4.85 | |
| C3F5O + C3F7 | Hole + Top | 4.84 | 4.90 | |
| Cu (1 1 0) | C5F9O + CF3 | Top + Top | 5.47 | 5.58 |
| C5F9O-(2) + CF3 | Top + Top | 5.96 | 6.08 | |
| C4F7O + C2F5 | Long Bridge + Top | 5.14 | 5.16 | |
| C3F5O + C3F7 | Long Bridge + Top | 5.25 | 5.26 | |
| Cu (1 1 1) | C5F9O + CF3 | Top + Top | 4.81 | 4.83 |
| C5F9O-(2) + CF3 | Top + Top | 4.89 | 5.29 | |
| C4F7O + C2F5 | Bridge + Top | 4.38 | 4.41 | |
| C3F5O + C3F7 | Bridge + Top | 4.39 | 4.36 |
| Surface | Reactions | TS | Eb/eV | /eV |
|---|---|---|---|---|
| Cu (1 0 0) | Pathway 1 | TS 1-1 | 2.25 | 0.03 |
| Pathway 2 | TS 1-2 | 2.49 | −0.42 | |
| Pathway 3 | TS 1-3 | 3.56 | −0.50 | |
| Pathway 4 | TS 1-4 | 3.70 | −0.59 | |
| Cu (1 1 0) | Pathway 1 | TS 2-1 | 2.77 | −0.12 |
| Pathway 2 | TS 2-2 | 2.57 | −0.57 | |
| Pathway 3 | TS 2-3 | 3.48 | −0.71 | |
| Pathway 4 | TS 2-4 | 3.36 | −0.84 | |
| Cu (1 1 1) | Pathway 1 | TS 3-1 | 2.75 | 0.32 |
| Pathway 2 | TS 3-2 | 2.92 | 0.27 | |
| Pathway 3 | TS 3-3 | 3.59 | −0.15 | |
| Pathway 4 | TS 3-4 | 3.50 | −0.23 | |
| Free C6F12O | Pathway 1 | 4.20 | ||
| Pathway 2 | 4.22 | |||
| Pathway 3 | 3.28 | |||
| Pathway 4 | 3.22 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xing, H.; Lu, S.; Zhang, H. Adsorption and Catalytic Decomposition Mechanism of C6F12O on Cu Surfaces: A Density Functional Theory Study. Catalysts 2025, 15, 1124. https://doi.org/10.3390/catal15121124
Xing H, Lu S, Zhang H. Adsorption and Catalytic Decomposition Mechanism of C6F12O on Cu Surfaces: A Density Functional Theory Study. Catalysts. 2025; 15(12):1124. https://doi.org/10.3390/catal15121124
Chicago/Turabian StyleXing, Haoran, Song Lu, and Heping Zhang. 2025. "Adsorption and Catalytic Decomposition Mechanism of C6F12O on Cu Surfaces: A Density Functional Theory Study" Catalysts 15, no. 12: 1124. https://doi.org/10.3390/catal15121124
APA StyleXing, H., Lu, S., & Zhang, H. (2025). Adsorption and Catalytic Decomposition Mechanism of C6F12O on Cu Surfaces: A Density Functional Theory Study. Catalysts, 15(12), 1124. https://doi.org/10.3390/catal15121124






