Recyclable Magnetic Fe3O4-Supported Copper Oxide as Efficient Catalyst for Oxidation of 5-Hydroxymethylfurfural to 2,5-Furanediformic Acid
Abstract
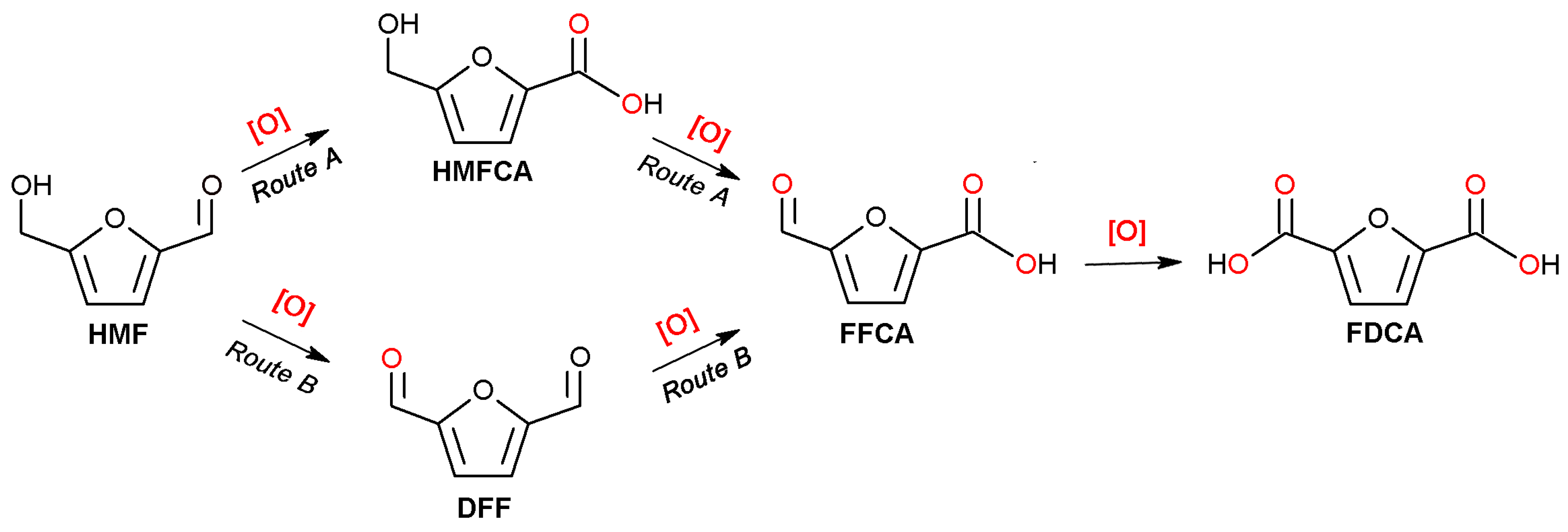
1. Introduction
2. Results
2.1. Catalyst Characterization
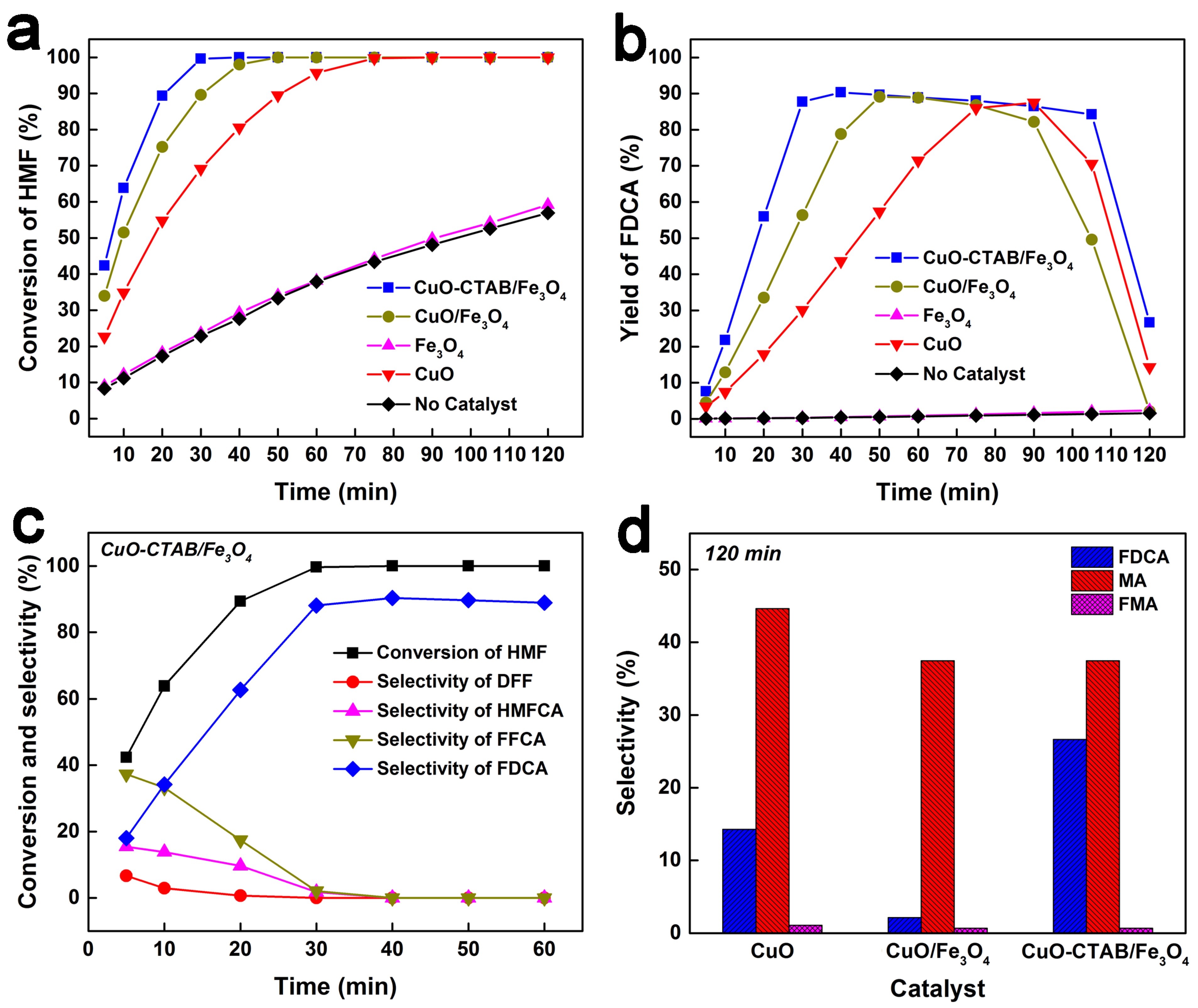
2.2. Catalytic Performance
3. Materials and Methods
3.1. Materials
3.2. Catalyst Preparation
3.3. Characterization Methods
3.4. Catalytic Activity Test
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Corma, A.; Iborra, S.; Velty, A. Chemical routes for the transformation of biomass into chemicals. Chem. Rev. 2007, 107, 2411–2502. [Google Scholar] [CrossRef]
- Mika, L.T.; Cséfalvay, E.; Németh, A. Catalytic conversion of carbohydrates to initial platform chemicals: Chemistry and Sustainability. Chem. Rev. 2018, 118, 505–613. [Google Scholar] [CrossRef]
- Iglesias, J.; Martínez-Salazar, I.; Maireles-Torres, P.; Alonso, D.M.; Mariscal, R.; Granados, M.L. Advances in catalytic routes for the production of carboxylic acids from biomass: A step forward for sustainable polymers. Chem. Soc. Rev. 2020, 49, 5704–5771. [Google Scholar] [CrossRef]
- Bozell, J.J.; Petersen, G.R. Technology development for the production of biobased products from biorefinery carbohydrates-the US Department of Energy’s “Top 10” revisited. Green. Chem. 2010, 12, 539–554. [Google Scholar] [CrossRef]
- Sheldon, R.A. Green and sustainable manufacture of chemicals from biomass: State of the art. Green. Chem. 2014, 16, 950–963. [Google Scholar] [CrossRef]
- Chícharo, B.; Fadlallah, S.; Moraru, D.; Darney, L.; Sangermano, M.; Aricòa, F.; Allais, F. Bio-based epoxy thermosets from 2,5-furan dicarboxylic acid derivates with tunable chain length. Polymer 2025, 336, 128880. [Google Scholar] [CrossRef]
- Burgess, S.K.; Leisen, J.E.; Kraftschik, B.E.; Mubarak, C.R.; Kriegel, R.M.; Koros, W.J. Chain mobility, thermal, and mechanical properties of poly(ethylene furanoate) compared to poly(ethylene terephthalate). Macromolecules 2014, 47, 1383–1391. [Google Scholar] [CrossRef]
- An, J.H.; Sun, G.H.; Xia, H.A. Aerobic oxidation of 5-hydroxymethylfurfural to high-yield 5-hydroxymethyl-2-furancarboxylic acid by poly(vinylpyrrolidone)-capped Ag nanoparticle catalysts. ACS Sustain. Chem. Eng. 2019, 7, 6696–6706. [Google Scholar] [CrossRef]
- Totaro, G.; Sisti, L.; Marchese, P.; Colonna, M.; Romano, A.; Gioia, C.; Vannini, M.; Celli, A. Current advances in the sustainable conversion of 5-hydroxymethylfurfural into 2,5-furandicarboxylic acid. ChemSusChem 2022, 15, e202200501. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Cibin, G.; Walton, R.I. Selective oxidation of biomass-derived 5-hydroxymethylfurfural catalyzed by an iron-grafted metal-organic framework with a sustainably sourced ligand. ACS Sustain. Chem. Eng. 2024, 12, 5575–5585. [Google Scholar] [CrossRef]
- Partenheimer, W.; Grushin, V.V. Synthesis of 2,5-diformylfuran and furan-2,5-dicarboxylic acid by catalytic air-oxidation of 5-hydroxymethylfurfural. Unexpectedly selective aerobic oxidation of benzyl alcohol to benzaldehyde with metal/bromide catalysts. Adv. Synth. Catal. 2001, 343, 102–111. [Google Scholar] [CrossRef]
- Davis, S.E.; Houk, L.R.; Tamargo, E.C.; Datye, A.K.; Davis, R.J. Oxidation of 5-hydroxymethylfurfural over supported Pt, Pd and Au catalysts. Catal. Today 2011, 160, 55–60. [Google Scholar] [CrossRef]
- Saha, B.; Gupta, D.; Abu-Omar, M.M.; Modak, A.; Bhaumik, A. Porphyrin-based porous organic polymer-supported iron(III) catalyst for efficient aerobic oxidation of 5-hydroxymethyl-furfural into 2,5-furandicarboxylic acid. J. Catal. 2013, 299, 316–320. [Google Scholar] [CrossRef]
- Kerdi, F.; Rass, H.A.; Pinel, C.; Besson, M.; Peru, G.; Leger, B.; Rio, S.; Monflier, E.; Ponchel, A. Evaluation of surface properties and pore structure of carbon on the activity of supported Ru catalysts in the aqueous-phase aerobic oxidation of HMF to FDCA. Appl. Catal. A-Gen. 2015, 506, 206–219. [Google Scholar] [CrossRef]
- Zuo, X.B.; Venkitasubramanian, P.; Busch, D.H.; Subramaniam, B. Optimization of Co/Mn/Br-catalyzed oxidation of 5-hydroxymethylfurfural to enhance 2,5-furandicarboxylic acid yield and minimize substrate burning. ACS Sustain. Chem. Eng. 2016, 4, 3659–3668. [Google Scholar] [CrossRef]
- Zhang, Z.H.; Huber, G.W. Catalytic oxidation of carbohydrates into organic acids and furan chemicals. Chem. Soc. Rev. 2018, 47, 1351–1390. [Google Scholar] [CrossRef]
- Zhang, S.; Sun, X.Z.; Zheng, Z.H.; Zhang, L. Nanoscale center-hollowed hexagon MnCo2O4 spinel catalyzed aerobic oxidation of 5-hydroxymethylfurfural to 2,5-furandicarboxylic acid. Catal. Commun. 2018, 113, 19–22. [Google Scholar] [CrossRef]
- Chen, S.B.; Cheng, Y.W.; Ban, H.; Zhang, Y.D.; Zheng, L.P.; Wang, L.J.; Li, X. Liquid-phase aerobic oxidation of 5-hydroxymethylfurfural to 2,5-furandicarboxylic acid over Co/Mn/Br catalyst. Ind. Eng. Chem. Res. 2020, 59, 17076–17084. [Google Scholar] [CrossRef]
- Zhu, P.; Zhang, W.Z.; Li, Q.F.; Xia, H.A. Visible-light-driven photocatalytic oxidation of 5-hydroxymethylfurfural to 2,5-furandicarboxylic acid over plasmonic Au/ZnO catalyst. ACS Sustain. Chem. Eng. 2022, 10, 8778–8787. [Google Scholar] [CrossRef]
- Xie, W.Z.; Liu, H.; Tang, X.; Zeng, X.H.; Sun, Y.; Ke, X.X.; Li, T.Y.; Fang, H.Y.; Lin, L. Efficient synthesis of 2,5-furandicarboxylic acid from biomass-derived 5-hydroxymethylfurfural in 1,4-dioxane/H2O mixture. Appl. Catal A-Gen. 2022, 630, 118463. [Google Scholar] [CrossRef]
- Mani, M.; Mariyaselvakumar, M.; Tothadi, S.; Panda, A.B.; Srinivasan, K.; Konwar, L.J. Base free HMF oxidation over Ru-MnO2 catalysts revisited: Evidence of Mn leaching to Mn-FDCA complexation and its implications on catalyst performance. Mol. Catal. 2024, 554, 113811. [Google Scholar] [CrossRef]
- Zhu, B.; Wang, Q.G.; Wang, J.G.; Yu, X.; Zhang, J.; Chen, C.L. Co@NC Chainmail nanowires for thermo- and electrocatalytic oxidation of 2,5-bis(hydroxymethyl)furan to 2,5-furandicarboxylic acid. ChemSusChem 2025, 18, e202401422. [Google Scholar] [CrossRef]
- Yang, P.; Hu, H.L.; Yang, Y.; Lu, G.W.; Fang, Q.Q.; Lan, G.J.; Zhang, J.; Chen, C.L. Efficient oxidation of 5-hydroxymethylfurfural to 2,5-furandicarboxylic acid by nanocopper oxide catalysts. ChemPlusChem 2025, 90, e202500181. [Google Scholar] [CrossRef]
- Ren, J.; Song, K.H.; Li, Z.H.; Wang, Q.; Li, J.; Wang, Y.X.; Li, D.B.; Kim, C.K. Activation of formyl C-H and hydroxyl O-H bonds in HMF by the CuO (111) and Co3O4(110) surfaces: A DFT study. Appl. Surf. Sci. 2018, 456, 174–183. [Google Scholar] [CrossRef]
- Liu, H.; Li, W.L.; Zuo, M.; Tang, X.; Zeng, X.H.; Sun, Y.; Lei, T.Z.; Fang, H.Y.; Li, T.Y.; Lin, L. Facile and efficient two-step formation of a renewable monomer 2,5-furandicarboxylic acid from carbohydrates over the NiOx catalyst. Ind. Eng. Chem. Res. 2020, 59, 4895–4904. [Google Scholar] [CrossRef]
- Jiang, W.; Wang, D.J.; Deng, X.L.; Gao, Y.X.; Wang, W.Z.; Ge, T.J.; Zhao, C.K.; Sun, Y. Oxygen vacancy boosted oxidation of 5-hydroxymethylfurfural to 2, 5-furandicarboxylic acid over CuCoOx. Mol. Catal. 2024, 556, 113917. [Google Scholar] [CrossRef]
- Liu, M.Y.; Ye, Y.Y.; Ye, J.M.; Gao, T.; Wang, D.H.; Chen, G.; Song, Z.J. Recent advances of magnetite (Fe3O4)-based magnetic materials in catalytic applications. Magnetochemistry 2023, 9, 110. [Google Scholar] [CrossRef]
- Gawande, M.B.; Branco, P.S.; Varm, R.S. Nano-magnetite (Fe3O4) as a support for recyclable catalysts in the development of sustainable methodologies. Chem. Soc. Rev. 2013, 42, 3371–3393. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, M.D.; Tran, H.V.; Xu, S.J.; Lee, T.R. Fe3O4 Nanoparticles: Structures, synthesis, magnetic properties, surface functionalization, and emerging applications. Appl. Sci. 2021, 11, 11301. [Google Scholar] [CrossRef] [PubMed]
- Rahdar, A.; Taboada, P.; Aliahmad, M.; Hajinezhad, M.R.; Sadeghfar, F. Iron oxide nanoparticles: Synthesis, physical characterization, and intraperitoneal biochemical studies in Rattus norvegicus. J. Mol. Struct. 2018, 1173, 240–245. [Google Scholar] [CrossRef]
- Rajpu, S.; Pittman Jr, C.U.; Mohan, D. Magnetic magnetite (Fe3O4) nanoparticle synthesis and applications for lead (Pb2+) and chromium (Cr6+) removal from water. J. Colloid. Interf. Sci. 2016, 468, 334–346. [Google Scholar] [CrossRef]
- Daou, T.J.; Pourroy, G.; Bégin-Colin, S.; Grenèche, J.M.; Ulhaq-Bouillet, C.; Legaré, P.; Bernhardt, P.; Leuvrey, C.; Rogez, G. Hydrothermal synthesis of monodisperse magnetite nanoparticles. Chem. Mater. 2006, 18, 4399–4404. [Google Scholar] [CrossRef]
- Xu, J.; Yang, H.B.; Fu, W.Y.; Du, K.; Sui, Y.M.; Chen, J.J.; Zeng, Y.; Li, M.H.; Zou, G.T. Preparation and magnetic properties of magnetite nanoparticles by sol-gel method. J. Magn. Magn. Mater. 2007, 309, 307–311. [Google Scholar] [CrossRef]
- Yang, Q.Y.; Zhang, B.Z.; Chen, D.; Li, Y.; Feng, C.D.; Du, W.A. Mechanochemistry strategy in metal/Fe3O4 with high stability for superior chemoselective catalysis. ACS Appl. Mater. Interfaces 2024, 16, 66219–66229. [Google Scholar] [CrossRef] [PubMed]
- Serga, V.; Burve, R.; Maiorov, M.; Krumina, A.; Skaudžius, R.; Zarkov, A.; Kareiva, A.; Popov, A.I. Impact of gadolinium on the structure and magnetic properties of nanocrystalline powders of iron oxides produced by the extraction-pyrolytic method. Materials 2020, 13, 4147. [Google Scholar] [CrossRef]
- Friščić, T.; Mottillo, C.; Titi, H.M. Mechanochemistry for synthesis. Angew. Chem. Int. Ed. 2020, 59, 1018–1029. [Google Scholar] [CrossRef]
- Zhao, J.H.; Shu, Y.; Zhang, P.F. Solid-state CTAB-assisted synthesis of mesoporous Fe3O4 and Au@Fe3O4 by mechanochemistry. Chin. J. Catal. 2019, 40, 1078–1084. [Google Scholar] [CrossRef]
- Klimakow, M.; Klobes, P.; Rademann, K.; Emmerling, F. Characterization of mechanochemically synthesized MOFs. Micropor. Mesopor. Mat. 2012, 154, 113–118. [Google Scholar] [CrossRef]
- Hamzehpoor, E.; Effaty, F.; Borchers, T.H.; Stein, R.S.; Wahrhaftig-Lewis, A.; Ottenwaelder, X.; Friščić, T.; Perepichka, D.F. Mechanochemical synthesis of boroxine-linked covalent organic frameworks. Angew. Chem. Int. Ed. 2024, 63, e202404539. [Google Scholar] [CrossRef] [PubMed]
- Joshi, H.; Ochoa-Hernández, C.; Nürenberg, E.; Kang, L.Q.; Wang, F.R.; Weidenthaler, C.; Schmidt, W.; Schüth, F. Insights into the mechanochemical synthesis of Sn-β: Solid-state metal incorporation in beta zeolite. Micropor. Mesopor. Mat. 2020, 309, 110566. [Google Scholar] [CrossRef]
- Schneidermann, C.; Jäckel, N.; Oswald, S.; Giebeler, L.; Presser, V.; Borchardt, L. Solvent-free mechanochemical synthesis of nitrogen-doped nanoporous carbon for electrochemical energy storage. ChemSusChem 2017, 10, 2416–2424. [Google Scholar] [CrossRef]
- Tian, C.; Yue, Y.H.; Miao, C.X.; Hua, W.M.; Gao, Z. Mesoporous silica-encapsulated Cu nanoparticles with enhanced performance for ethanol dehydrogenation to acetaldehyde. ACS Sustain. Chem. Eng. 2025, 13, 1401–1408. [Google Scholar] [CrossRef]
- Zhu, K.K.; Sun, J.M.; Liu, J.; Wang, L.Q.; Wan, H.Y.; Hu, J.Z.; Wang, Y.; Peden, C.H.F.; Nie, Z.M. Solvent evaporation assisted preparation of oriented nanocrystalline mesoporous MFI zeolites. ACS Catal. 2011, 1, 682–690. [Google Scholar] [CrossRef]
- Hu, H.L.; Xue, T.T.; Zhang, Z.X.; Gan, J.; Chen, L.Q.; Zhang, J.; Qu, F.Z.; Cai, W.J.; Wang, L. Direct conversion of 5-hydroxymethylfurfural to furanic diether by copper-loaded hierarchically structured ZSM-5 catalyst in a fixed-bed Reactor. ChemCatChem 2021, 13, 3461–3469. [Google Scholar] [CrossRef]
- Zhou, Q.; Jia, X.H.; Liu, R.; Liu, F.F.; Zhang, H.; Hou, D.; Wang, L.; Luo, Y.K.; Huang, B.C.; Huang, H.; et al. CTAB-Assisted, controlled preparation of a CeZrOx/CuSAPO-34 catalyst and its performance for the synergistic removal of NOx and toluene from flue gas. J. Environ. Chem. Eng. 2025, 13, 118561. [Google Scholar] [CrossRef]
- Witoon, T.; Chalorngtham, J.; Dumrongbunditkul, P.; Chareonpanich, M.; Limtrakul, J. CO2 hydrogenation to methanol over Cu/ZrO2 catalysts: Effects of zirconia phases. Chem. Eng. J. 2016, 293, 327–336. [Google Scholar] [CrossRef]
- Xu, Y.X.; Huang, H.J.; Kong, L.X.; Ma, X.B. Effect of calcination temperature on the Cu-ZrO2 interfacial structure and its catalytic behavior in the hydrogenation of dimethyl oxalate. Catal. Sci. Technol. 2022, 12, 6782–6794. [Google Scholar] [CrossRef]
- Xu, S.G.; Han, X.; Zhang, F.; Sun, Z.Y.; Chai, R.Y.; Xu, F.; Du, S.G.; Jiao, X.L.; Chen, D.R.; Zhang, J. Synergistic role of Cu0 and acidic sites in Cu/Al2O3 catalysts for enhanced furfural hydrogenation to furfuryl alcohol. Chem. Eng. J. 2025, 522, 167587. [Google Scholar] [CrossRef]
- Cai, F.F.; Zhu, W.; Xiao, G.M. Promoting effect of zirconium oxide on Cu-Al2O3 catalyst for the hydrogenolysis of glycerol to 1,2-propanediol. Catal. Sci. Technol. 2016, 6, 4889–4900. [Google Scholar] [CrossRef]
- Hu, D.X.; Hu, H.L.; Zhou, H.; Li, G.Z.; Chen, C.L.; Zhang, J.; Yang, Y.; Hu, Y.P.; Zhang, Y.J.; Wang, L. The effect of potassium on Cu/Al2O3 catalysts for the hydrogenation of 5-hydroxymethylfurfural to 2,5-bis(hydroxymethyl)furan in a fixed-bed reactor. Catal. Sci. Technol. 2018, 8, 6091–6099. [Google Scholar] [CrossRef]
- Sajid, M.; Zhao, X.B.; Liu, D.H. Production of 2,5-furandicarboxylic acid (FDCA) from 5-hydroxymethylfurfural (HMF): Recent progress focusing on the chemical-catalytic routes. Green. Chem. 2018, 20, 5427–5453. [Google Scholar] [CrossRef]
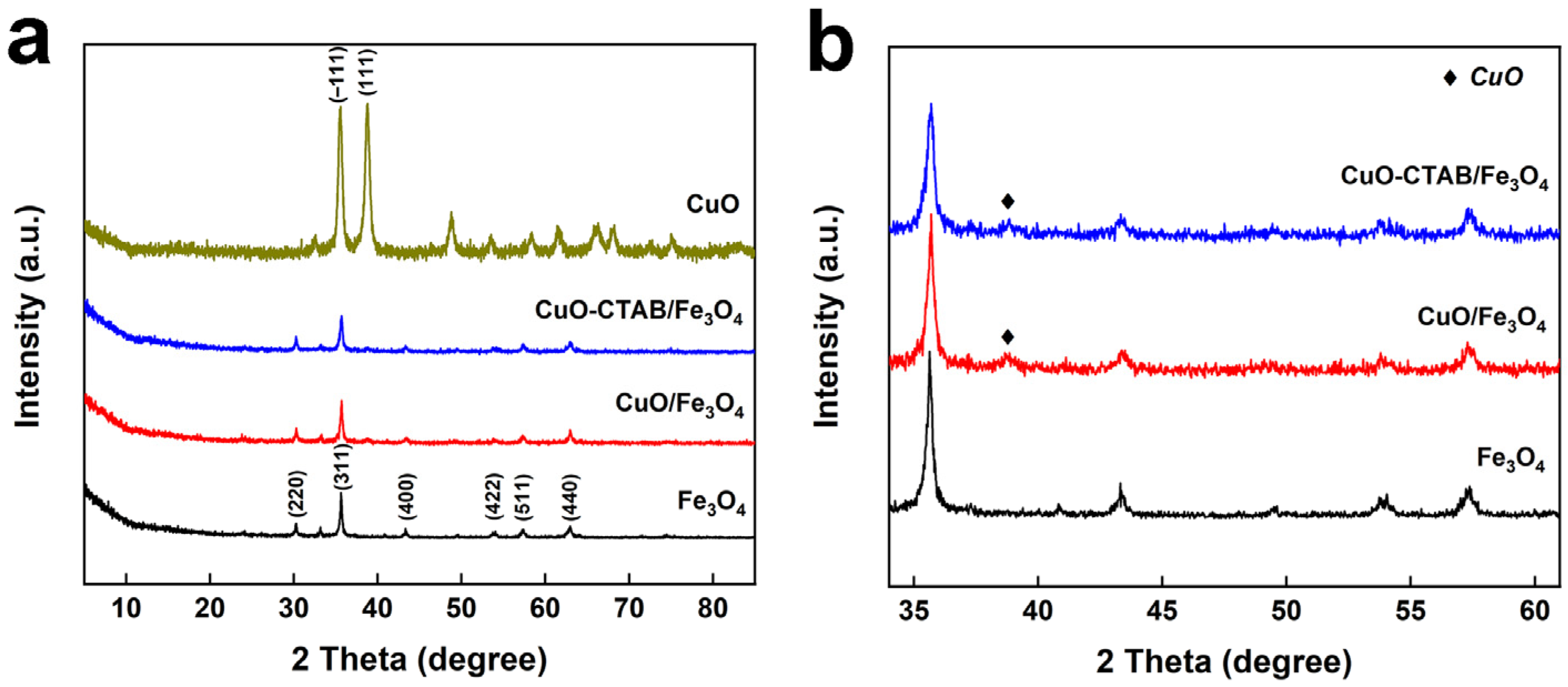
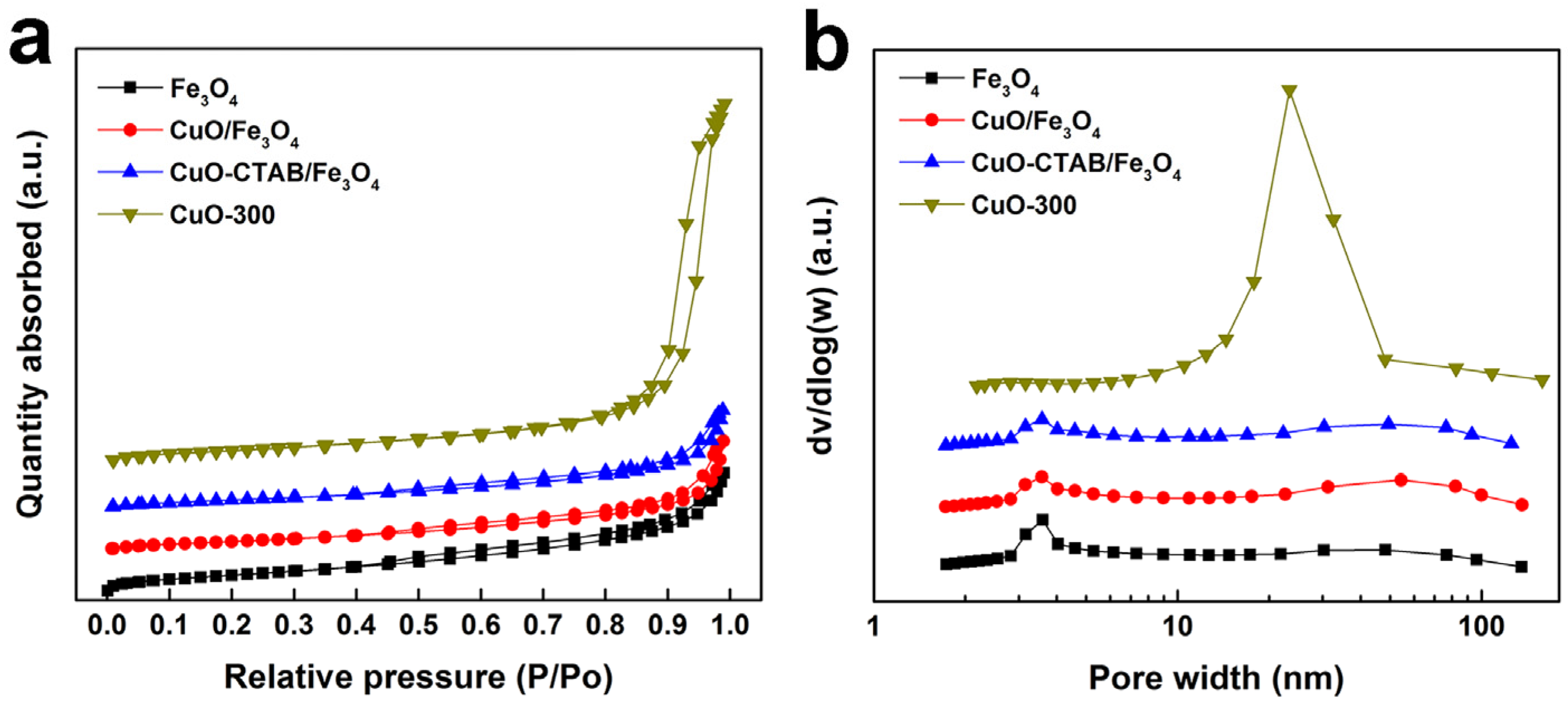

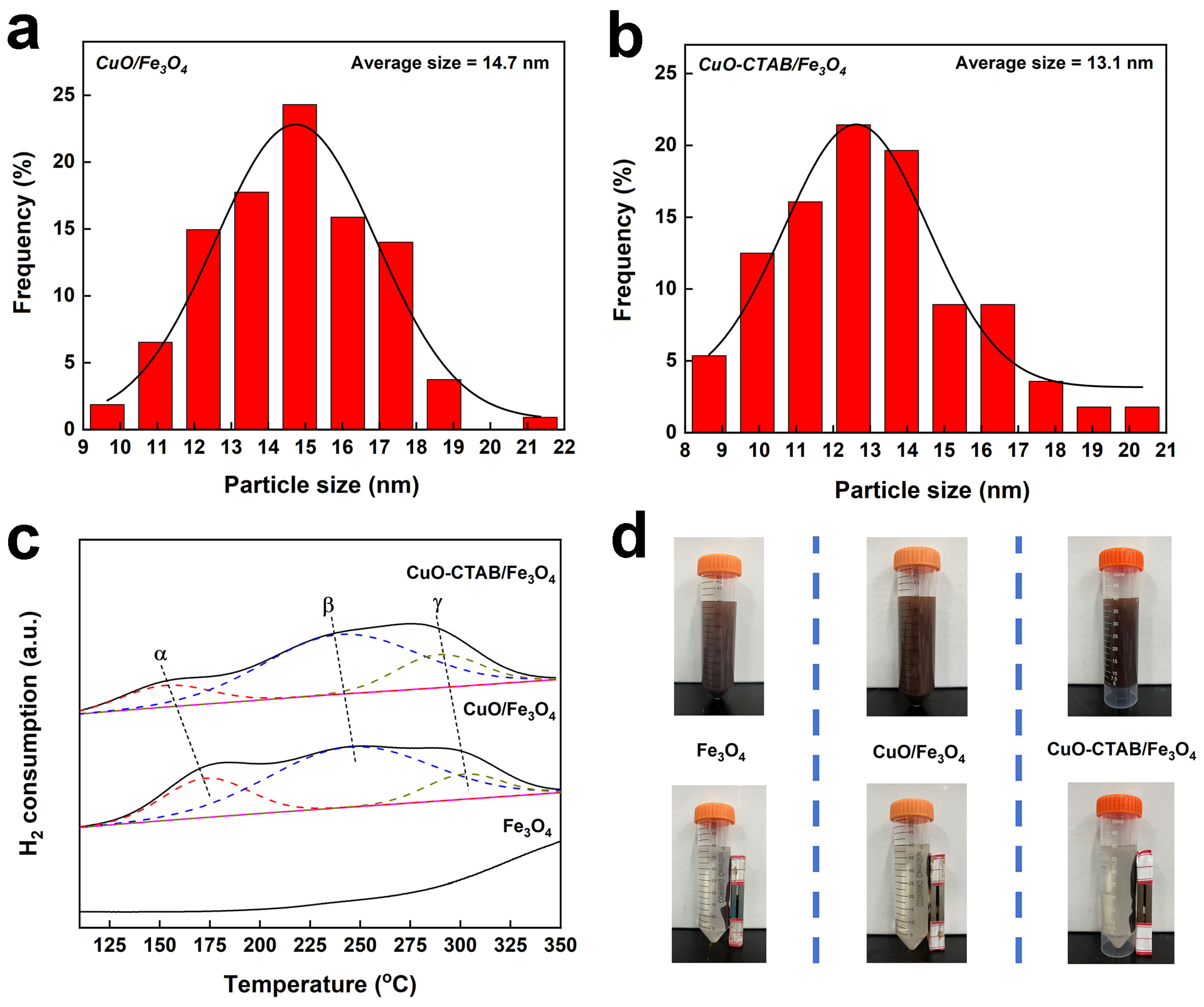

| Sample | SBET (m2·g−1) [a] | Pore Volume (cm3·g−1) [b] | Average Diameters (nm) [c] | CuO Content (wt%) [d] |
|---|---|---|---|---|
| Fe3O4 | 53.8 | 0.116 | 5.5 (Fe3O4) | / |
| CuO/Fe3O4 | 35.3 | 0.107 | 14.7 (CuO) | 9.1 |
| CuO-CTAB/Fe3O4 | 33.1 | 0.096 | 13.1 (CuO) | 9.3 |
| CuO | 46.7 | 0.34 | 16.0 (CuO) | / |
| Substrate | Reaction Conditions | Conversion | Yield |
|---|---|---|---|
| BHMF | 0.64 g BHMF, 1.575 g catalyst, 0.52 g NaOH, 45 mL NaClO solution, 80 mL water, 30 °C, 40 min | 100% | 90.5% FDCA |
| FAL | 0.49 g FAL, 1.575 g catalyst, 0.52 g NaOH, 45 mL NaClO solution, 80 mL water, 30 °C, 20 min | 100% | 82.2% FA |
| FFA | 0.50 g FA, 1.575 g catalyst, 0.52 g NaOH, 45 mL NaClO solution, 80 mL water, 30 °C, 30 min | 100% | 84.9% FA |
| Entry | Reaction Conditions | Conversion (%) | Selectivity of FDCA (%) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| HMF (g) | Catalyst (g) | NaOH (g) | NaClO/HMF Molar Ratio [a] | Water (mL) | Temperature (°C) | Time (min) | HMF | NaClO [b] | ||
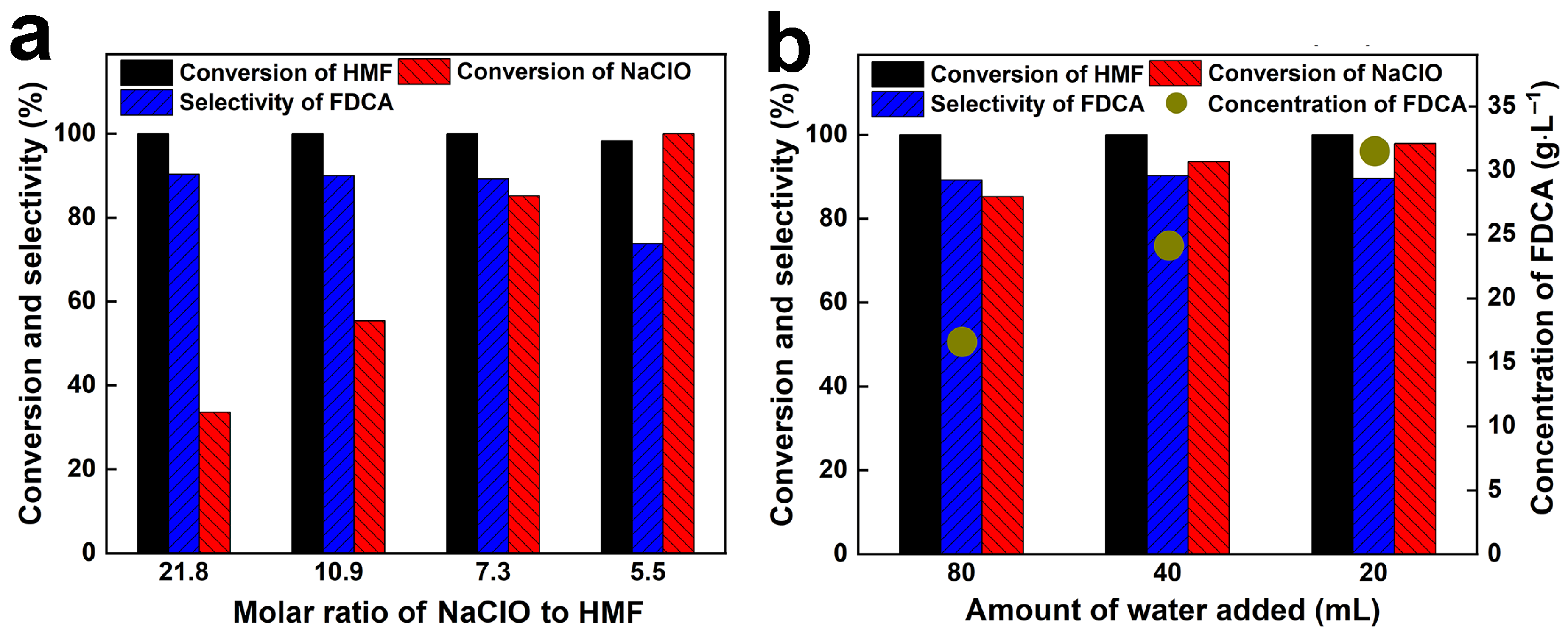
| 1 | 0.63 | 1.575 | 0.52 | 21.8 | 80 | 30 | 40 | 100 | 33.6 | 90.3 |
| 2 | 1.26 | 3.15 | 1.04 | 10.9 | 80 | 30 | 30 | 100 | 55.4 | 90.0 |
| 3 | 1.89 | 4.725 | 1.56 | 7.3 | 80 | 30 | 60 | 100 | 85.2 | 89.2 |
| 4 | 2.52 | 6.3 | 2.08 | 5.5 | 80 | 30 | 120 | 100 | 100 | 73.8 |
| 5 | 1.89 | 4.725 | 1.56 | 7.3 | 40 | 30 | 60 | 100 | 93.6 | 90.2 |
| 6 | 1.89 | 4.725 | 1.56 | 7.3 | 20 | 30 | 60 | 100 | 97.9 | 89.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, P.; Hu, H.; Yang, Y.; Lan, G.; El-Hout, S.I.; Fang, Q.; Lu, G.; Chen, C.; Zhang, J. Recyclable Magnetic Fe3O4-Supported Copper Oxide as Efficient Catalyst for Oxidation of 5-Hydroxymethylfurfural to 2,5-Furanediformic Acid. Catalysts 2025, 15, 1120. https://doi.org/10.3390/catal15121120
Yang P, Hu H, Yang Y, Lan G, El-Hout SI, Fang Q, Lu G, Chen C, Zhang J. Recyclable Magnetic Fe3O4-Supported Copper Oxide as Efficient Catalyst for Oxidation of 5-Hydroxymethylfurfural to 2,5-Furanediformic Acid. Catalysts. 2025; 15(12):1120. https://doi.org/10.3390/catal15121120
Chicago/Turabian StyleYang, Peng, Hualei Hu, Yong Yang, Guojun Lan, Soliman I. El-Hout, Qianquan Fang, Guowen Lu, Chunlin Chen, and Jian Zhang. 2025. "Recyclable Magnetic Fe3O4-Supported Copper Oxide as Efficient Catalyst for Oxidation of 5-Hydroxymethylfurfural to 2,5-Furanediformic Acid" Catalysts 15, no. 12: 1120. https://doi.org/10.3390/catal15121120
APA StyleYang, P., Hu, H., Yang, Y., Lan, G., El-Hout, S. I., Fang, Q., Lu, G., Chen, C., & Zhang, J. (2025). Recyclable Magnetic Fe3O4-Supported Copper Oxide as Efficient Catalyst for Oxidation of 5-Hydroxymethylfurfural to 2,5-Furanediformic Acid. Catalysts, 15(12), 1120. https://doi.org/10.3390/catal15121120







