Bi-Dentate Pyridyl Amine-Derived Complexes of Aluminium: Synthesis, Structure and ROP Capability
Abstract
1. Introduction
2. Results and Discussion
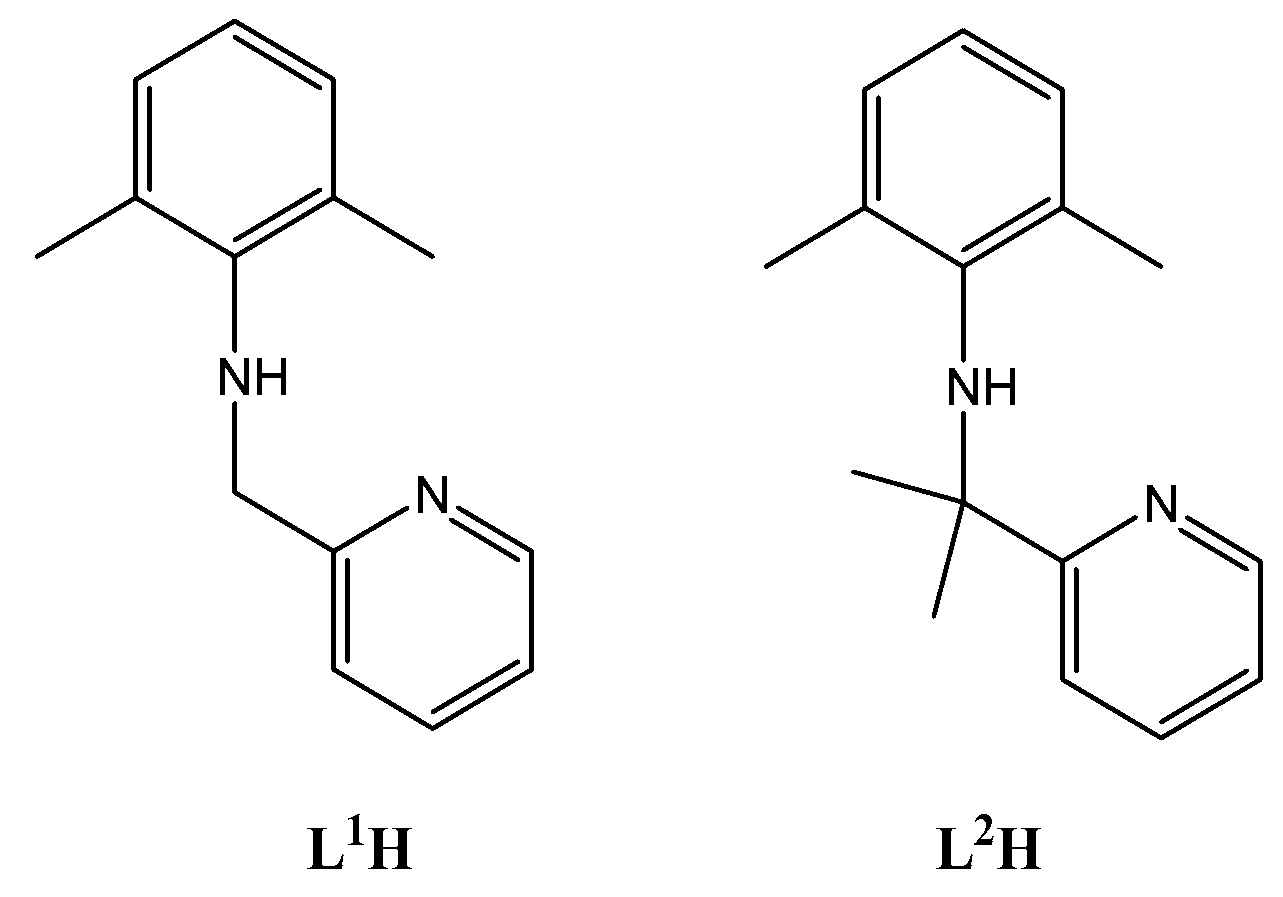
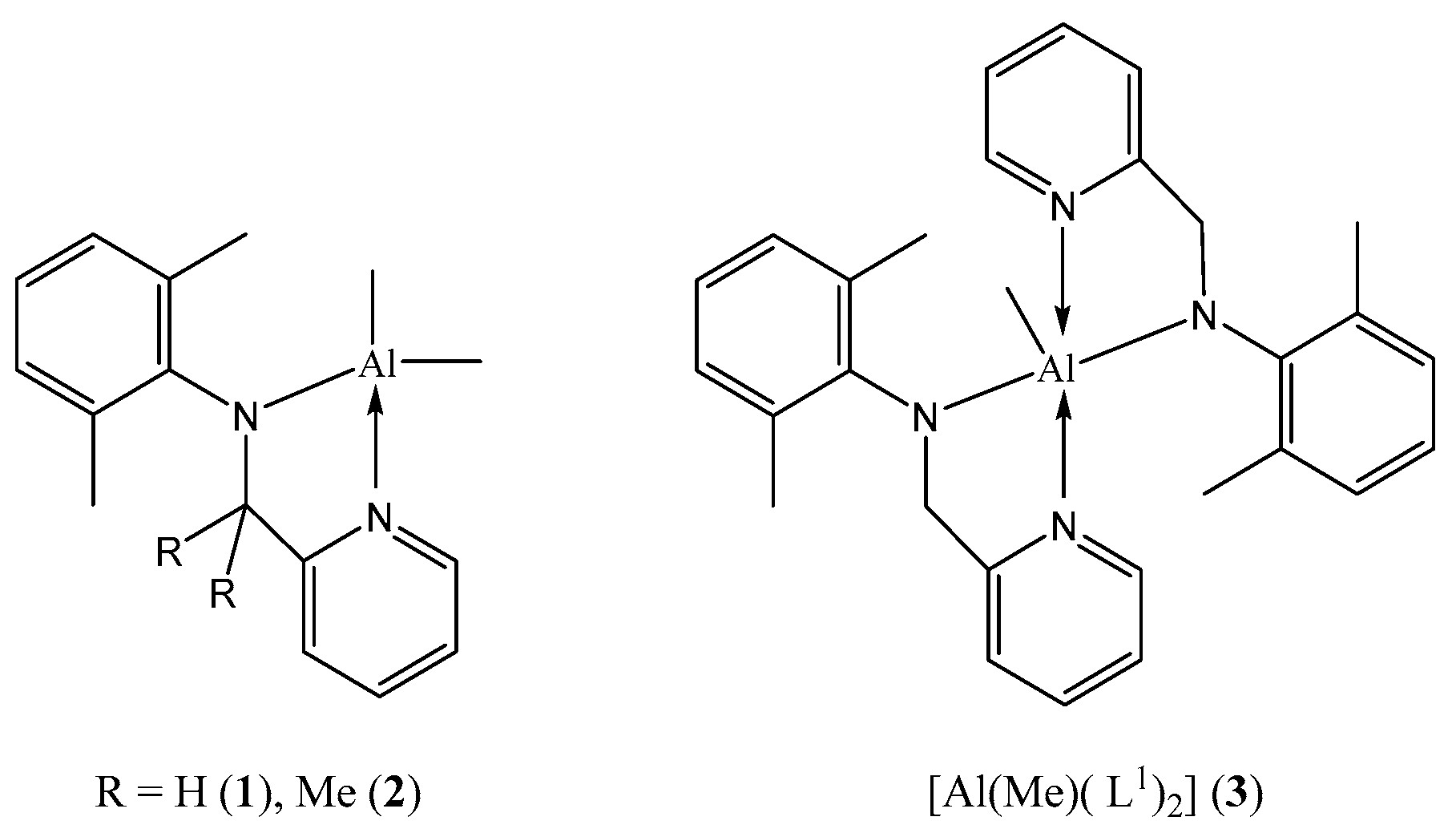
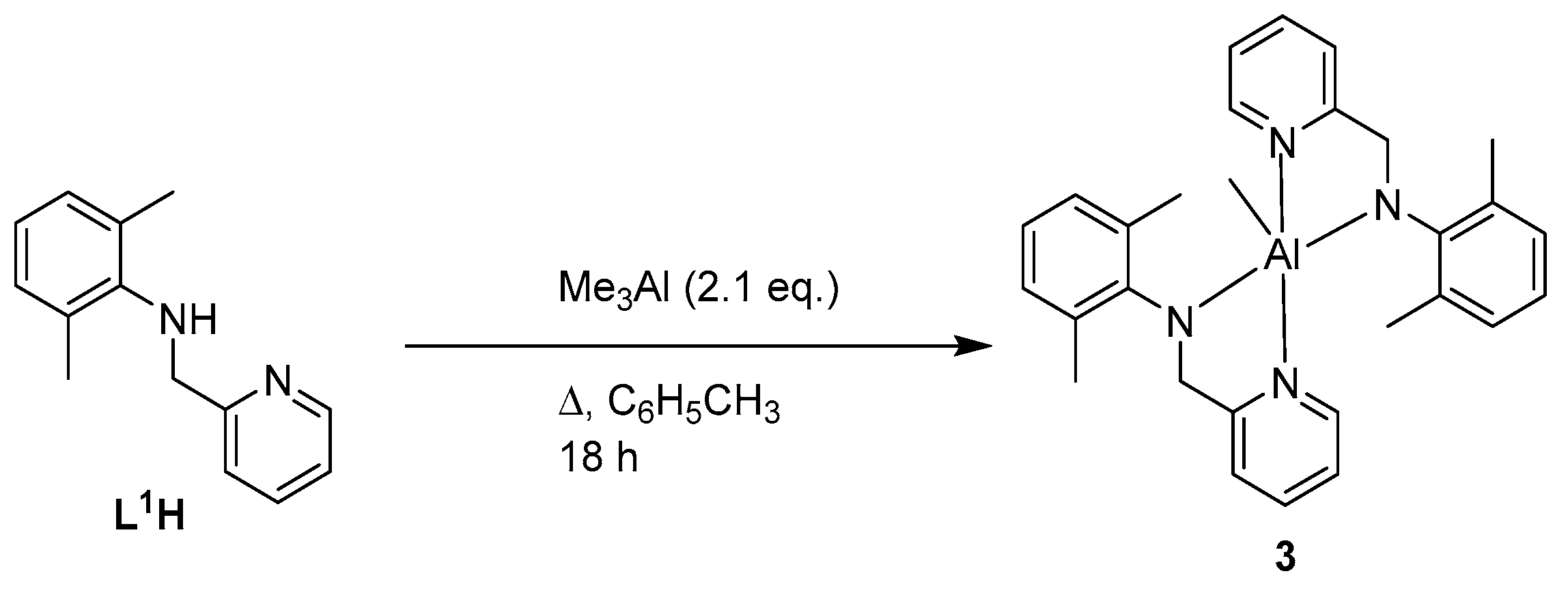
2.1. Synthesis of Ligands and Complexes
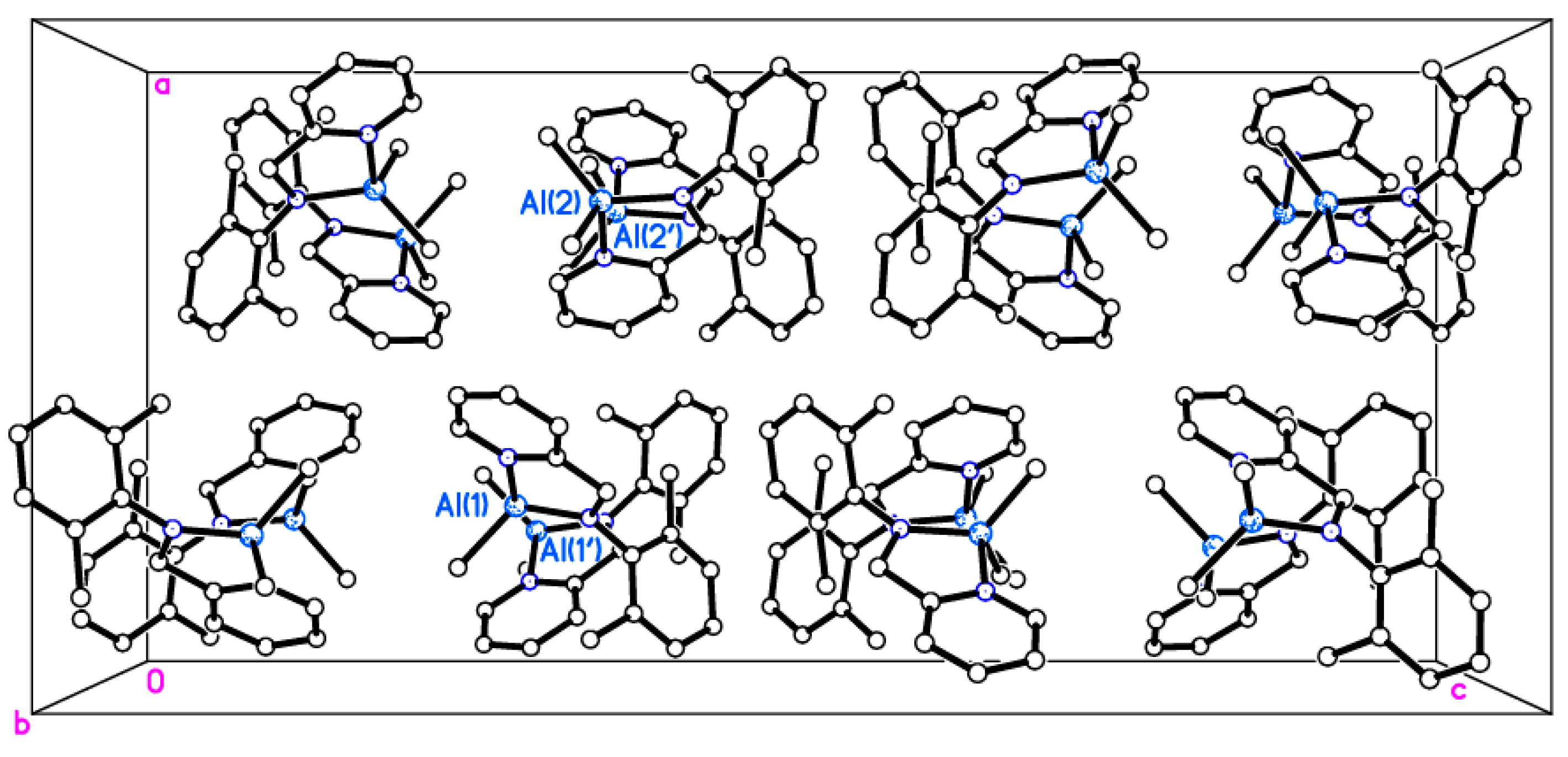
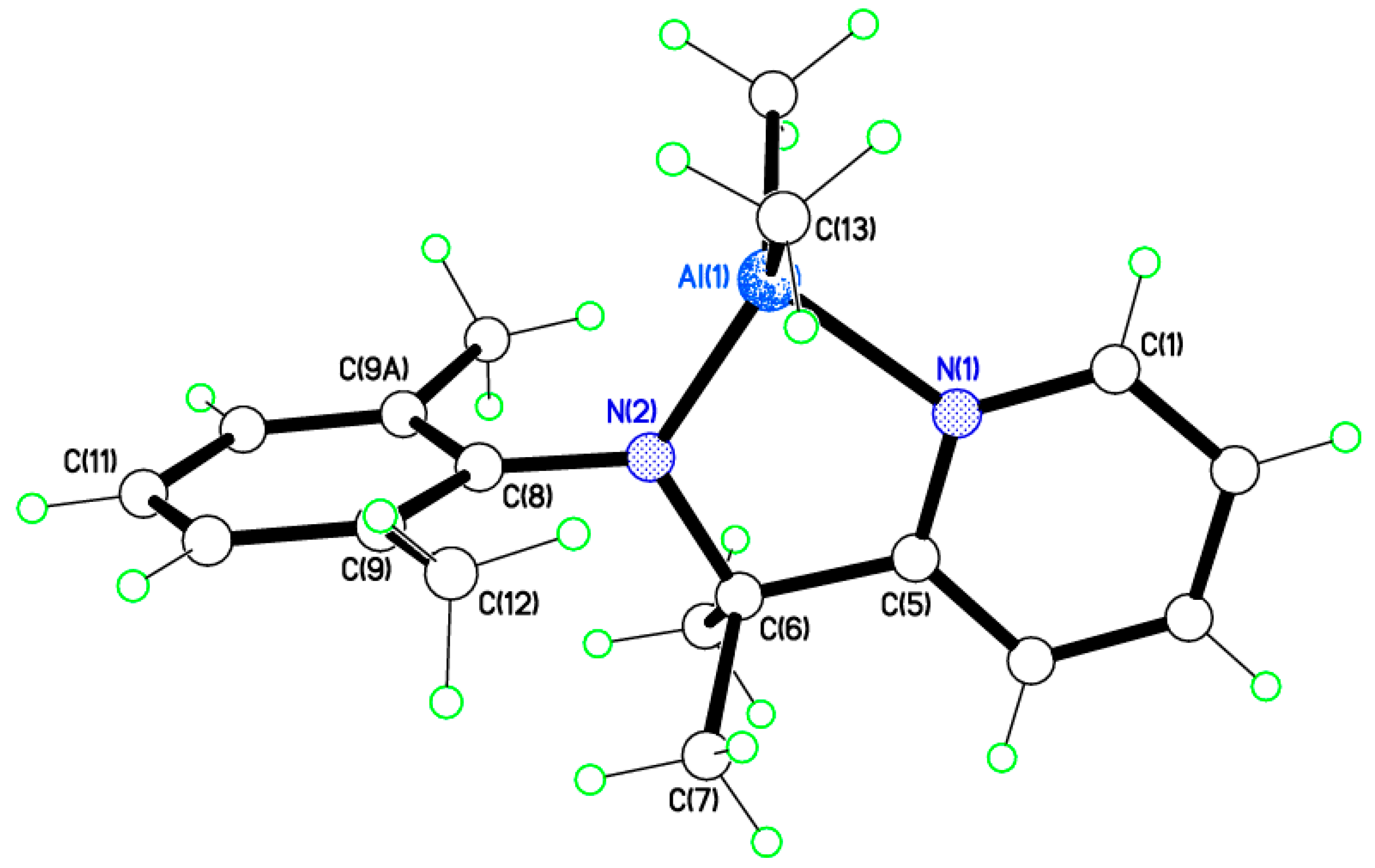
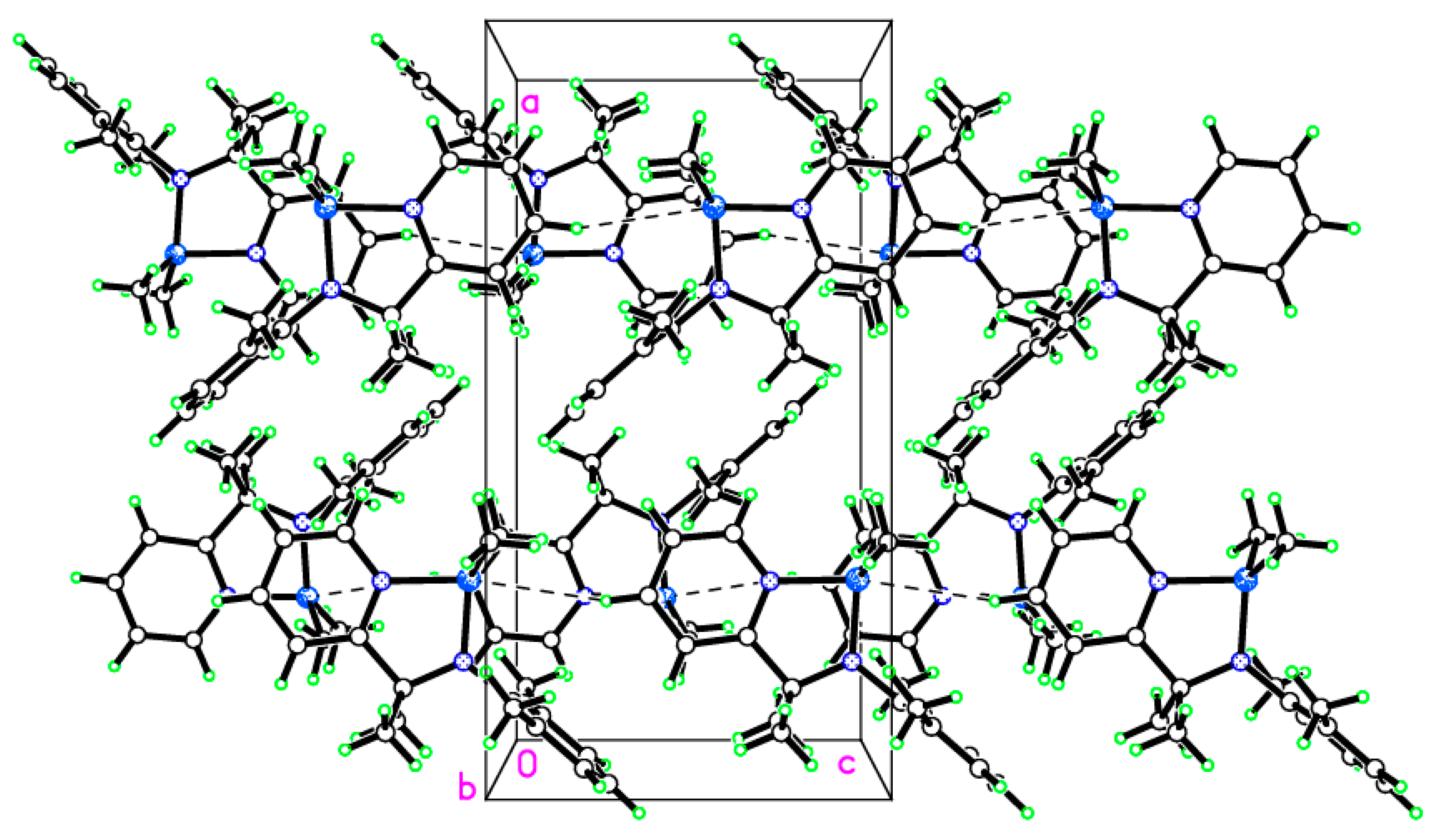
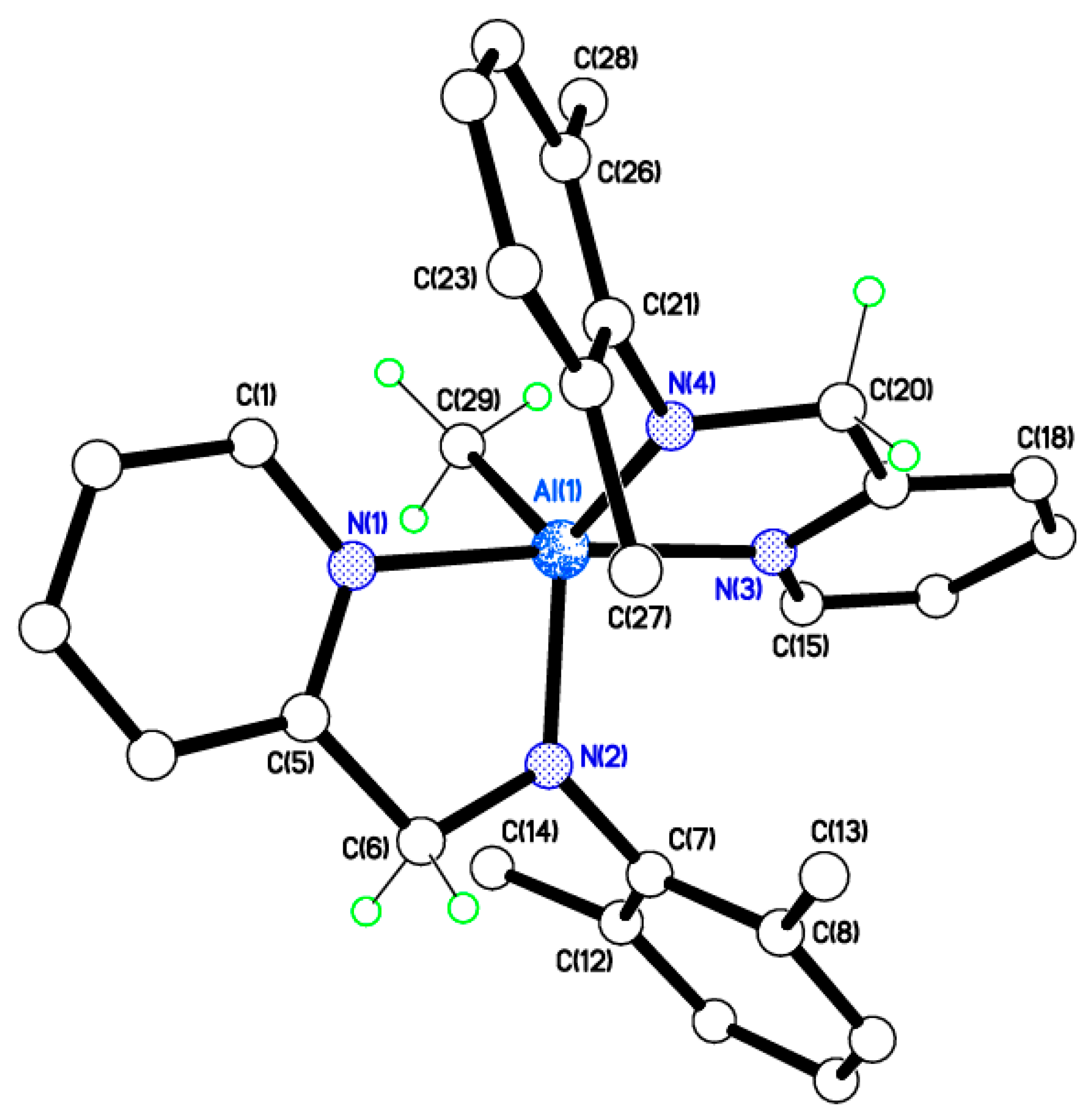
2.2. Molecular Structures of Complexes
3. Ring Opening Polymerization (ROP)
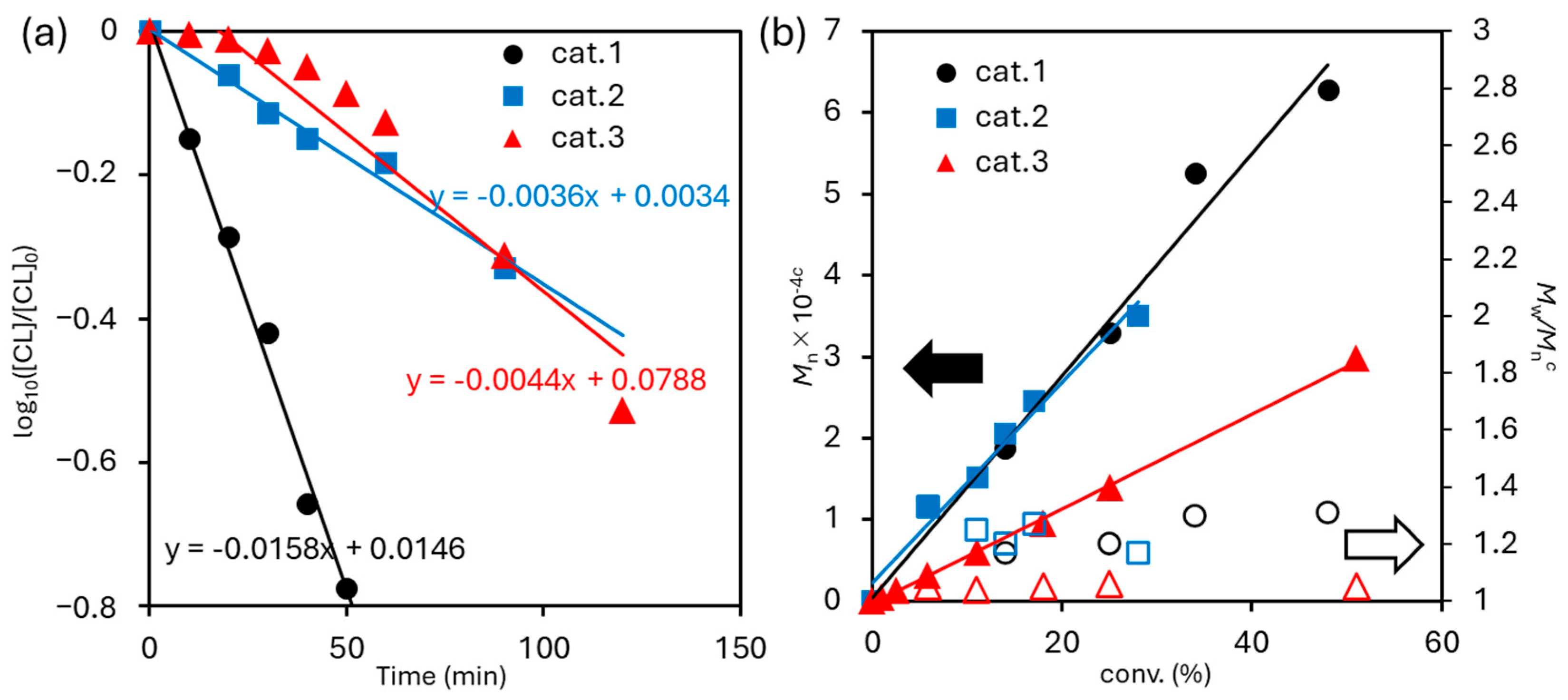
3.1. Ring Opening Polymerization of ε-Caprolactone (ε-CL)
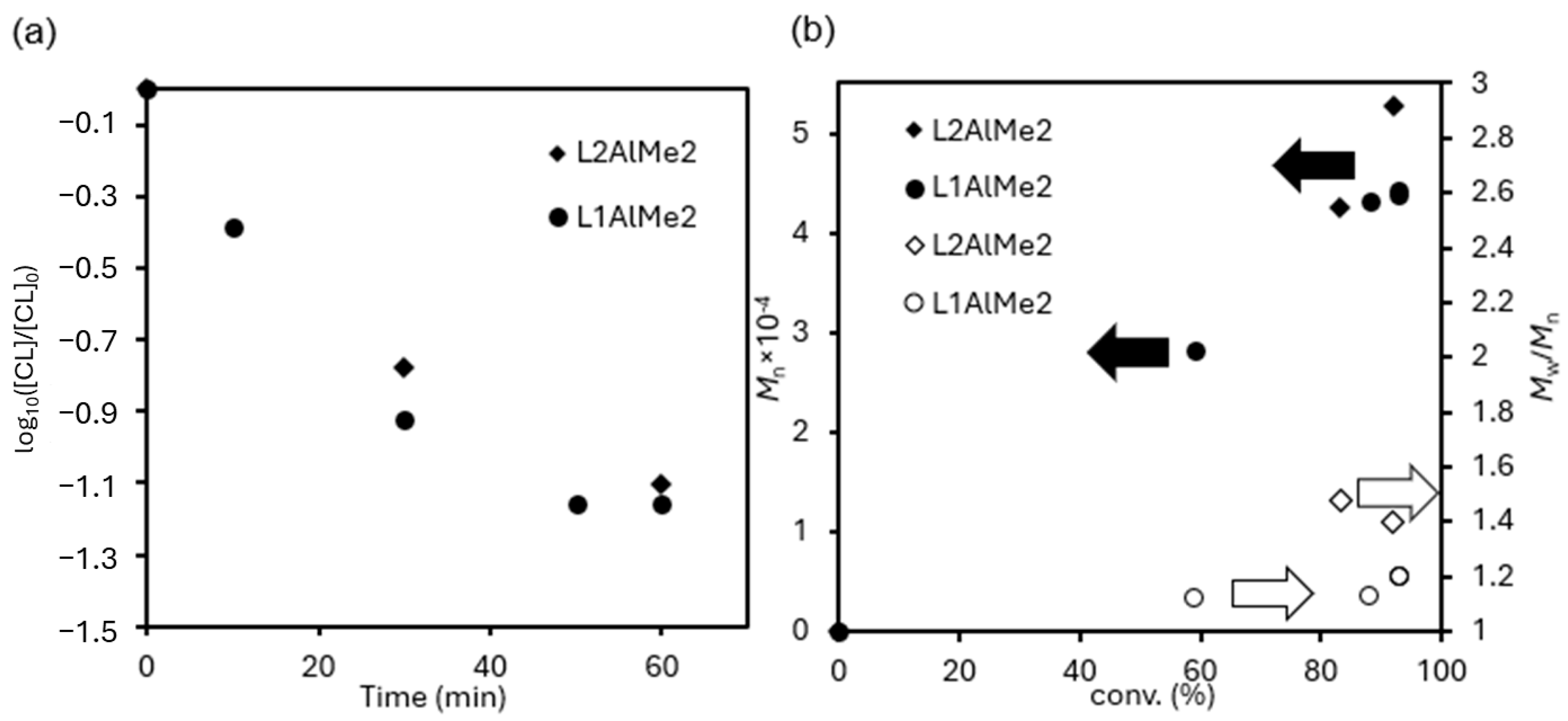
Kinetics
3.2. δ-Valerolactone (δ-VL)
3.2.1. Kinetics
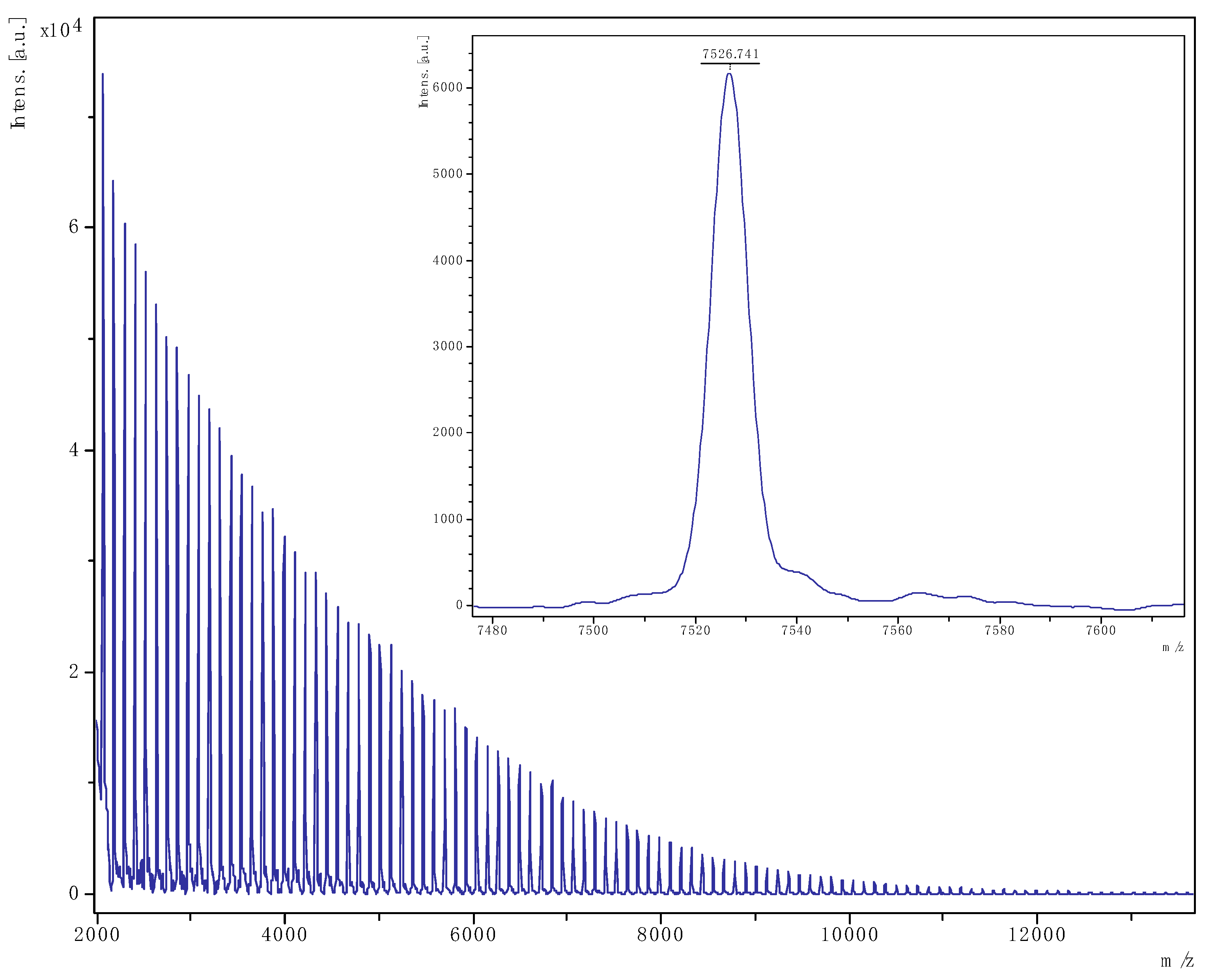
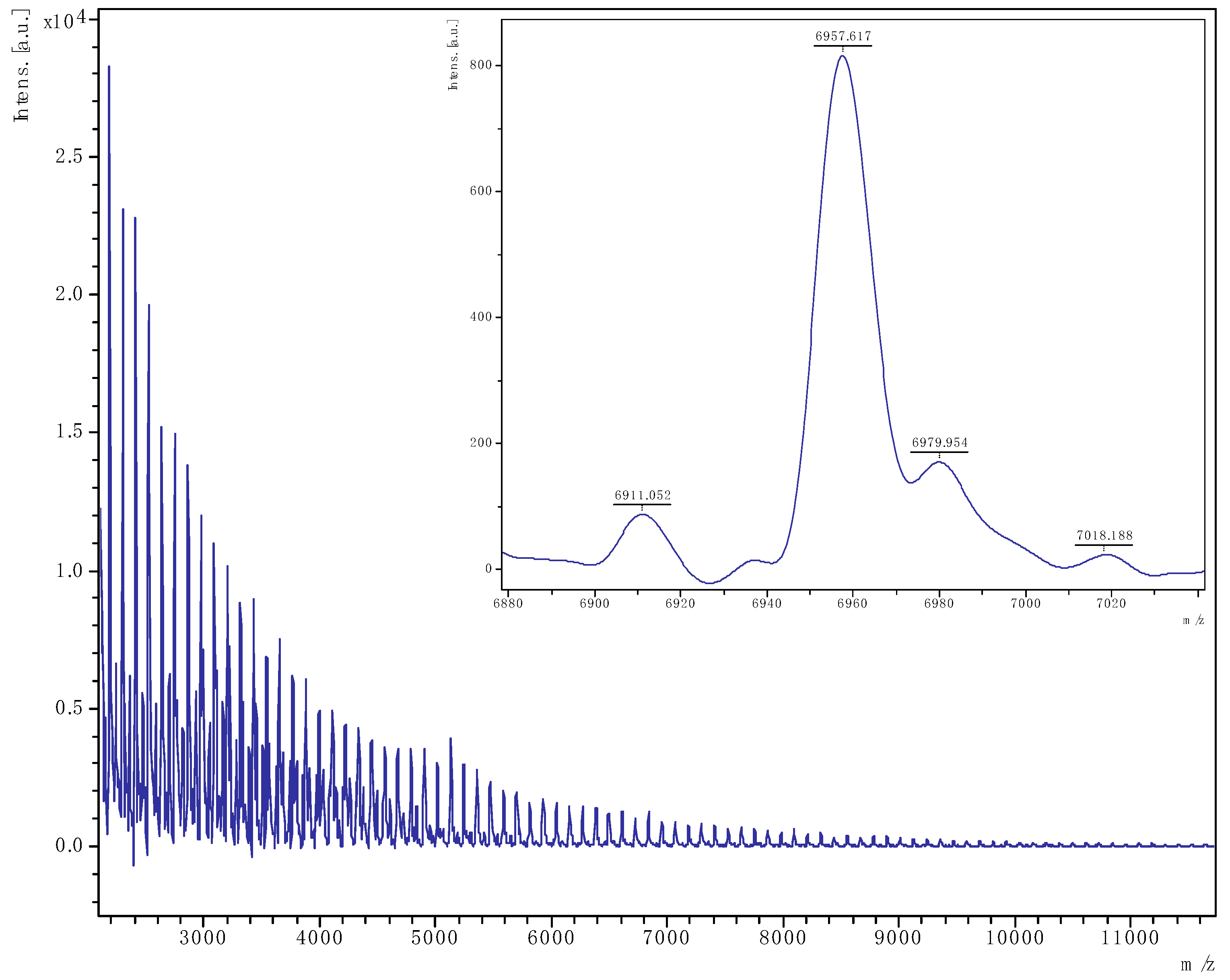
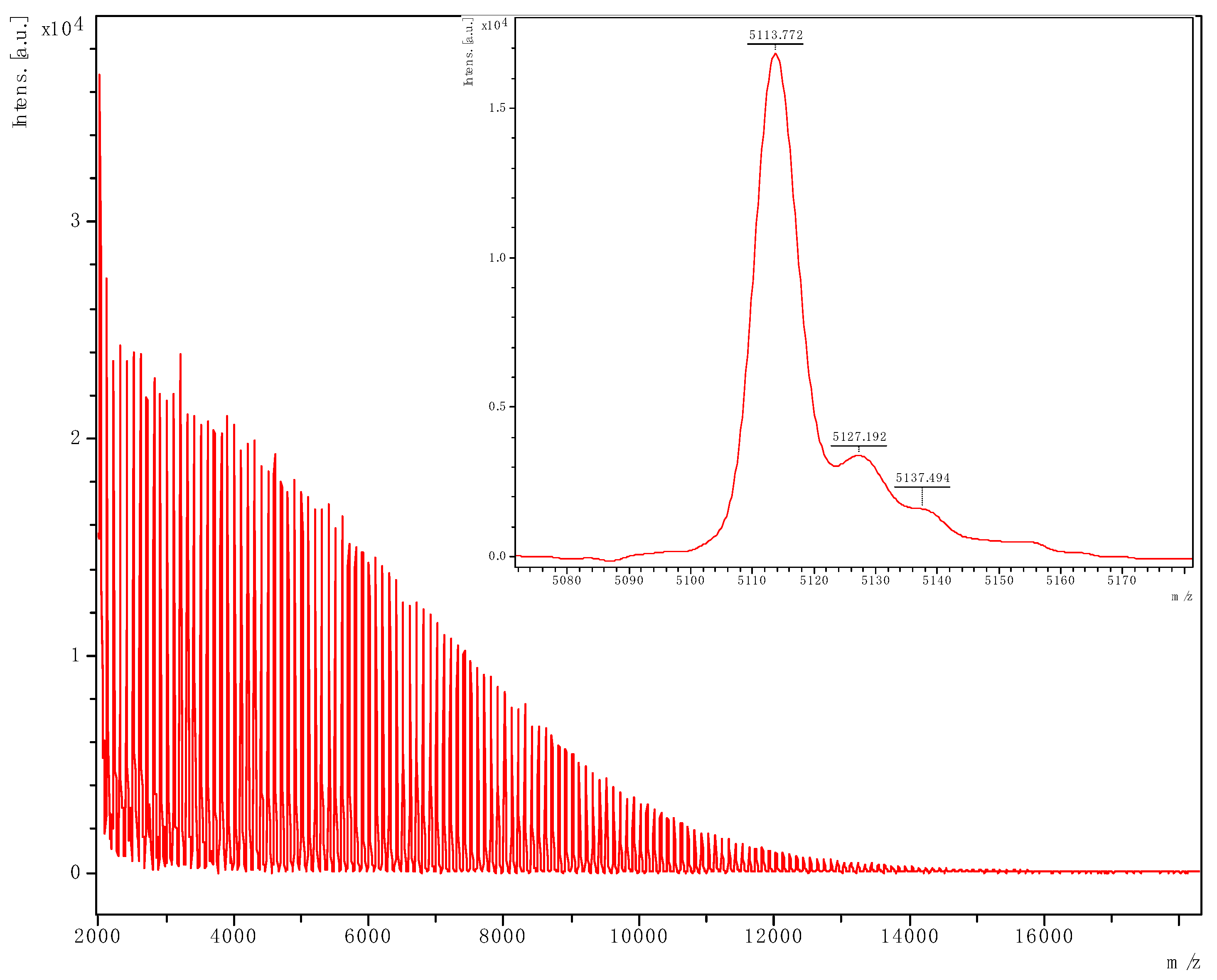
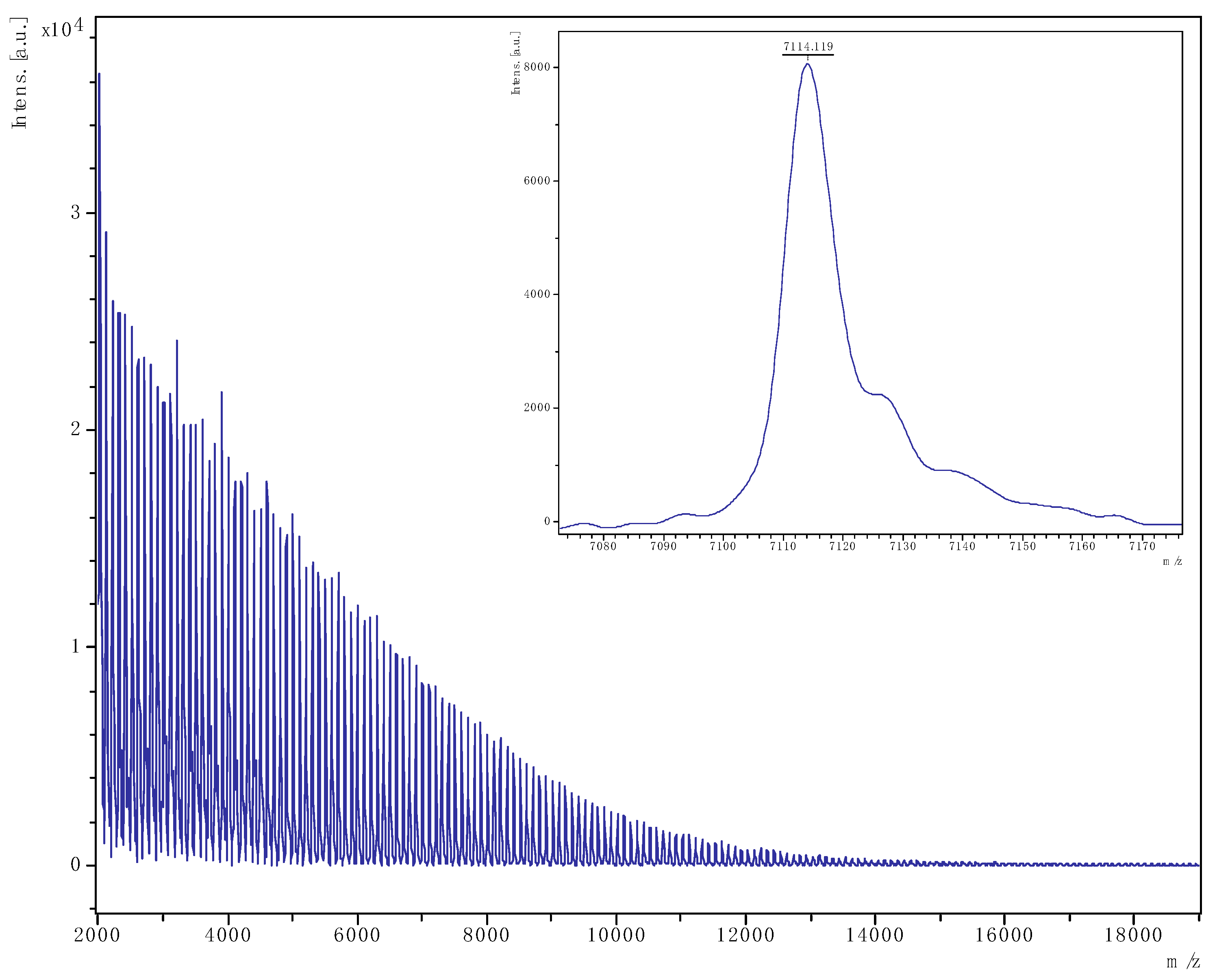
3.2.2. MALDI-ToF Spectra
4. Experimental
4.1. General
4.1.1. Synthesis of L2H
4.1.2. Synthesis of [Me2Al(L1)] (1)
4.1.3. Synthesis of [Me2Al(L2)] (2)
4.1.4. Synthesis of [MeAl(L1)2] (3)
4.2. Procedure for ROP of ε-Caprolactone or δ-Valerolacone
4.2.1. Kinetic Studies
4.2.2. Crystallography
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Labet, M.; Thielemans, W. Synthesis of polycaprolactone: A review. Chem. Soc. Rev. 2009, 38, 3484–3504. [Google Scholar] [CrossRef]
- Arbaoui, A.; Redshaw, C. Metal Catalysts for ε-caprolactone polymerisation. Polym. Chem. 2010, 1, 801–826. [Google Scholar] [CrossRef]
- Thomas, C.M. Stereo-controlled ring-opening polymerization of cyclic esters: Synthesis of new polyester microstructures. Chem. Soc. Rev. 2010, 39, 165–173. [Google Scholar] [CrossRef]
- Gao, J.; Zhu, D.; Zhang, W.; Solan, G.A.; Ma, Y.; Sun, W.-H. Recent progress in the application of group 1, 2 & 13 metal complexes as catalysts for the ring opening polymerization of cyclic esters. Inorg. Chem. Front. 2019, 6, 2619–2652. [Google Scholar] [CrossRef]
- Santoro, O.; Zhang, X.; Redshaw, C. Synthesis of biodegradable polymers: A review on the use of Schiff-base metal complexes as catalysts for the Ring Opening Polymerization (ROP) of cyclic esters. Catalysts 2020, 10, 800. [Google Scholar] [CrossRef]
- Wu, L.-J.; Lee, W.; Ganta, P.K.; Chang, Y.-L.; Chang, Y.-C.; Chen, H.-Y. Multinuclear metal catalysts in ring-opening polymerization of ε caprolactone and lactide: Cooperative and electronic effects between metal centers. Coord. Chem. Rev. 2023, 475, 214847. [Google Scholar] [CrossRef]
- D’Auria, I.; D’Alterio, M.C.; Tedesco, C.; Pellecchia, C. Tailor-made block copolymers of L-, D- and rac-lactides and e-caprolactone via one-pot sequential ring opening polymerization by pyridylamidozinc(II) catalysts. RSC Adv. 2019, 9, 32771–32779. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, J.; Sarker, A.; Panda, T.K. Alkali and alkali earth metal complexes as versatile catalysts for ring-opening polymerization of cyclic esters. Chem. Rec. 2021, 21, 1898–1911. [Google Scholar] [CrossRef]
- Hashmi, O.H.; Capet, F.; Visseaux, M.; Champouret, Y. Homoleptic and heteroleptic substituted amidomethylpyridine iron complexes: Synthesis, structure and polymerization of rac-lactide. Eur. J. Inorg. Chem. 2022, 2022, e2022000073. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, X.; Zhang, W.; Sun, W.-H. Progress of ring-opening polymerization of cyclic esters by iron compounds. Organometallics 2023, 42, 1680–1692. [Google Scholar] [CrossRef]
- Gravina, G.; Pierri, G.; Pellecchia, C. New highly acive Fe(II) pyridylamido catalyst for the ring opening polymerization and copolymerization of cyclic esters. Mol. Catal. 2024, 555, 113891. [Google Scholar] [CrossRef]
- Iwasa, N.; Fujiki, M.; Nomura, K. Ring-opening polymerization of various cyclic esters by Al complex catalysts containing a series of phenoxy-imine ligands: Effect of the imino substituents for the catalytic activity. J. Mol. Catal. A Chem. 2008, 292, 67–75. [Google Scholar] [CrossRef]
- Iwasa, N.; Katao, S.; Liu, J.; Fujiki, M.; Furukawa, Y.; Nomura, K. Notable effect of fluoro substituents in the imino group in ring-opening polymerization of ε-caprolactone by Al complexes containing phenoxyimine ligands. Organometallics 2009, 28, 2179–2187. [Google Scholar] [CrossRef]
- Liu, J.; Iwasa, N.; Nomura, K. Synthesis of Al complexes containing phenoxy-imine ligands and their use as the catalyst precursors for efficient living ring-opening polymerisation of ε-caprolactone. Dalton Trans. 2008, 3978–3988. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, K.-Q.; Elsegood, M.R.J.; Prior, T.J.; Sun, X.; Mo, S.; Redshaw, C. Organoaluminium complexes of o-,m-,p-anisidines: Synthesis, structural studies and ROP of ε-caprolactone. Cat. Sci. Tech. 2014, 4, 3025–3031. [Google Scholar] [CrossRef]
- Fuoco, T.; Pappalardo, D. Alkyl aluminum complexes bearing salicylaldiminato ligands: Initiators in the ring-opening polymerization of cyclic esters. Catalysts 2017, 7, 64. [Google Scholar] [CrossRef]
- Wang, K.; Zhao, K.-Q.; Mo, S.; Al-Khafaji, Y.; Prior, T.J.; Elsegood, M.R.J.; Redshaw, C. Organoaluminium complexes derived from Anilines or Schiff bases for ring opening polymerization of ε-caprolactone, δ-valerolactone and rac-lactide. Eur. J. Inorg. Chem. 2017, 2017, 1951–1965. [Google Scholar] [CrossRef]
- García-Valle, F.M.; Cuenca, T.; Mosquera, M.E.G.; Millone, S.; Cano, J. Ring-opening polymerization (ROP) of cyclic esters by versatile aluminum diphenoxyimine complexes: From polylactide to random copolymers. Eur. Polym. J. 2020, 125, 109527. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhang, W.; Han, M.; Wang, X.; Solan, G.A.; Wang, R.; Ma, Y.; Sun, W.-H. Phenoxy-imine/-amide aluminum complexes with pendant or coordinated pyridine moieties: Solvent effects on structural type and catalytic capability for the ROP of cyclic esters. Polymer 2022, 242, 124602. [Google Scholar] [CrossRef]
- Sagar, S.; Nath, P.; Bano, K.; Karmakar, H.; Sharma, J.; Sarkar, A.; Panda, T.K. Binunclear and mononuclear aluminum complexes as quick and controlled initiators of well-orderd ROP of cyclic esters. ChemCatChem 2024, 16, e202300972. [Google Scholar] [CrossRef]
- Sumrit, P.; Kamavichanurat, S.; Joopor, W.; Wattanathana, W.; Nakornkhet, C.; Hormnirun, P. Aluminium complexes of phenoxy-azo ligands in the catalysis of rac-lactide polymerisation. Dalton Trans. 2024, 53, 13854–13870. [Google Scholar] [CrossRef] [PubMed]
- García-Lόpez, J.D.; García-Álvarez, A.-C.; Hernández-Balderas, U.; Gallardo-Garibay, A.; Jancik, V.; Martínez-Otero, D.; Moya-Cabrera, M. Ligand-directed assembly of multimetallic aluminum complexes: Synthesis, structure and ROP catalysis. Eur. J. Inorg. Chem. 2024, 27, e202400466. [Google Scholar] [CrossRef]
- Annunziata, L.; Pragliola, S.; Pappalardo, D.; Tedesco, C.; Pellecchia, C. New (anilidomethyl)pyridine Titanium(IV) and Zirconium(IV) catalyst precursors for the Highly Chemo- and Stereoselective cis-1,4-polymerization of 1,3-buadiene. Macromolecules 2011, 44, 1934–1941. [Google Scholar] [CrossRef]
- Zhang, S.; Katao, S.; Sun, W.-H.; Nomura, K. Synthesis of (arylimido)vanadium(V) complexes containing (2-anilidomethyl)pyridine ligands and their use as the catalyst precursors for olefin polymerization. Organometallics 2009, 28, 5925–5933. [Google Scholar] [CrossRef]
- Zhang, S.; Nomura, K. Highly efficient dimerization of ethylene by (imido)vanadium complexes containing (2-anilidomethyl)pyridine ligand: Notable ligand effect toward activity and selectivity. J. Am. Chem. Soc. 2010, 132, 4960–4965. [Google Scholar] [CrossRef]
- Nomura, K.; Mitsudome, T.; Igarashi, A.; Nagai, G.; Tsutsumi, K.; Ina, T.; Omiya, T.; Takaya, H.; Yamazoe, S. Synthesis of (Adamantylimido)vanadium(V) Dimethyl Complex Containing (2-Anilidomethyl)pyridine Ligand and Selected Reactions: Exploring the Oxidation State of the Catalytically Active Species in Ethylene Dimerization. Organometallics 2017, 36, 530–542. [Google Scholar] [CrossRef]
- Kuboki, M.; Nomura, K. (Arylimido)niobium(V) Complexes Containing 2-Pyridylmethylanilido Ligand as Catalyst Precursors for Ethylene Dimerization That Proceeds via Cationic Nb(V) Species. Organometallics 2019, 38, 1544–1559. [Google Scholar] [CrossRef]
- Armitage, A.P.; Boyron, O.; Champouret, Y.D.M.; Patel, M.; Singh, K.; Solan, G.A. Dimethyl-Aluminium Complexes Bearing Naphthyl-Substituted Pyridine-Alkylamides as Pro-Initiators for the Efficient ROP of ε-Caprolactone. Catalysts 2015, 5, 1425–1444. [Google Scholar] [CrossRef]
- Nienkemper, K.; Kehr, G.; Kehr, S.; Fröhlich, R.; Erker, G. (Amidomethyl)pyridine zirconium and hafnium complexes: Synthesis and structural characterization. J. Organomet. Chem. 2008, 693, 1572–1589. [Google Scholar] [CrossRef]
- Knijnenburg, Q.; Smits, J.M.M.; Budzelaar, P.H.M. Reaction of the Diimine Pyridine Ligand with Aluminum Alkyls: An Unexpectedly Complex Reaction. Organometallics 2006, 25, 1036–1046. [Google Scholar] [CrossRef]
- Davies, C.J.; Gregory, A.; Griffith, P.; Perkins, T.; Singh, K.; Solan, G.A. Use of Suzuki cross-coupling as a route to 2-phenoxy-6-iminopyridines and chiral 2-phenoxy-6-(methanamino) pyridines. Tetrahedron 2008, 64, 9857–9864. [Google Scholar] [CrossRef]
- Wei, Y.; Wang, S.; Zhou, S.; Feng, Z.; Guo, L.; Zhu, X.; Mu, X.; Yao, F. Aluminum Alkyl Complexes Supported by Bidentate N,N Ligands: Synthesis, Structure, and Catalytic Activity for Guanylation of Amines. Organometallics 2015, 34, 1882–1889. [Google Scholar] [CrossRef]
- Liu, R.; Yang, S.; Ding, Y.; Xia, D. Study on the effect of substituents on the structure, volatility, and fluorescence of N-(Alkyl or TMS)-2-pyridinamine diethyl aluminum complexes. J. Organomet. Chem. 2021, 933, 121646. [Google Scholar] [CrossRef]
- Addison, A.W.; Rao, T.N.; Reedijk, J.; van Rijn, J.; Verschoor, G.C. Synthesis, structure, and spectroscopic properties of copper(II) compounds containing nitrogen–sulphur donor ligands; the crystal and molecular structure of aqua[1,7-bis(N-methylbenzimidazol-2′-yl)-2,6-dithiaheptane]copper(II) perchlorate. J. Chem. Soc. Dalton Trans. 1984, 1349–1356. [Google Scholar] [CrossRef]
- Huang, C.-H.; Wang, F.-C.; Ko, B.-T.; Yu, T.-L.; Lin, C.-C. Ring-Opening Polymerization of ε-Caprolactone and L-Lactide Using Aluminum Thiolates as Initiator. Macromolecules 2001, 34, 356–361. [Google Scholar] [CrossRef]
- Save, M.; Schappacher, M.; Soum, A. Controlled Ring-Opening Polymerization of Lactones and Lactides Initiated by Lanthanum Isopropoxide, 1. General Aspects and Kinetics. Macromol. Chem. Phys. 2002, 203, 889–899. [Google Scholar] [CrossRef]
- Dove, A.P. Organic Catalysis for Ring Opening Polymerization. ACS Macro Lett. 2012, 1, 1409–1412. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT–Integrated space-group and crystal structure determination. Acta Cryst. 2015, A71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. 2015, C71, 3–8. [Google Scholar] [CrossRef]



| Entry | Cat. (μmol) | Temp. (°C) | Time (min) | Conv. b (%) | Mn(GPC) c ×10−4 | Mn(calc) d ×10−4 | Mw/Mn c | N e (μmol) |
|---|---|---|---|---|---|---|---|---|
| 1 | 1 (20) | 50 | 10 | 14 | 1.52 | 1.88 | 1.17 | 14.9 |
| 2 | 1 (20) | 50 | 20 | 25 | 1.80 | 3.30 | 1.20 | 13.2 |
| 3 | 1 (20) | 50 | 30 | 34 | 1.50 | 5.26 | 1.30 | 13.2 |
| 4 | 1 (20) | 50 | 40 | 48 | 2.98 | 6.28 | 1.31 | 15.6 |
| 5 | 1 (20) | 80 | 30 | 98 | 9.51 | 12.4 | 1.23 | 16.1 |
| 6 | 2 (20) | 50 | 20 | 5.9 | 0.43 | 1.15 | 1.33 | 7.94 |
| 7 | 2 (20) | 50 | 30 | 11 | 0.61 | 1.52 | 1.25 | 10.6 |
| 8 | 2 (20) | 50 | 40 | 14 | 1.23 | 2.06 | 1.20 | 11.4 |
| 9 | 2 (20) | 50 | 60 | 17 | 1.11 | 2.46 | 1.27 | 13.8 |
| 10 | 2 (20) | 50 | 90 | 28 | 2.27 | 3.51 | 1.17 | 16.3 |
| 11 | 2 (20) | 80 | 30 | 77 | 3.66 | 8.98 | 1.15 | 17.5 |
| 12 | 2 (20) | 80 | 60 | >99 | 6.72 | 11.0 | 1.16 | 18.4 |
| 13 | 3 (20) | 50 | 10 | 0.89 | 0.05 | trace | - | - |
| 14 | 3 (20) | 50 | 20 | 2.5 | 0.13 | trace | - | - |
| 15 | 3 (20) | 50 | 30 | 5.8 | 0.31 | 0.49 | 1.05 | 24.3 |
| 16 | 3 (20) | 50 | 40 | 11 | 0.60 | 1.25 | 1.04 | 17.4 |
| 17 | 3 (20) | 50 | 50 | 18 | 0.95 | 1.83 | 1.05 | 20.1 |
| 18 | 3 (20) | 50 | 60 | 25 | 1.39 | 2.77 | 1.06 | 18.6 |
| 19 | 3 (20) | 50 | 90 | 51 | 2.98 | 5.62 | 1.05 | 18.6 |
| 20 | 3 (20) | 80 | 30 | 81 | 3.46 | 7.81 | 1.08 | 21.1 |
| 21 | 3 (20) | 80 | 60 | 98 | 4.45 | 9.93 | 1.16 | 20.1 |
| 22 | L1H (40) | 80 | 60 | trace | - | - | - | - |
| 23 | L2H (40) | 80 | 60 | trace | - | - | - | - |
| Run a | Cat. (μmol) | Temp. (°C) | Time (min) | Conv. b (%) | Mn(GPC) c ×10−4 | Mn(calc) d ×10−4 | Mw/Mn c | N e (μmol) |
|---|---|---|---|---|---|---|---|---|
| 24 | 1 (20) | 50 | 60 | 22 | 1.23 | 4.94 | 1.05 | 20.3 |
| 25 | 1 (40) | 50 | 10 | 59 | 1.60 | 2.40 | 1.12 | 41.9 |
| 26 | 1 (40) | 50 | 30 | 88 | 2.46 | 2.48 | 1.13 | 40.6 |
| 27 | 1 (40) | 50 | 50 | 93 | 2.50 | 2.37 | 1.2 | 42.4 |
| 28 | 1 (40) | 50 | 60 | 93 | 2.52 | 2.39 | 1.2 | 42 |
| 29 | 2 (40) | 50 | 30 | 83 | 2.43 | 2.53 | 1.48 | 39.7 |
| 30 | 2 (40) | 50 | 60 | 92 | 3.00 | 2.88 | 1.4 | 34.9 |
| 31 | 3 (40) | 50 | 30 | 62 | 0.91 | 1.47 | 1.11 | 68.4 |
| 32 | 3 (40) | 50 | 60 | 91 | 1.72 | 1.90 | 1.12 | 52.9 |
| Compound | 1 | 2 | 3 |
|---|---|---|---|
| Formula weight | 268.33 | 296.38 | 464.57 |
| Crystal system | Orthorhombic | Orthorhombic | Monoclinic |
| Space group | Pbca | Pnma | P21/c |
| Unit cell dimensions | |||
| a (Å) | 14.8183(2) | 16.71447(18) | 15.36793(12) |
| b (Å) | 12.9284(2) | 11.79057(15) | 8.16407(6) |
| c (Å) | 32.4960(5) | 8.71683(10) | 21.64688(17) |
| β (º) | 90 | 90 | 110.0738(9) |
| V (Å3) | 6225.48(16) | 1717.85(3) | 2550.93(4) |
| Z | 16 | 4 | 4 |
| Temperature (K) | 93(2) | 100(2) | 100(2) |
| Wavelength (Å) | 1.54184 | 0.71073 | 1.54178 |
| Calculated density (g.cm−3) | 1.145 | 1.146 | 1.210 |
| Absorption coefficient (mm−1) | 1.03 | 0.11 | 0.87 |
| Transmission factors (min./max.) | 0.709 and 1.000 | 0.928 and 1.000 | 0.808 and 1.000 |
| Crystal size (mm3) | 0.27 × 0.09 × 0.06 | 0.20 × 0.08 × 0.07 | 0.22 × 0.20 × 0.05 |
| θ(max) (°) | 73.7 | 36.0 | 73.5 |
| Reflections measured | 38,334 | 69,199 | 84,510 |
| Unique reflections | 6191 | 4115 | 4918 |
| Rint | 0.074 | 0.024 | 0.024 |
| Reflections with F2 > 2σ(F2) | 4632 | 3717 | 4850 |
| Number of parameters | 352 | 115 | 313 |
| R1 [F2 > 2σ(F2)] | 0.068 | 0.034 | 0.034 |
| wR2 (all data) | 0.136 | 0.102 | 0.087 |
| GOOF, S | 1.03 | 1.05 | 1.05 |
| Largest difference peak and hole (e Å−3) | 0.34 and −0.30 | 0.47 and −0.23 | 0.26 and −0.24 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sato, S.; Motuzis, I.; Elsegood, M.R.J.; Nomura, K.; Redshaw, C. Bi-Dentate Pyridyl Amine-Derived Complexes of Aluminium: Synthesis, Structure and ROP Capability. Catalysts 2025, 15, 1119. https://doi.org/10.3390/catal15121119
Sato S, Motuzis I, Elsegood MRJ, Nomura K, Redshaw C. Bi-Dentate Pyridyl Amine-Derived Complexes of Aluminium: Synthesis, Structure and ROP Capability. Catalysts. 2025; 15(12):1119. https://doi.org/10.3390/catal15121119
Chicago/Turabian StyleSato, Shunsuke, Ignas Motuzis, Mark R. J. Elsegood, Kotohiro Nomura, and Carl Redshaw. 2025. "Bi-Dentate Pyridyl Amine-Derived Complexes of Aluminium: Synthesis, Structure and ROP Capability" Catalysts 15, no. 12: 1119. https://doi.org/10.3390/catal15121119
APA StyleSato, S., Motuzis, I., Elsegood, M. R. J., Nomura, K., & Redshaw, C. (2025). Bi-Dentate Pyridyl Amine-Derived Complexes of Aluminium: Synthesis, Structure and ROP Capability. Catalysts, 15(12), 1119. https://doi.org/10.3390/catal15121119








