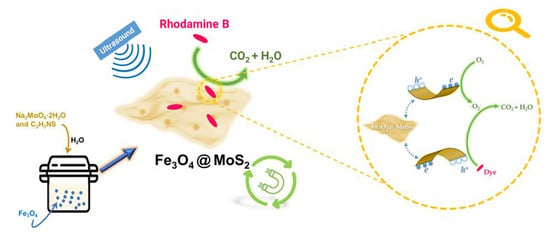
Separable Magnetic Fe3O4@MoS2 Composite for Adsorption and Piezo-Catalytic Degradation of Dye
Abstract
:1. Introduction
2. Results and Discussion
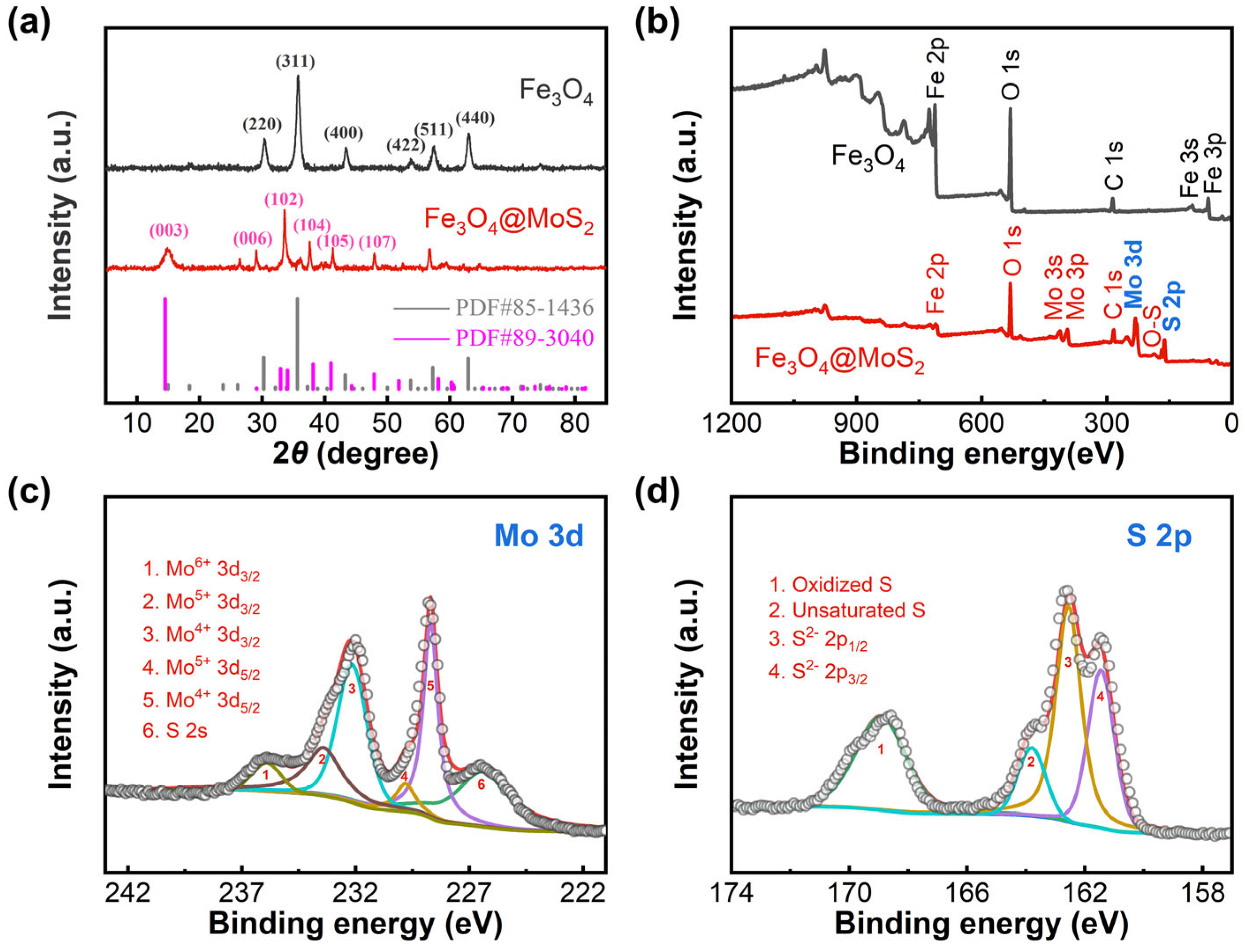
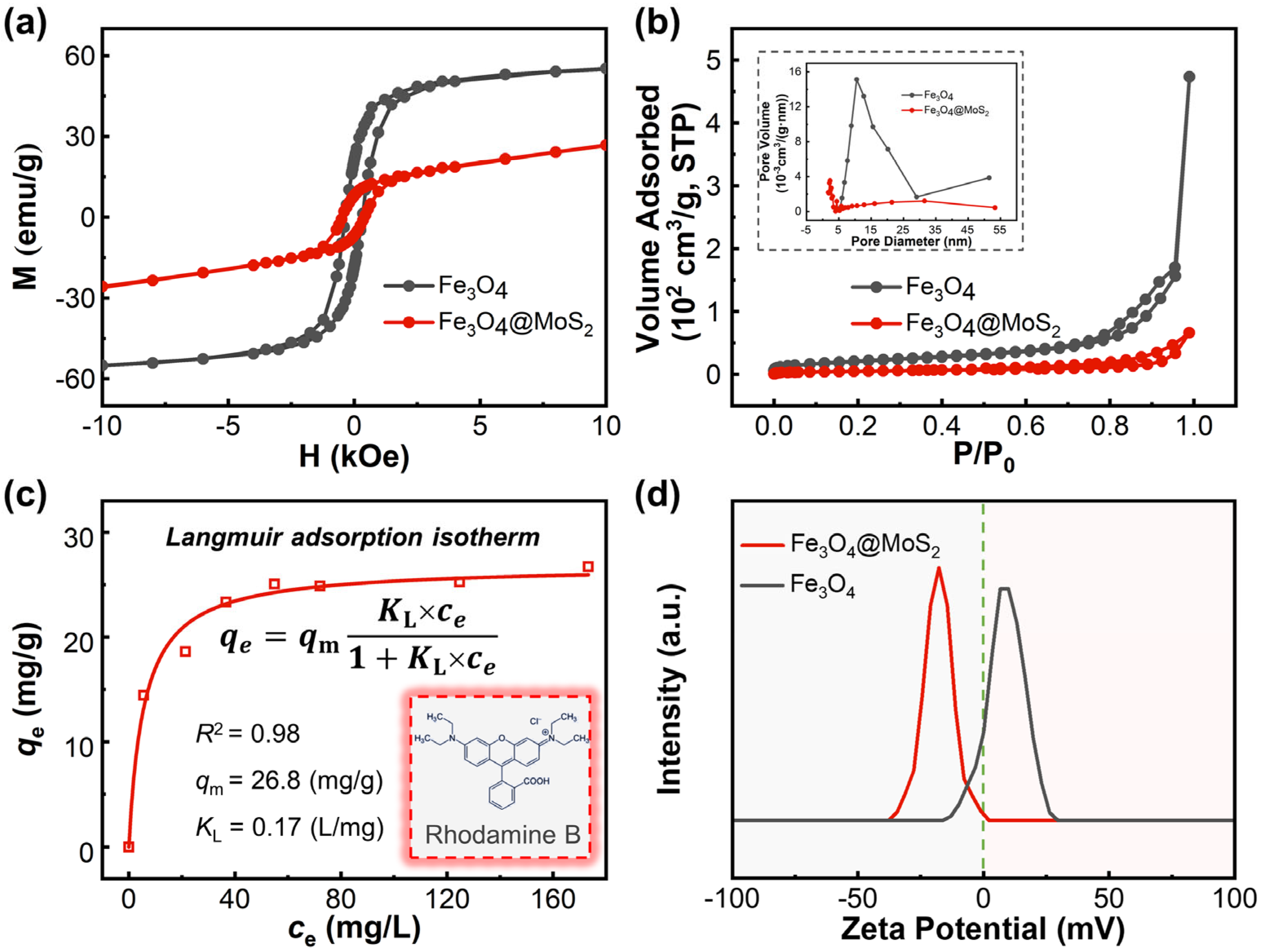
2.1. Structure and Composition of Materials
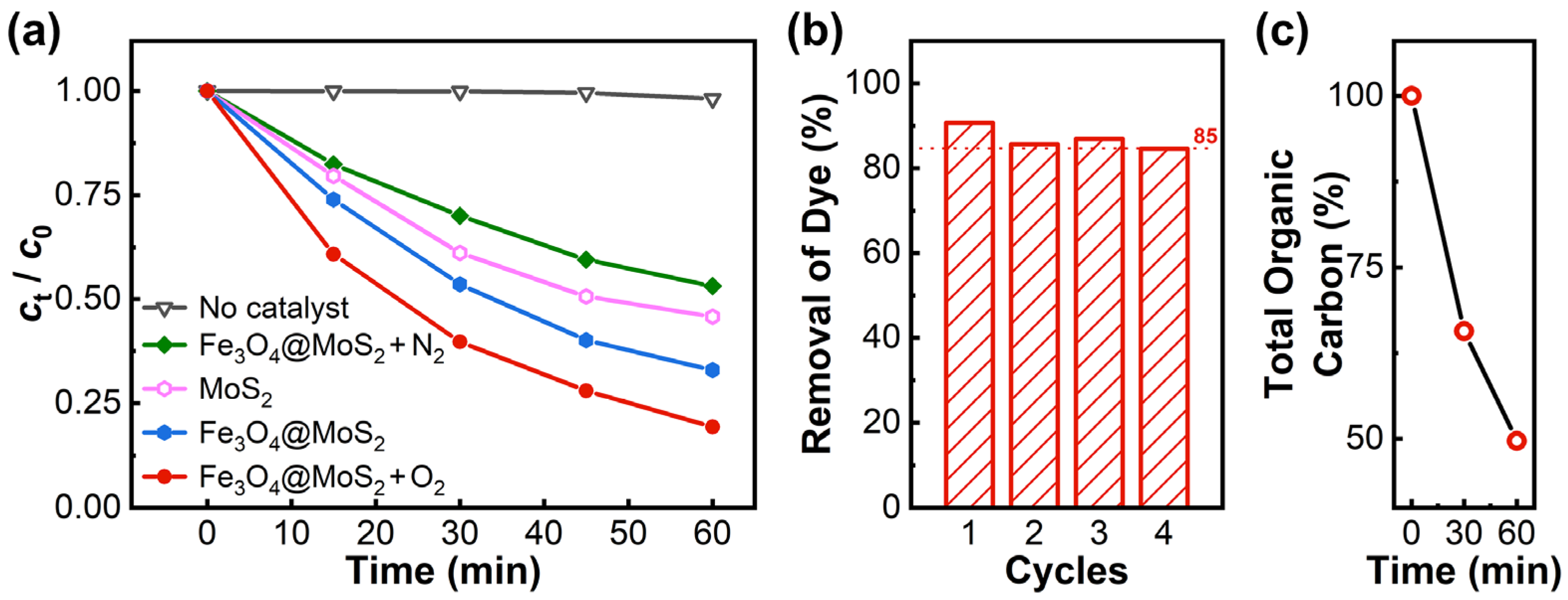
2.2. Adsorption and Piezo-Catalytic Degradation of Rhodamine B over Materials
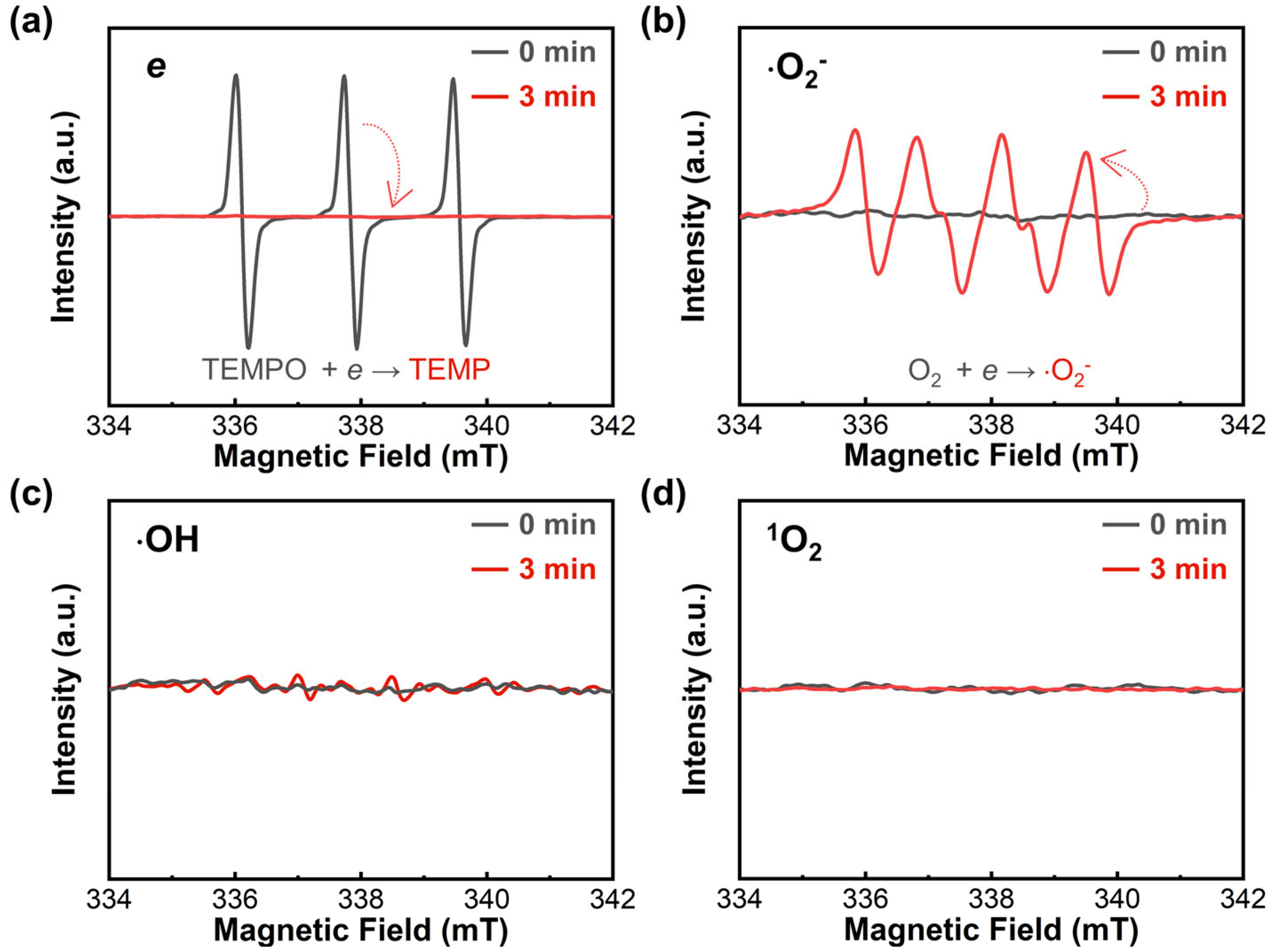
2.3. Mechanism of Piezo-Catalytic Degradation
3. Materials and Methods
3.1. Catalyst Preparation
3.2. Charaterizations
3.3. Measurement of Adsorption and Piezo-Catalytic Activity
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ye, A.; Fan, W.; Zhang, Q.; Deng, W.; Wang, Y. CdS–graphene and CdS–CNT nanocomposites as visible-light photocatalysts for hydrogen evolution and organic dye degradation. Catal. Sci. Technol. 2012, 2, 969–978. [Google Scholar] [CrossRef]
- Curri, M.; Comparelli, R.; Cozzoli, P.; Mascolo, G.; Agostiano, A. Colloidal oxide nanoparticles for the photocatalytic degradation of organic dye. Mater. Sci. Eng. C 2003, 23, 285–289. [Google Scholar] [CrossRef]
- Xu, M.; Wang, Z.; Wang, F.; Hong, P.; Wang, C. Fabrication of cerium doped Ti/nanoTiO2/PbO2 electrode with improved electrocatalytic activity and its application in organic degradation. Electrochim. Acta 2016, 201, 240–250. [Google Scholar] [CrossRef]
- Ma, H.; Zhuo, Q.; Wang, B. Electro-catalytic degradation of methylene blue wastewater assisted by Fe2O3-modified kaolin. Chem. Eng. J. 2009, 155, 248–253. [Google Scholar] [CrossRef]
- Chen, C.C.; Liao, H.J.; Cheng, C.Y.; Yen, C.Y.; Chung, Y.C. Biodegradation of crystal violet by pseudomonas putida. Biotechnol. Lett. 2007, 29, 391–396. [Google Scholar] [CrossRef]
- Rajaguru, P.; Kalaiselvi, K.; Palanivel, M.; Subburam, V. Biodegradation of azo dyes in a sequential anaerobic-aerobic system. Appl. Microbiol. Biotechnol. 2000, 54, 268–273. [Google Scholar] [CrossRef]
- Wang, Z.L. The new field of nanopiezotronics. Mater. Today 2007, 10, 20–28. [Google Scholar] [CrossRef]
- Wang, Z.L. Piezotronics and Piezo-Phototronics; Springer: Berlin/Heidelberg, Germany, 2012; Volume 24. [Google Scholar]
- Tu, S.; Huang, H.; Zhang, T.; Zhang, Y. Controllable synthesis of multi-responsive ferroelectric layered perovskite-like Bi4Ti3O12: Photocatalysis and piezoelectric-catalysis and mechanism insight. Appl. Catal. B 2017, 219, 550–562. [Google Scholar] [CrossRef]
- You, H.; Jia, Y.; Wu, Z.; Xu, X.; Qian, W. Strong piezo-electrochemical effect of multiferroic BiFeO3 square micro-sheets for mechanocatalysis. Electrochem. Commun. 2017, 79, 55–58. [Google Scholar] [CrossRef]
- Li, S.; Zhang, M.; Gao, Y.; Bao, B.; Wang, S. ZnO–Zn/CNT hybrid film as light-free nanocatalyst for degradation reaction. Nano Energy 2013, 2, 1329–1336. [Google Scholar] [CrossRef]
- Li, S.; Zhao, Z.; Zhao, Z.; Zhang, Z.; Li, X.; Zhang, Z. Recent Advances of Ferro-, Piezo-, and Pyroelectric Nanomaterials for Catalytic Applications. ACS Appl. Nano Mater. 2020, 3, 1063–1079. [Google Scholar] [CrossRef]
- Liang, Z.; Yan, C.; Rtimi, S.; Bandara, J. Piezoelectric materials for catalytic/photocatalytic removal of pollutants: Recent advances and outlook. Appl. Catal. B 2019, 241, 256–269. [Google Scholar] [CrossRef]
- You, H.; Wu, Z.; Zhang, L.; Ying, Y.; Liu, Y.; Fei, L.; Chen, X.; Jia, Y.; Wang, Y.; Wang, F.; et al. Harvesting the Vibration Energy of BiFeO3 Nanosheets for Hydrogen Evolution. Angew. Chem. Int. Ed. 2019, 58, 11779–11784. [Google Scholar] [CrossRef]
- Xu, Q.; Gao, X.; Zhao, S.; Liu, Y.N.; Zhang, D.; Zhou, K.; Khanbareh, H.; Chen, W.; Zhang, Y.; Bowen, C. Construction of Bio-Piezoelectric Platforms: From Structures and Synthesis to Applications. Adv. Mater. 2021, 33, 2008452. [Google Scholar] [CrossRef]
- Yu, T.; Wu, W.; Zhang, J.; Gao, C.; Yang, T.; Wang, X. Piezoelectricity catalyzed ROS generation of MoS2 only by aeration for wastewater purification. Res. Chem. Intermed. 2021, 47, 4763–4777. [Google Scholar] [CrossRef]
- Nie, G.; Yao, Y.; Duan, X.; Xiao, L.; Wang, S. Advances of piezoelectric nanomaterials for applications in advanced oxidation technologies. Curr. Opin. Chem. Eng. 2021, 33, 100693. [Google Scholar] [CrossRef]
- Bößl, F.; Tudela, I. Piezocatalysis: Can catalysts really dance? Curr. Opin. Green Sustain. 2021, 32, 100537. [Google Scholar] [CrossRef]
- Duerloo, K.-A.N.; Ong, M.T.; Reed, E.J. Intrinsic piezoelectricity in two-dimensional materials. J. Phys. Chem. Lett. 2012, 3, 2871–2876. [Google Scholar] [CrossRef]
- Zhu, H.; Wang, Y.; Xiao, J.; Liu, M.; Xiong, S. Observation of piezoelectricity in free-standing monolayer MoS2. Nat. Nanotechnol. 2015, 10, 151–155. [Google Scholar] [CrossRef]
- Wu, J.M.; Chang, W.E.; Chang, Y.T.; Chang, C.K. Piezo-catalytic effect on the enhancement of the ultra-high degradation activity in the dark by single-and few-layers MoS2 nanoflowers. Adv. Mater. 2016, 28, 3718–3725. [Google Scholar] [CrossRef]
- Su, Y.; Zhang, L.; Wang, W.; Li, X.; Zhang, Y.; Shao, D. Enhanced H2 evolution based on ultrasound-assisted piezo-catalysis of modified MoS2. J. Mater. Chem. A 2018, 6, 11909–11915. [Google Scholar] [CrossRef]
- Tu, S.C.; Guo, Y.X.; Zhang, Y.H.; Hu, C.; Zhang, T.R.; Ma, T.Y.; Huang, H.W. Piezocatalysis and Piezo-Photocatalysis: Catalysts Classification and Modification Strategy, Reaction Mechanism, and Practical Application. Adv. Funct. Mater. 2020, 30, 2005158. [Google Scholar] [CrossRef]
- Ding, X.; Xiao, D.; Ji, L.; Jin, D.; Dai, K.; Yang, Z.X.; Wang, S.Y.; Chen, H. Simple fabrication of Fe3O4/C/g-C3N4 two-dimensional composite by hydrothermal carbonization approach with enhanced photocatalytic performance under visible light. Catal. Sci. Technol. 2018, 8, 3484–3492. [Google Scholar] [CrossRef]
- Chen, Y.; Deng, X.M.; Wen, J.Y.; Zhu, J.; Bian, Z.F. Piezo-promoted the generation of reactive oxygen species and the photodegradation of organic pollutants. Appl. Catal. B 2019, 258, 118024. [Google Scholar] [CrossRef]
- Bystrzejewska-Piotrowska, G.; Golimowski, J.; Urban, P.L. Nanoparticles: Their potential toxicity, waste and environmental management. Waste Manag. 2009, 29, 2587–2595. [Google Scholar] [CrossRef]
- Ma, M.; Zhang, Q.; Yin, D.; Dou, J.; Zhang, H. Preparation of high-magnetization Fe3O4-NH2-Pd(0) catalyst for Heck reaction. Catal. Commun. 2012, 17, 168–172. [Google Scholar] [CrossRef]
- Xu, P.; Zeng, G.M.; Huang, D.L.; Feng, C.L.; Hu, S.; Zhao, M.H.; Lai, C.; Wei, Z.; Huang, C.; Xie, G.X.; et al. Use of iron oxide nanomaterials in wastewater treatment: A review. Sci. Total Environ. 2012, 424, 1–10. [Google Scholar] [CrossRef]
- Song, H.J.; You, S.; Jia, X.H.; Yang, J. MoS2 nanosheets decorated with magnetic Fe3O4 nanoparticles and their ultrafast adsorption for wastewater treatment. Ceram. Int. 2015, 41, 13896–13902. [Google Scholar] [CrossRef]
- Verwey, E.J.W. Electronic Conduction of Magnetite (Fe3O4) and its Transition Point at Low Temperatures. Nature 1939, 144, 327–328. [Google Scholar] [CrossRef]
- Oroujeni, M.; Kaboudin, B.; Xia, W.; Jönsson, P.; Ossipov, D.A. Conjugation of cyclodextrin to magnetic Fe3O4 nanoparticles via polydopamine coating for drug delivery. Prog. Org. Coat. 2018, 114, 154–161. [Google Scholar] [CrossRef]
- Ma, W.; Yao, B.; Zhang, W.; He, Y.; Yu, Y. A novel multi-flaw MoS2 nanosheet piezocatalyst with superhigh degradation efficiency for ciprofloxacin. Environ. Sci. Nano 2018, 5, 2876–2887. [Google Scholar] [CrossRef]
- Lu, J.; Zhou, Y.; Zhou, Y.B. Efficiently activate peroxymonosulfate by Fe3O4@MoS2 for rapid degradation of sulfonamides. Chem. Eng. J. 2021, 422, 130126. [Google Scholar] [CrossRef]
- Qi, K.Y.; Yuan, Z.M.; Hou, Y.; Zhao, R.C.; Zhang, B.W. Facile synthesis and improved Li-storage performance of Fe-doped MoS2/reduced graphene oxide as anode materials. Appl. Surf. Sci. 2019, 483, 688–695. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhou, L.; Zhou, Y.B.; Xia, M.Y.; Zhang, J.L. Z-scheme photo-Fenton system for efficiency synchronous oxidation of organic contaminants and reduction of metal ions. Appl. Catal. B 2020, 279, 119365. [Google Scholar] [CrossRef]
- Peng, X.D.; Xiong, C.; Lin, Y.K.; Zhao, C.; Zhao, T.S. Honeycomb-like hierarchical porous silicon composites with dual protection for ultrastable Li-ion battery anodes. SmartMat 2021, 1–12. [Google Scholar] [CrossRef]
- Tan, I.A.W.; Ahmad, A.L.; Hameed, B.H. Adsorption of basic dye on high-surface-area activated carbon prepared from coconut husk: Equilibrium, kinetic and thermodynamic studies. J. Hazard. Mater. 2008, 154, 337–346. [Google Scholar] [CrossRef]
- Xiao, Y.; Tian, G.; Li, W.; Xie, Y.; Jiang, B.; Tian, C.; Zhao, D.; Fu, H. Molecule Self-Assembly Synthesis of Porous Few-Layer Carbon Nitride for Highly Efficient Photoredox Catalysis. J. Am. Chem. Soc. 2019, 141, 2508–2515. [Google Scholar] [CrossRef]
- Wu, J.M.; Sun, Y.G.; Chang, W.E.; Lee, J.T. Piezoelectricity induced water splitting and formation of hydroxyl radical from active edge sites of MoS2 nanoflowers. Nano Energy 2018, 46, 372–382. [Google Scholar] [CrossRef]
- Zhou, W.; Yin, Z.; Du, Y.; Huang, X.; Zeng, Z. Synthesis of few-layer MoS2 nanosheet-coated TiO2 nanobelt heterostructures for enhanced photocatalytic activities. Small 2013, 9, 140–147. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, C.; Liu, W.; Li, H.; Yang, M.; Yang, Z. Separable Magnetic Fe3O4@MoS2 Composite for Adsorption and Piezo-Catalytic Degradation of Dye. Catalysts 2021, 11, 1403. https://doi.org/10.3390/catal11111403
Zhou C, Liu W, Li H, Yang M, Yang Z. Separable Magnetic Fe3O4@MoS2 Composite for Adsorption and Piezo-Catalytic Degradation of Dye. Catalysts. 2021; 11(11):1403. https://doi.org/10.3390/catal11111403
Chicago/Turabian StyleZhou, Chi, Wencheng Liu, Hanqing Li, Miao Yang, and Zixin Yang. 2021. "Separable Magnetic Fe3O4@MoS2 Composite for Adsorption and Piezo-Catalytic Degradation of Dye" Catalysts 11, no. 11: 1403. https://doi.org/10.3390/catal11111403