Improved Brønsted to Lewis (B/L) Ratio of Co- and Mo-Impregnated ZSM-5 Catalysts for Palm Oil Conversion to Hydrocarbon-Rich Biofuels
Abstract
:1. Introduction
2. Results and Discussion
2.1. Catalysts Characterizations
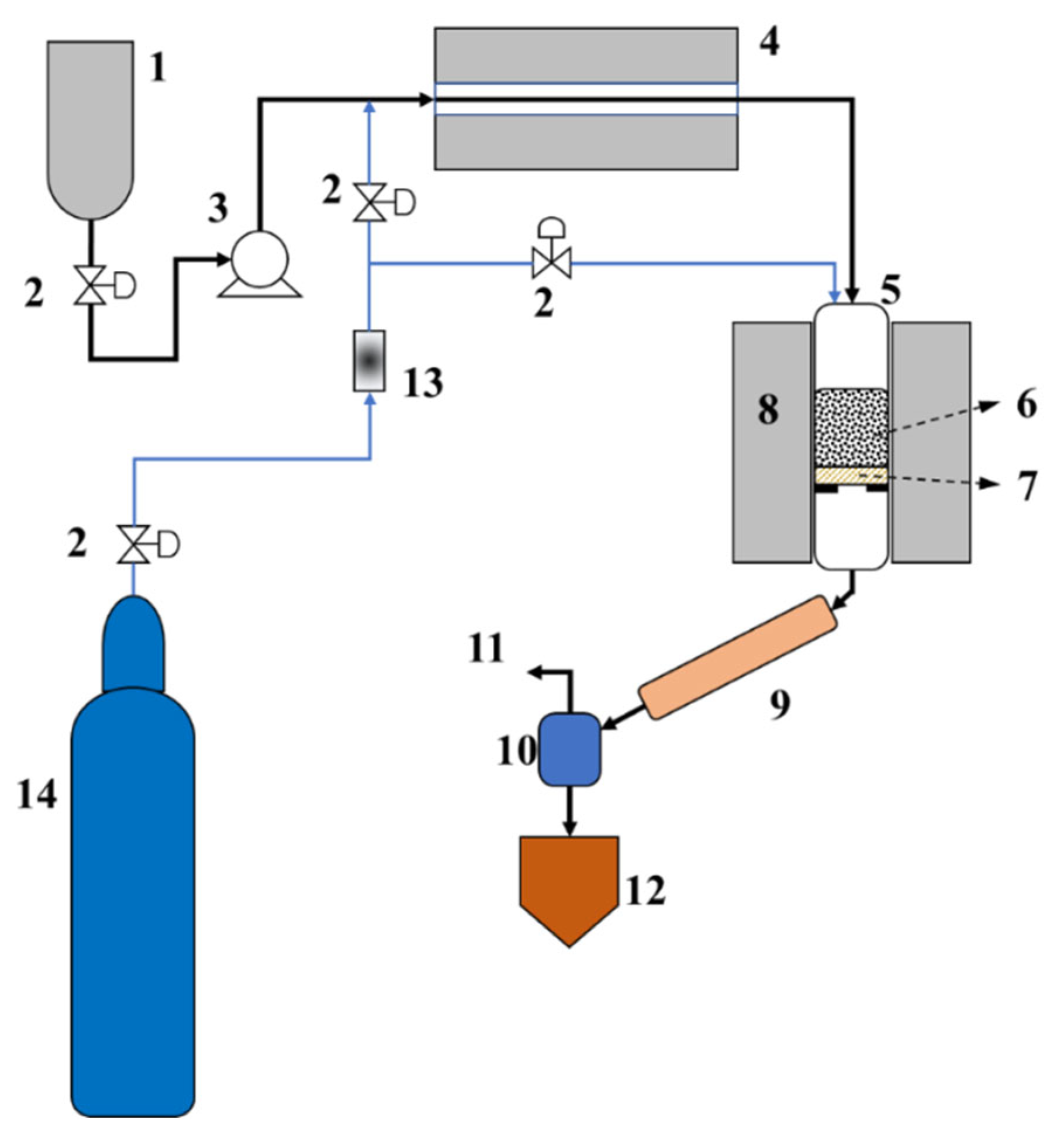
2.2. Catalytic Reaction Process Performance Test
2.3. Organic Liquid Product Distribution
2.4. Hydrocarbons Product Distributions
3. Materials and Methods
3.1. Materials of Research
3.2. Catalysts Preparation and Characterizations
3.3. Catalytic Cracking Test
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Musa, M.L.; Mat, R.; Abdullah, T.A.T. Catalytic Conversion of Residual Palm Oil in Spent Bleaching Earth (SBE) by HZSM-5 Zeolite based-Catalysts. Bull. Chem. React. Eng. Catal. 2018, 13, 456–465. [Google Scholar] [CrossRef] [Green Version]
- Istadi, I.; Riyanto, T.; Buchori, L.; Anggoro, D.D.; Pakpahan, A.W.S.; Pakpahan, A.J. Biofuels Production from Catalytic Cracking of Palm Oil Using Modified HY Zeolite Catalysts over A Continuous Fixed Bed Catalytic Reactor. Int. J. Renew. Energy Dev. 2021, 10, 149–156. [Google Scholar] [CrossRef]
- Ramya, G.; Sudhakar, R.; Joice, J.A.I.; Ramakrishnan, R.; Sivakumar, T. Liquid hydrocarbon fuels from jatropha oil through catalytic cracking technology using AlMCM-41/ZSM-5 composite catalysts. Appl. Catal. A Gen. 2012, 433–434, 170–178. [Google Scholar] [CrossRef]
- Riyanto, T.; Istadi, I.; Buchori, L.; Anggoro, D.D.; Dani Nandiyanto, A.B. Plasma-Assisted Catalytic Cracking as an Advanced Process for Vegetable Oils Conversion to Biofuels: A Mini Review. Ind. Eng. Chem. Res. 2020, 59, 17632–17652. [Google Scholar]
- Buzetzki, E.; Sidorová, K.; Cvengrošová, Z.; Kaszonyi, A.; Cvengroš, J. The influence of zeolite catalysts on the products of rapeseed oil cracking. Fuel Process. Technol. 2011, 92, 1623–1631. [Google Scholar] [CrossRef]
- Gurdeep Singh, H.K.; Yusup, S.; Quitain, A.T.; Abdullah, B.; Ameen, M.; Sasaki, M.; Kida, T.; Cheah, K.W. Biogasoline production from linoleic acid via catalytic cracking over nickel and copper-doped ZSM-5 catalysts. Environ. Res. 2020, 186, 109616. [Google Scholar] [CrossRef] [PubMed]
- Istadi, I.; Buchori, L.; Anggoro, D.D.; Riyanto, T.; Indriana, A.; Khotimah, C.; Setiawan, F.A.P. Effects of Ion Exchange Process on Catalyst Activity and Plasma-Assisted Reactor Toward Cracking of Palm Oil into Biofuels. Bull. Chem. React. Eng. Catal. 2019, 14, 459–467. [Google Scholar] [CrossRef] [Green Version]
- Ibarra, Á.; Hita, I.; Azkoiti, M.J.; Arandes, J.M.; Bilbao, J. Catalytic cracking of raw bio-oil under FCC unit conditions over different zeolite-based catalysts. J. Ind. Eng. Chem. 2019, 78, 372–382. [Google Scholar] [CrossRef]
- Hu, C.; Xiao, R.; Zhang, H. Ex-situ catalytic fast pyrolysis of biomass over HZSM-5 in a two-stage fluidized-bed/fixed-bed combination reactor. Bioresour. Technol. 2017, 243, 1133–1140. [Google Scholar] [CrossRef] [PubMed]
- Botas, J.A.; Serrano, D.P.; García, A.; de Vicente, J.; Ramos, R. Catalytic conversion of rapeseed oil into raw chemicals and fuels over Ni- and Mo-modified nanocrystalline ZSM-5 zeolite. Catal. Today 2012, 195, 59–70. [Google Scholar] [CrossRef]
- Haji Morni, N.A.; Yeung, C.M.; Tian, H.; Yang, Y.; Phusunti, N.; Abu Bakar, M.S.; Azad, A.K. Catalytic fast Co-Pyrolysis of sewage sludge−sawdust using mixed metal oxides modified with ZSM-5 catalysts on dual-catalysts for product upgrading. J. Energy Inst. 2021, 94, 387–397. [Google Scholar] [CrossRef]
- Ding, J.; Chen, P.; Fan, S.; Zhang, Z.; Han, L.; Zhao, G.; Liu, Y.; Lu, Y. Microfibrous-structured SS-fiber@meso-HZSM-5 catalyst for methanol-to-propylene: Steam-assisted crystallization synthesis and insight into the stability enhancement. ACS Sustain. Chem. Eng. 2017, 5, 1840–1853. [Google Scholar] [CrossRef]
- Huang, Z.; Zhang, J.; Li, P.; Xu, L.; Zhang, X.; Yuan, Y.; Xu, L. Tert-Butylation of naphthalene by tertiary butanol over HY zeolite and cerium-modified HY catalysts. Catal. Sci. Technol. 2017, 7, 4700–4709. [Google Scholar] [CrossRef]
- Azzi, H.; Rekkab-Hammoumraoui, I.; Chérif-Aouali, L.; Choukchou-Braham, A. Mesoporous Co3O4 as a new catalyst for allylic oxidation of cyclohexene. Bull. Chem. React. Eng. Catal. 2019, 14, 112–123. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Jin, R.; Qiao, Y.; Wu, Y.; Wang, X.; Wang, Y. Determination of Lead(II) using glassy carbon electrode modified with hexagonal Co3O4 microparticles. Int. J. Electrochem. Sci. 2018, 13, 10415–10426. [Google Scholar] [CrossRef]
- Al-Senani, G.M.; Deraz, N.M.; Abd-Elkader, O.H. Magnetic and Characterization Studies of CoO/Co3O4 Nanocomposite. Processes 2020, 8, 844. [Google Scholar] [CrossRef]
- Wang, L.; Li, M.C.; Zhang, G.H.; Xue, Z.L. Morphology evolution and quantitative analysis of β-MoO3 and α-MoO3. High Temp. Mater. Process. 2020, 39, 620–626. [Google Scholar] [CrossRef]
- Sen, S.K.; Dutta, S.; Khan, M.R.; Manir, M.S.; Dutta, S.; Al Mortuza, A.; Razia, S.; Hakim, M.A. Characterization and Antibacterial Activity Study of Hydrothermally Synthesized h-MoO3 Nanorods and α-MoO3 Nanoplates. Bionanoscience 2019, 9, 873–882. [Google Scholar] [CrossRef]
- Kheffache, O.; Lopez-Olmos, C.; Rodriguez-Ramos, I.; Cherifi, O. Clean 3,4-dihydropyrimidones synthesis via biginelli reaction over supported molybdenum: Structural and textural characteristic of αMoO3. Bull. Chem. React. Eng. Catal. 2020, 15, 698–713. [Google Scholar] [CrossRef]
- Al-Wadaani, F.; Omer, A.; Abboudi, M.; Oudghiri Hassani, H.; Rakass, S.; Messali, M.; Benaissa, M. High Catalytic Efficiency of Nanostructured β-CoMoO4 in the Reduction of the Ortho-, Meta- and Para-Nitrophenol Isomers. Molecules 2018, 23, 364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, W.; Wang, X.; Hu, Y.; Sun, L.; Gao, C.; Zhang, C.; Liu, H.; Duan, M. Hydrothermal Synthesized of CoMoO4 Microspheres as Excellent Electrode Material for Supercapacitor. Nanoscale Res. Lett. 2018, 13, 120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Araujo, C.P.B.; De Souza, C.P.; Maia, L.M.D.; Souto, M.V.M.; Barbosa, C.M. On the synthesis of molybdenum carbide with cobalt addition via gas-solid reactions in a CH4/H2 atmosphere. Brazilian J. Chem. Eng. 2016, 33, 577–588. [Google Scholar] [CrossRef] [Green Version]
- Trisunaryanti, W.; Triyono, T.; Ghoni, M.A.; Fatmawati, D.A.; Mahayuwati, P.N.; Suarsih, E. Hydrocracking of Calophyllum inophyllum Oil Employing Co and/or Mo Supported on γ-Al2O3 for Biofuel Production. Bull. Chem. React. Eng. Catal. 2020, 15, 743–751. [Google Scholar] [CrossRef]
- Yigezu, Z.D.; Muthukumar, K. Biofuel production by catalytic cracking of sunflower oil using vanadium pentoxide. J. Anal. Appl. Pyrolysis 2015, 112, 341–347. [Google Scholar] [CrossRef]
- Lowell, S.; Shields, J.E.; Thomas, M.A.; Thommes, M. Surface Area Analysis from the Langmuir and BET Theories. In Characterization of Porous Solids and Powders: Surface Area, Pore Size and Density. Particle Technology Series; Springer: Dordrecht, The Netherlands, 2004; pp. 58–81. ISBN 1205420207. [Google Scholar]
- Khabtou, S.; Chevreau, T.; Lavalley, J.C. Quantitative infrared study of the distinct acidic hydroxyl groups contained in modified Y zeolites. Microporous Mater. 1994, 3, 133–148. [Google Scholar] [CrossRef]
- Devassy, B.M.; Halligudi, S.B. Effect of calcination temperature on the catalytic activity of zirconia-supported heteropoly acids. J. Mol. Catal. A Chem. 2006, 253, 8–15. [Google Scholar] [CrossRef]
- Istadi, I.; Riyanto, T.; Khofiyanida, E.; Buchori, L.; Anggoro, D.D.; Sumantri, I.; Putro, B.H.S.; Firnanda, A.S. Low-oxygenated biofuels production from palm oil through hydrocracking process using the enhanced Spent RFCC catalysts. Bioresour. Technol. Reports 2021, 14, 100677. [Google Scholar] [CrossRef]
- Vieira, S.S.; Magriotis, Z.M.; Ribeiro, M.F.; Graça, I.; Fernandes, A.; Lopes, J.M.F.M.; Coelho, S.M.; Santos, N.A.V.; Saczk, A.A. Use of HZSM-5 modified with citric acid as acid heterogeneous catalyst for biodiesel production via esterification of oleic acid. Microporous Mesoporous Mater. 2015, 201, 160–168. [Google Scholar] [CrossRef]
- Stratiev, D.; Shishkova, I.; Ivanov, M.; Dinkov, R.; Georgiev, B.; Argirov, G.; Atanassova, V.; Vassilev, P.; Atanassov, K.; Yordanov, D.; et al. Role of Catalyst in Optimizing Fluid Catalytic Cracking Performance during Cracking of H-Oil-Derived Gas Oils. ACS Omega 2021, 6, 7626–7637. [Google Scholar] [CrossRef] [PubMed]
- Anggoro, D.D.; Buchori, L.; Silaen, G.C.; Utami, R.N. Preparation, characterization, and activation of Co-Mo/Y zeolite catalyst for coal tar conversion to liquid fuel. Bull. Chem. React. Eng. Catal. 2017, 12, 219–226. [Google Scholar] [CrossRef] [Green Version]
- Sadiq, M.; Sahibed-dine, A.; Baalala, M.; Nohair, K.; Abdennouri, M.; Bensitel, M.; Lamonier, C.; Leglise, J. Influence of acid-base properties of cobalt-molybdenum catalysts supported on magnesium orthophosphates in isomerization of 3,3-dimethylbut-1-ene. Arab. J. Chem. 2011, 4, 449–457. [Google Scholar] [CrossRef] [Green Version]
- Sridhar, A.; Rahman, M.; Infantes-Molina, A.; Wylie, B.J.; Borcik, C.G.; Khatib, S.J. Bimetallic Mo-Co/ZSM-5 and Mo-Ni/ZSM-5 catalysts for methane dehydroaromatization: A study of the effect of pretreatment and metal loadings on the catalytic behavior. Appl. Catal. A Gen. 2020, 589, 117247. [Google Scholar] [CrossRef]
- Yue, Y.; Fu, J.; Wang, C.; Yuan, P.; Bao, X.; Xie, Z.; Basset, J.M.; Zhu, H. Propane dehydrogenation catalyzed by single Lewis acid site in Sn-Beta zeolite. J. Catal. 2021, 395, 155–167. [Google Scholar] [CrossRef]
- Feng, R.; Bai, P.; Liu, S.; Zhang, P.; Liu, X.; Yan, Z.; Zhang, Z.; Gao, X. The application of mesoporous alumina with rich Brönsted acidic sites in FCC catalysts. Appl. Petrochemical Res. 2014, 4, 367–372. [Google Scholar] [CrossRef] [Green Version]
- Li, C.; Ma, J.; Xiao, Z.; Hector, S.B.; Liu, R.; Zuo, S.; Xie, X.; Zhang, A.; Wu, H.; Liu, Q. Catalytic cracking of Swida wilsoniana oil for hydrocarbon biofuel over Cu-modified ZSM-5 zeolite. Fuel 2018, 218, 59–66. [Google Scholar] [CrossRef]
- Wang, W.; Yang, Y.; Bao, J.; Luo, H. Characterization and catalytic properties of Ni–Mo–B amorphous catalysts for phenol hydrodeoxygenation. Catal. Commun. 2009, 11, 100–105. [Google Scholar] [CrossRef]
- Wang, W.; Yang, Y.; Luo, H.; Hu, T.; Liu, W. Amorphous Co–Mo–B catalyst with high activity for the hydrodeoxygenation of bio-oil. Catal. Commun. 2011, 12, 436–440. [Google Scholar] [CrossRef]
- Sahebdelfar, S.; Ravanchi, M.T. Deoxygenation of propionic acid: Thermodynamic equilibrium analysis of upgrading a bio-oil model compound. Renew. Energy 2017, 114, 1113–1122. [Google Scholar] [CrossRef]
- Simakova, I.; Rozmysłowicz, B.; Simakova, O.; Mäki-Arvela, P.; Simakov, A.; Murzin, D.Y. Catalytic deoxygenation of C18 fatty acids over mesoporous Pd/C catalyst for synthesis of biofuels. Top. Catal. 2011, 54, 460–466. [Google Scholar] [CrossRef]
- Simakova, I.; Simakova, O.; Mäki-Arvela, P.; Simakov, A.; Estrada, M.; Murzin, D.Y. Deoxygenation of palmitic and stearic acid over supported Pd catalysts: Effect of metal dispersion. Appl. Catal. A Gen. 2009, 355, 100–108. [Google Scholar] [CrossRef]
- Rane, N.; Kersbulck, M.; van Santen, R.A.; Hensen, E.J.M. Cracking of n-heptane over Brønsted acid sites and Lewis acid Ga sites in ZSM-5 zeolite. Microporous Mesoporous Mater. 2008, 110, 279–291. [Google Scholar] [CrossRef]
- Liu, C.; Deng, Y.; Pan, Y.; Gu, Y.; Qiao, B.; Gao, X. Effect of ZSM-5 on the aromatization performance in cracking catalyst. J. Mol. Catal. A Chem. 2004, 215, 195–199. [Google Scholar] [CrossRef]
- Kostyniuk, A.; Key, D.; Mdleleni, M. Effect of Fe-Mo promoters on HZSM-5 zeolite catalyst for 1-hexene aromatization. J. Saudi Chem. Soc. 2019, 23, 612–626. [Google Scholar] [CrossRef]

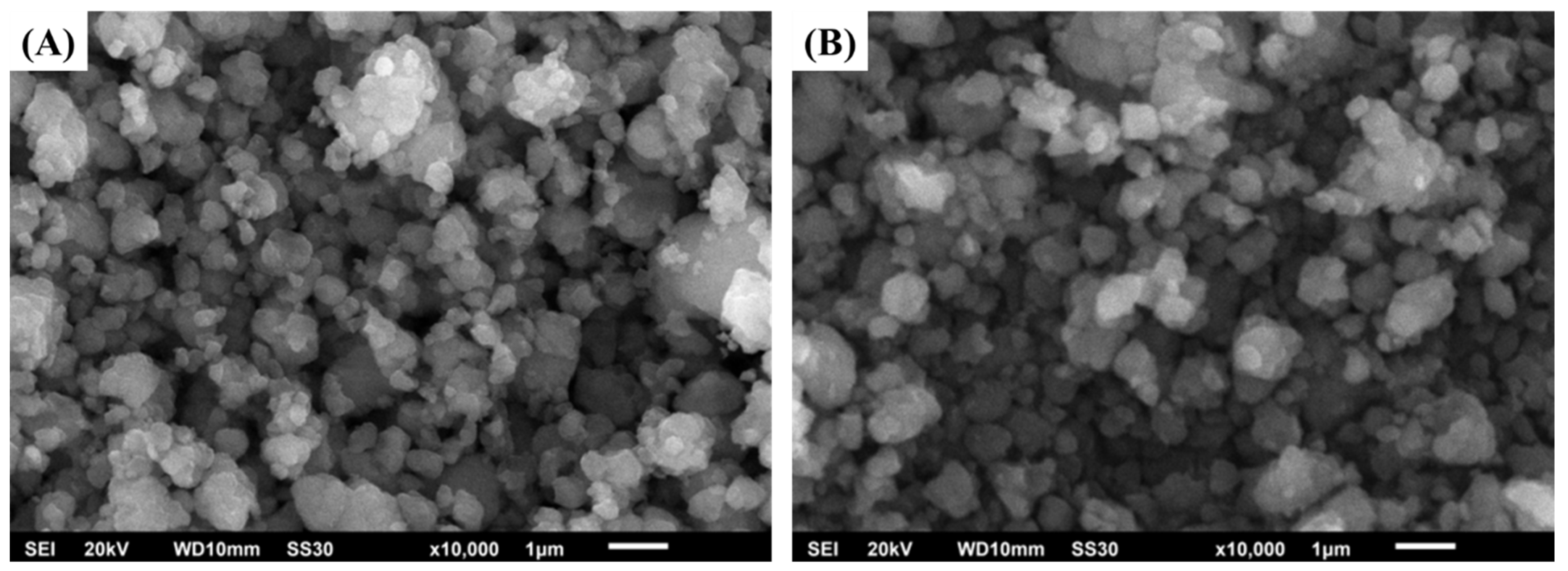
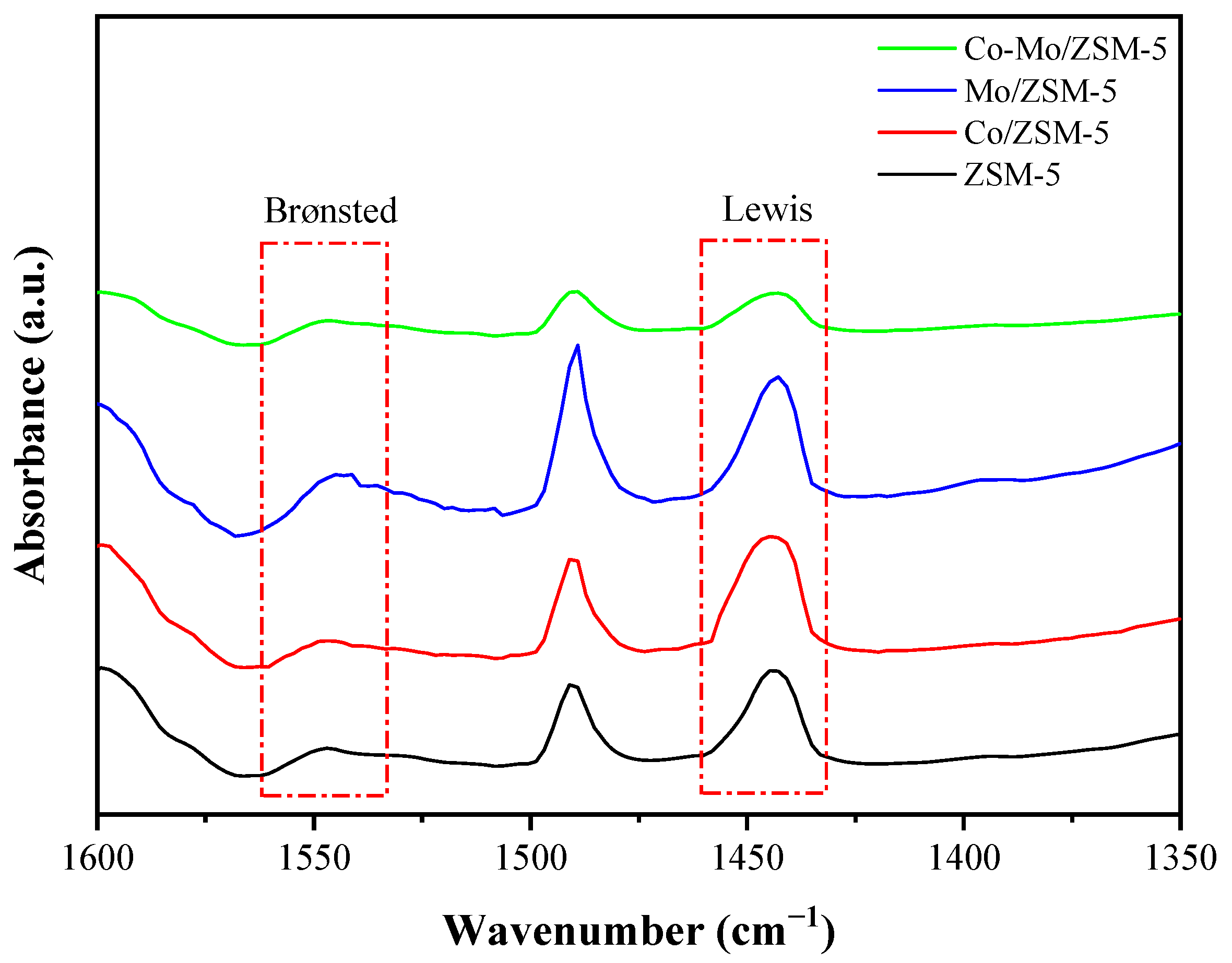
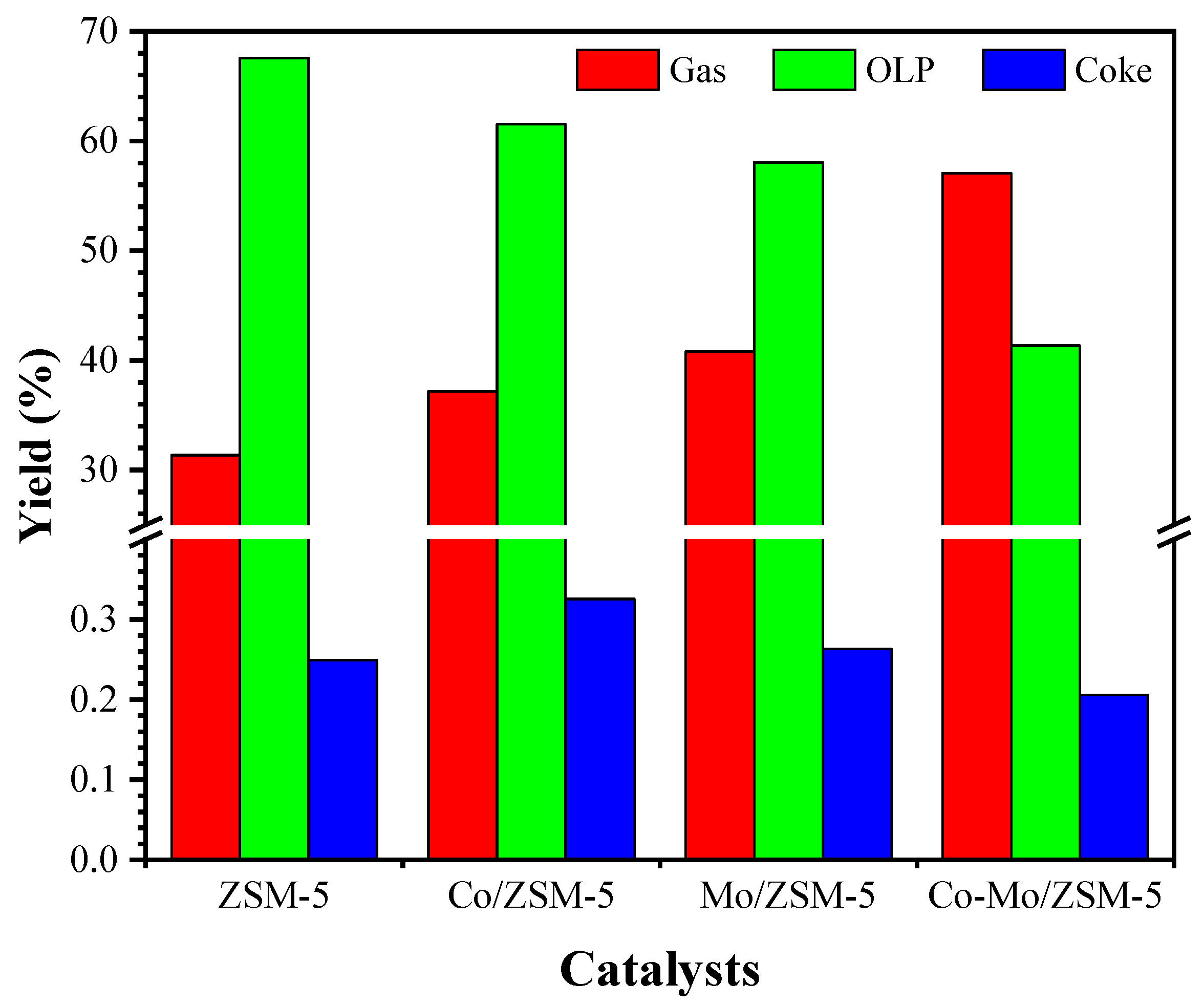

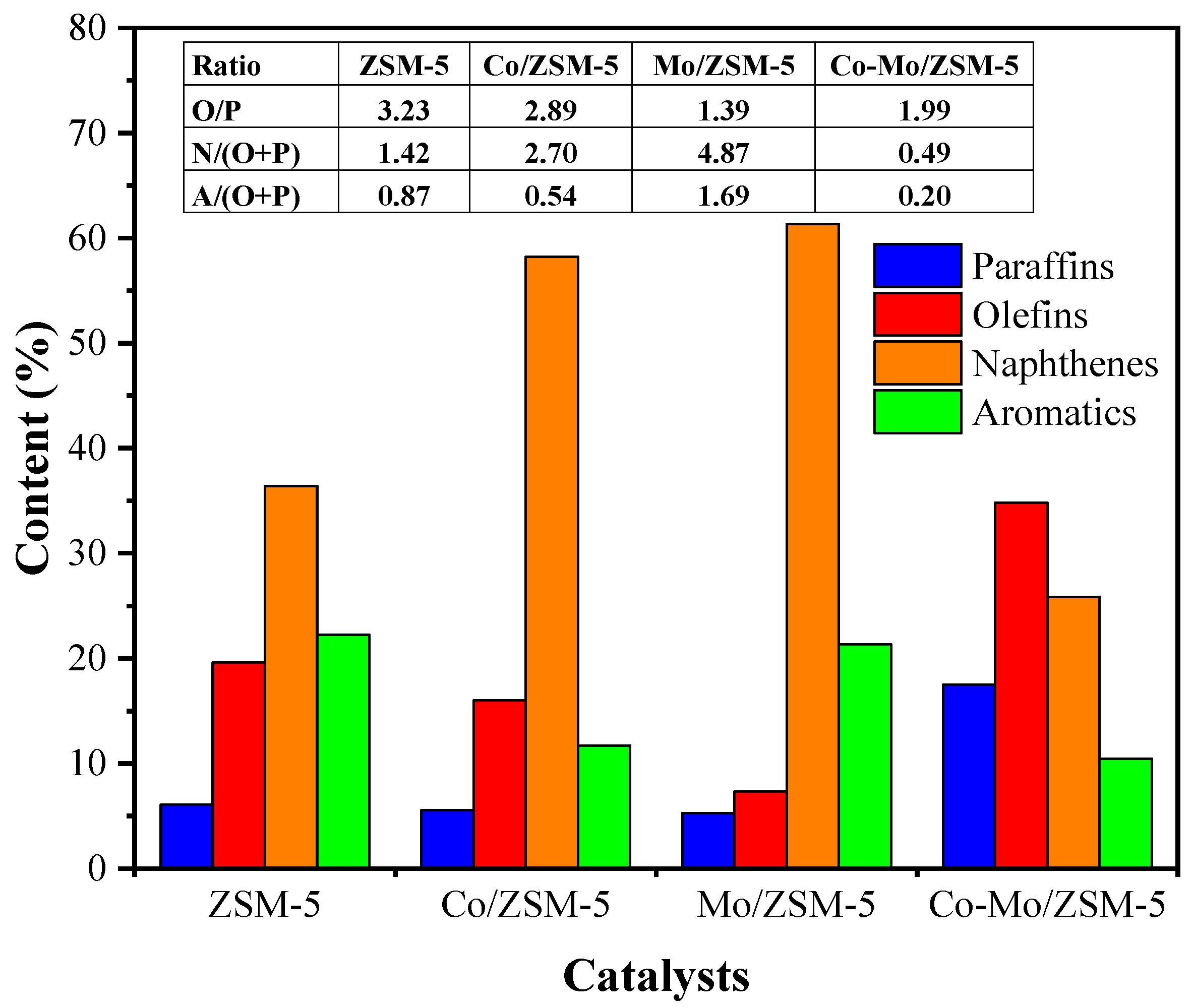
| Catalysts | Metal Content (%wt) | Surface Area (m2/g) | Acid Sites (µmol/g) | B/L Ratio ‡ | |||
|---|---|---|---|---|---|---|---|
| Co | Mo | Lewis | Brønsted | Total | |||
| ZSM-5 | - | - | 396.50 | 21.21 | 1.71 | 22.92 | 0.081 |
| Co/ZSM-5 | 4.85 | - | 360.46 | 41.93 | 1.46 | 43.39 | 0.035 |
| Mo/ZSM-5 | - | 4.98 | 331.07 | 22.68 | 1.81 | 24.49 | 0.080 |
| Co-Mo/ZSM-5 | 2.62 | 3.39 | 315.50 | 9.45 | 1.09 | 10.54 | 0.116 |
| Component | Composition (%) | |||
|---|---|---|---|---|
| ZSM-5 | Co/ZSM-5 | Mo/ZSM-5 | Co-Mo/ZSM-5 | |
| Hydrocarbons | 84.32 | 91.50 | 95.26 | 88.65 |
| Alcohols | 11.44 | 6.74 | 4.74 | 5.92 |
| Ketones | 1.81 | 0.97 | 0.00 | 3.38 |
| Esters | 2.43 | 0.78 | 0.00 | 2.05 |
| Components | Molecular Formula | Composition (%) |
|---|---|---|
| Palmitic acid (C16:0) | C16H32O2 | 43.30 |
| Oleic acid (C18:1) | C18H34O2 | 18.51 |
| 2-Monopalmitin | C19H38O4 | 3.25 |
| Z-13-Octadecenyl acetate | C20H38O2 | 2.03 |
| Oleic acid, 3-(oxtadecyloxy)propyl ester | C39H76O3 | 1.47 |
| 1-Nonadecene | C19H38 | 16.27 |
| 11-Hexacosyne | C26H50 | 5.80 |
| 9-Tricosene | C23H46 | 5.68 |
| 2-Pentadecanone | C15H30O | 1.34 |
| (Z,Z)-3,9-cis-6,7-epoxy-nonadecadiene | C19H34O | 1.18 |
| 1-Heptadecanol | C17H36O | 1.17 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Riyanto, T.; Istadi, I.; Jongsomjit, B.; Anggoro, D.D.; Pratama, A.A.; Faris, M.A.A. Improved Brønsted to Lewis (B/L) Ratio of Co- and Mo-Impregnated ZSM-5 Catalysts for Palm Oil Conversion to Hydrocarbon-Rich Biofuels. Catalysts 2021, 11, 1286. https://doi.org/10.3390/catal11111286
Riyanto T, Istadi I, Jongsomjit B, Anggoro DD, Pratama AA, Faris MAA. Improved Brønsted to Lewis (B/L) Ratio of Co- and Mo-Impregnated ZSM-5 Catalysts for Palm Oil Conversion to Hydrocarbon-Rich Biofuels. Catalysts. 2021; 11(11):1286. https://doi.org/10.3390/catal11111286
Chicago/Turabian StyleRiyanto, Teguh, Istadi Istadi, Bunjerd Jongsomjit, Didi D. Anggoro, Aryadita Ayu Pratama, and Muhammad Aviv Al Faris. 2021. "Improved Brønsted to Lewis (B/L) Ratio of Co- and Mo-Impregnated ZSM-5 Catalysts for Palm Oil Conversion to Hydrocarbon-Rich Biofuels" Catalysts 11, no. 11: 1286. https://doi.org/10.3390/catal11111286







