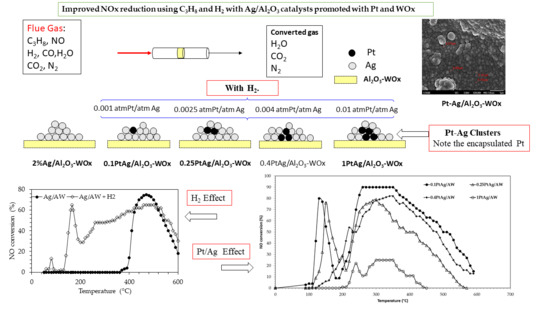
Improved NOx Reduction Using C3H8 and H2 with Ag/Al2O3 Catalysts Promoted with Pt and WOx
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization
2.1.1. Textural Properties
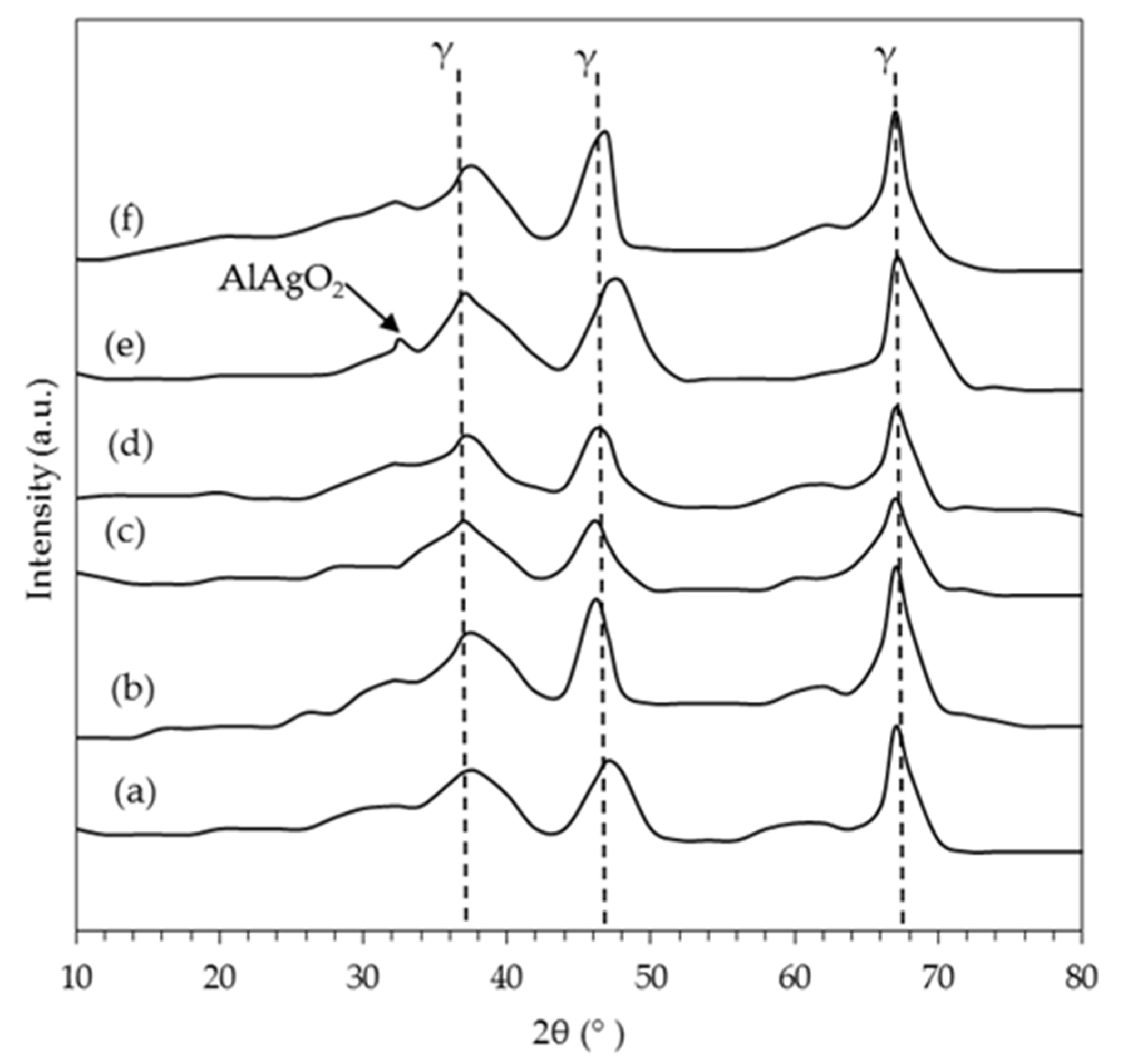
2.1.2. X-Ray Diffraction (XRD)
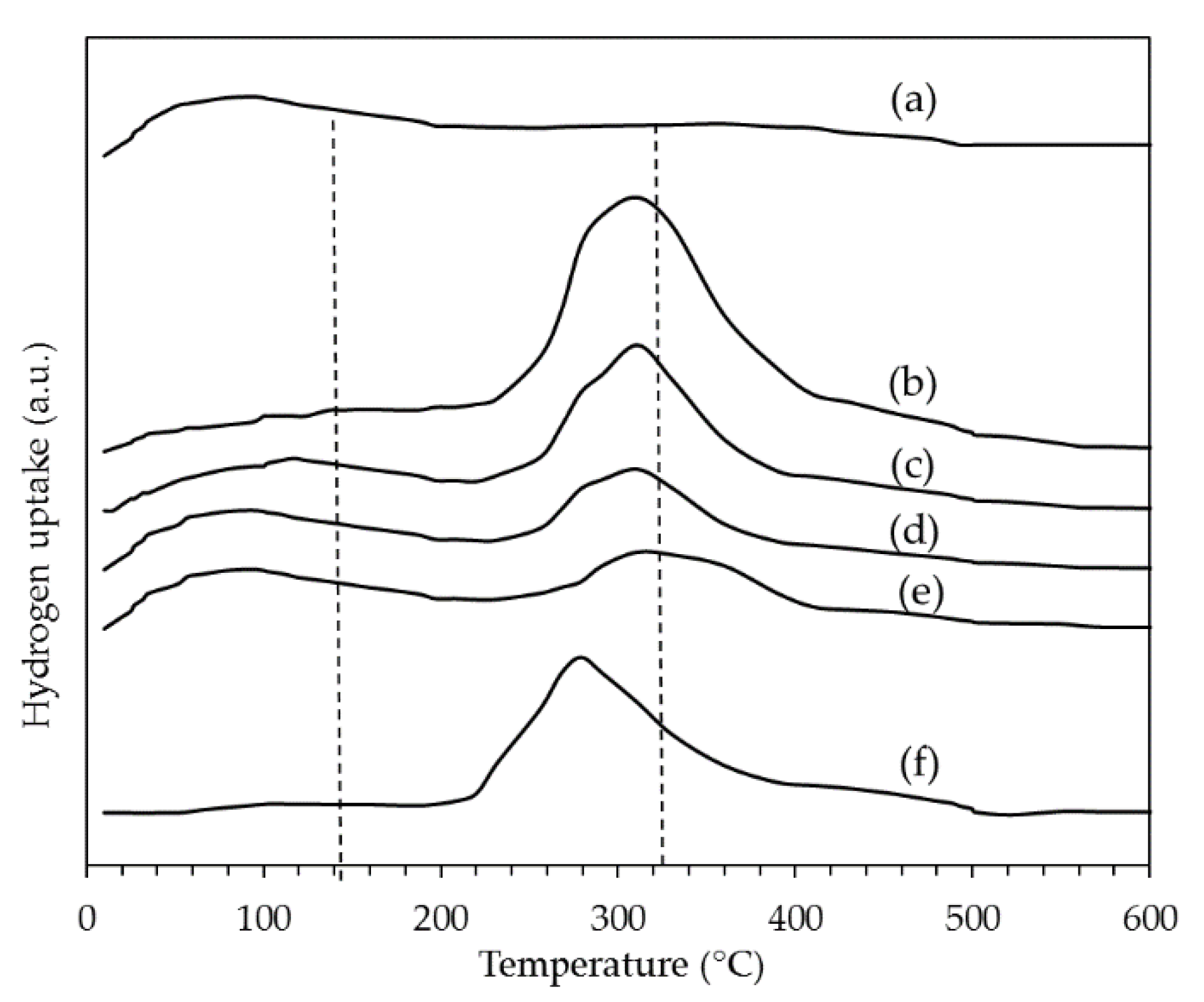
2.1.3. Temperature Programmed Reduction (TPR)
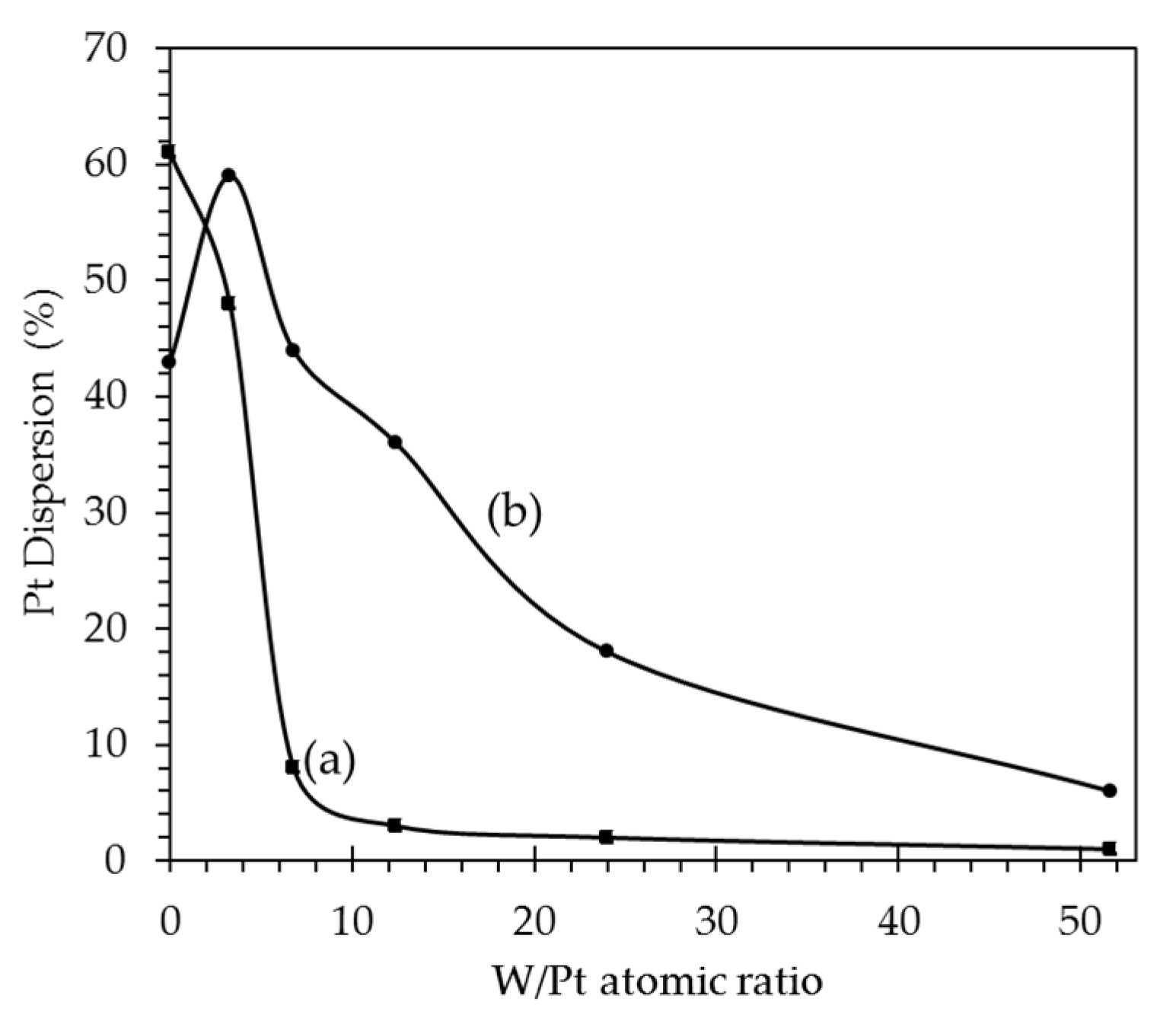
2.1.4. H2 Chemisorption
2.1.5. SEM of the Catalysts
2.1.6. UV–Vis Spectroscopy
2Ag/AW catalyst
0.4Pt/AW Catalyst
PtAg/AW Catalysts
2.2. Catalytic Activity
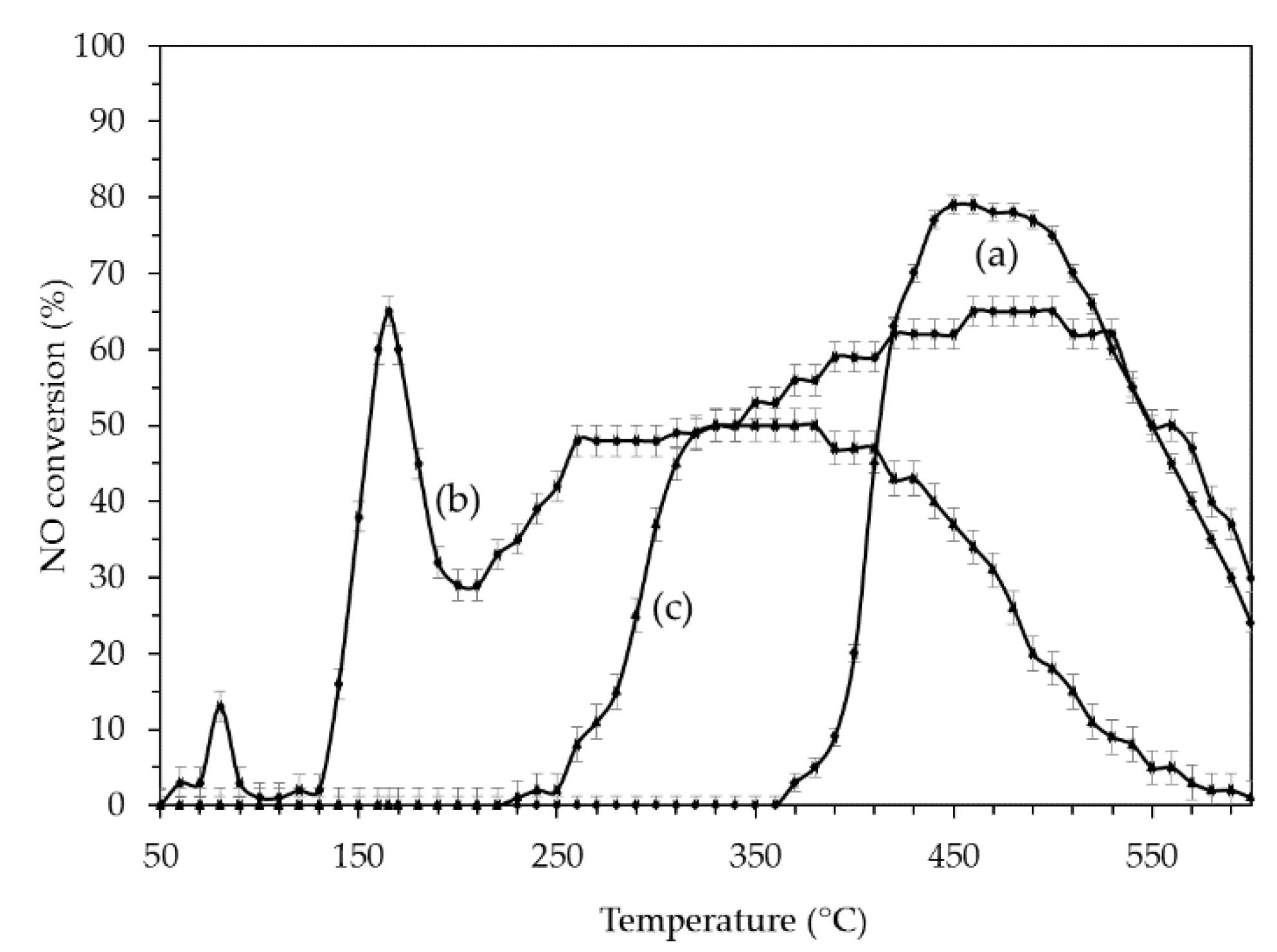
2.2.1. SCR of NO on Pt and Ag Catalysts
2.2.2. SCR of NO with C3H8 on PtAg Catalysts
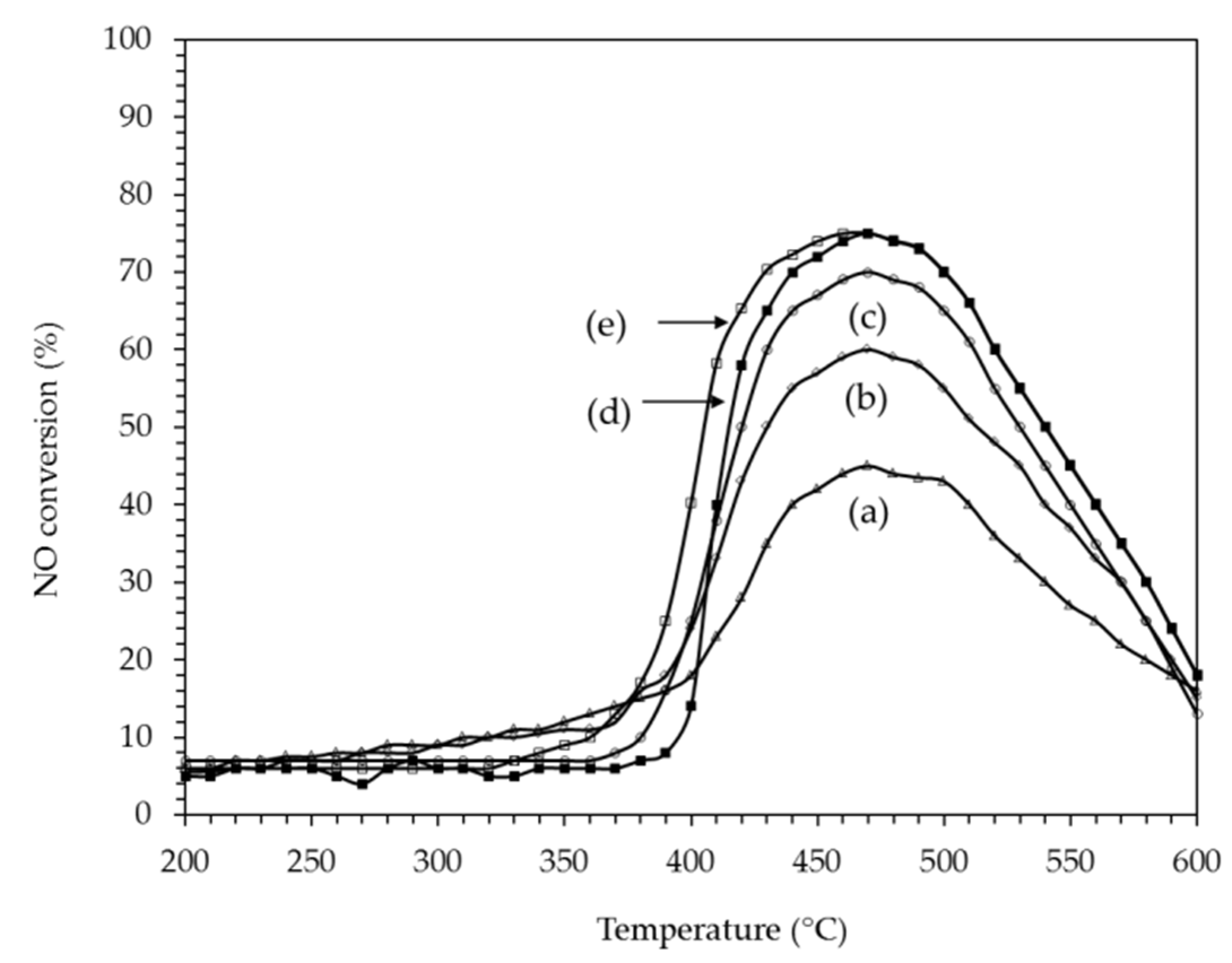
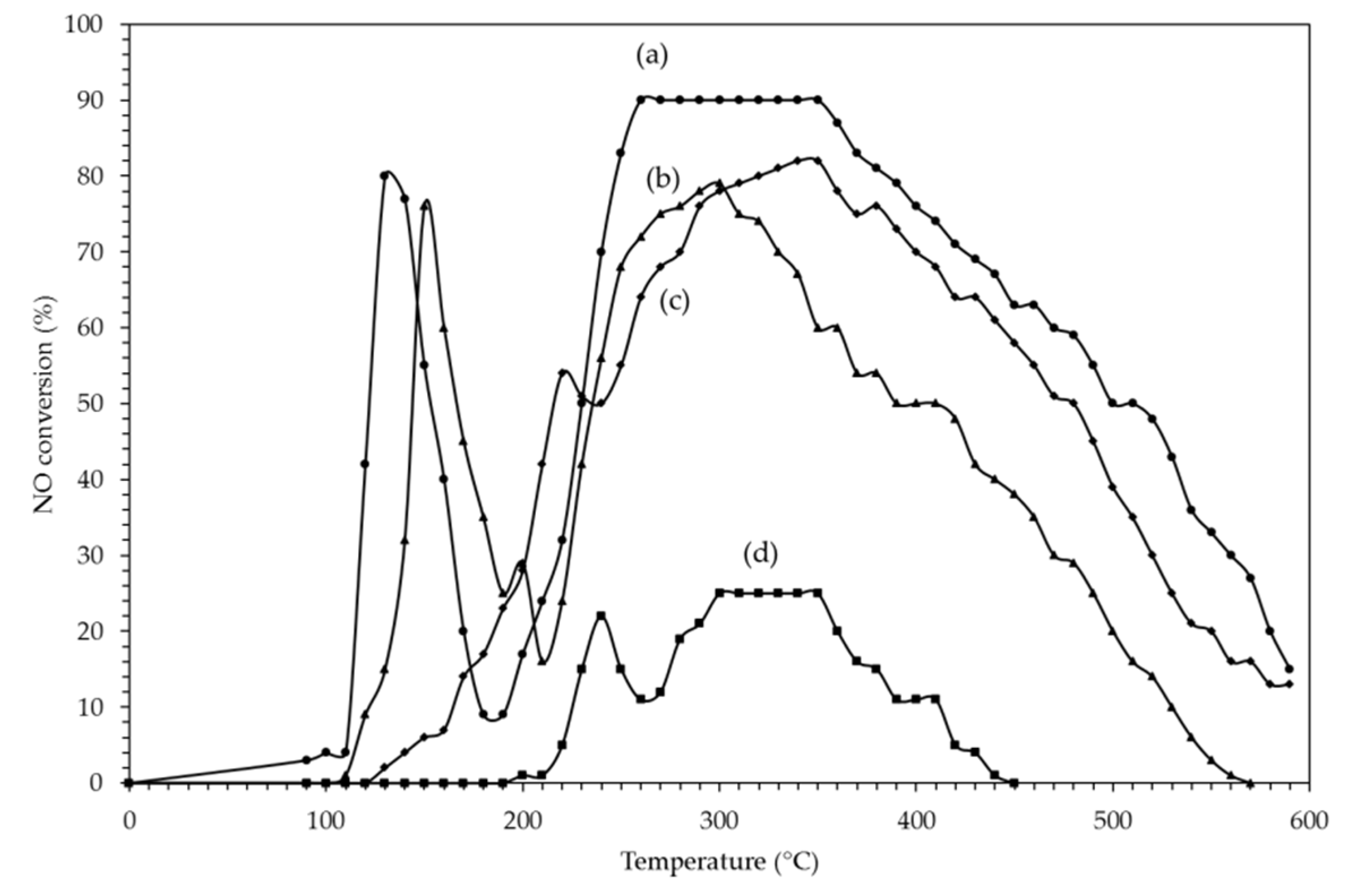
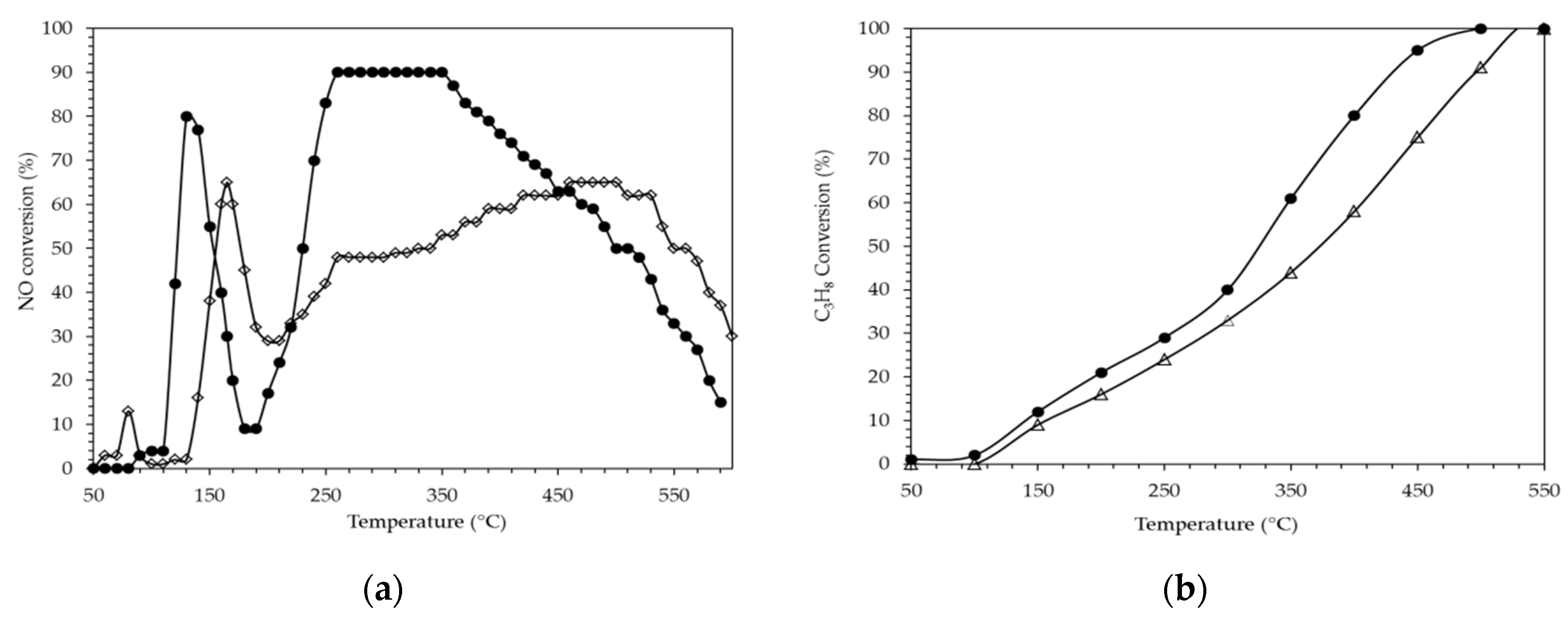
2.2.3. H2 Assisted SCR of NO on PtAg Catalysts
2.2.4. Effect of H2O on H2–C3H8–SCR of NO
3. Materials and Methods
3.1. Preparation of Catalysts
3.2. Catalyst Characterization
3.3. Catalytic Evaluation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A. Stabilization of Al2O3 and Pt/Al2O3 Catalysts

References
- Twigg, M.V. Progress and future challenges in controlling automotive exhaust gas emissions. Appl. Catal. B Environ. 2007, 70, 2–15. [Google Scholar] [CrossRef]
- Burch, R.; Breen, J.P.; Meunier, F.C. A review of the selective reduction of NOx with hydrocarbons under lean-burn conditions with non-zeolitic oxide and platinum group metal catalysts. Appl. Catal. B Environ. 2002, 39, 283–303. [Google Scholar] [CrossRef]
- Kannisto, H.; Ingelsten, H.H.; Skoglundh, M. Ag–Al2O3 catalysts for lean NOx reduction—Influence of preparation method and reductant. J. Mol. Catal. A Chem. 2009, 302, 86–96. [Google Scholar] [CrossRef]
- Nakatsuji, T.; Yasukawa, R.; Tabata, K.; Ueda, K.; Niwa, M. Catalytic reduction system of NOx in exhaust gases from diesel engines with secondary fuel injection. Appl. Catal. B Environ. 1998, 17, 333–345. [Google Scholar] [CrossRef]
- Miyadera, T.; Yoshida, K. Alumina-supported catalysts for the selective reduction of nitric oxide by propene. Chem. Lett. 1993, 9, 1483–1486. [Google Scholar] [CrossRef]
- Satokawa, S.; Yamaseki, K.; Uchida, H. Influence of low concentration of SO2 for selective reduction of NO by C3H8 in lean-exhaust conditions on the activity of Ag/Al2O3 catalyst. Appl. Catal. B Environ. 2001, 34, 299–306. [Google Scholar] [CrossRef]
- Kyriienko, P.; Popovych, N.; Soloviev, S.; Orlyk, S.; Dzwigaj, S. Remarkable activity of Ag/Al2O3/cordierite catalysts in SCR of NO with ethanol and butanol. Appl. Catal. B Environ. 2013, 140–141, 691–699. [Google Scholar] [CrossRef]
- Popovych, N.O.; Soloviev, S.O.; Orlyk, S.M. Selective reduction of nitrogen oxides (NOx) with oxygenates and hydrocarbons over bifunctional, silver–alumina catalysts: A review. Theor. Exp. Chem. 2016, 52, 133–151. [Google Scholar] [CrossRef]
- Männikkö, M.; Wang, X.; Skoglundh, M.; Härelind, H. Characterization of the active species in the silver/alumina system for lean NOx reduction with methanol. Catal. Today 2016, 267, 76–81. [Google Scholar] [CrossRef]
- Deng, H.; Yu, Y.; He, H. Adsorption states of typical intermediates on Ag/Al2O3 catalyst employed in the selective catalytic reduction of NOx by ethanol. Chin. J. Catal. 2015, 36, 1312–1320. [Google Scholar] [CrossRef]
- Bethke, K.A.; Kung, H.H. Supported Ag catalysts for the lean reduction of NO with C3H6. J. Catal. 1997, 172, 93–102. [Google Scholar] [CrossRef]
- Hoost, T.E.; Kudla, R.J.; Collins, K.M.; Chattha, M.S. Characterization of Ag/γ-Al2O3 catalysts and their lean-NOx properties. Appl. Catal. B Environ. 1997, 13, 59–67. [Google Scholar] [CrossRef]
- Kung, M.C.; Kung, H.H. Lean NOx catalysis over alumina-supported catalysts. Top. Catal. 2000, 10, 21–26. [Google Scholar] [CrossRef]
- Meunier, F.C.; Breen, J.P.; Zuzaniuk, V.; Olsson, M.; Ross, J.R.H. Mechanistic aspects of the selective reduction of NO by propene over alumina and silver-alumina catalysts. J. Catal. 1999, 187, 493–505. [Google Scholar] [CrossRef]
- Martínez-Arias, A.; Fernández-García, M.; Iglesias-Juez, A.; Anderson, J.A.; Conesa, J.C.; Soria, J. Study of the lean NOx reduction with C3H6 in the presence of water over silver/alumina catalysts prepared from inverse microemulsions. Appl. Catal. B Environ. 2000, 28, 29–41. [Google Scholar] [CrossRef]
- Shimizu, K.I.; Shibata, J.; Yoshida, H.; Satsuma, A.; Hattori, T. Silver-alumina catalysts for selective reduction of NO by higher hydrocarbons: Structure of active sites and reaction mechanism. Appl. Catal. B Environ. 2001, 30, 151–162. [Google Scholar] [CrossRef]
- Bogdanchikova, N.; Meunier, F.C.; Avalos-Borja, M.; Breen, J.P.; Pestryakov, A. On the nature of the silver phases of Ag/Al2O3 catalysts for reactions involving nitric oxide. Appl. Catal. B Environ. 2002, 36, 287–297. [Google Scholar] [CrossRef]
- Iglesias-Juez, A.; Hungría, A.B.; Martínez-Arias, A.; Fuerte, A.; Fernández-García, M.; Anderson, J.A. Nature and catalytic role of active silver species in the lean NOx reduction with C3H6 in the presence of water. J. Catal. 2003, 217, 310–323. [Google Scholar] [CrossRef]
- Arve, K.; Capek, L.; Klingstedt, F.; Eränen, K.; Lindfors, L.E.; Murzin, D.Y. Preparation and characterization of Ag/alumina catalysts for the removal of NOx emissions under oxygen rich conditions. Top. Catal. 2004, 30, 91–95. [Google Scholar] [CrossRef]
- Mrabet, D.; Manh-Hiep, V.; Kaliaguine, S.; Trong-On, D. A new route to the shape-controlled synthesis of nano-sized γ-alumina and Ag/γ-alumina for selective catalytic reduction of NO in the presence of propene. J. Colloid Interface Sci. 2017, 485, 144–151. [Google Scholar] [CrossRef]
- Keshavaraja, A.; She, X.; Flytzani-Stephanopoulos, M. Selective catalytic reduction of NO with methane over Ag-alumina catalysts. Appl. Catal. B Environ. 2000, 27, L1–L9. [Google Scholar] [CrossRef]
- Shimizu, K.; Satsuma, A.; Hattori, T. Catalytic performance of Ag–Al2O3 catalyst for the selective catalytic reduction of NO by higher hydrocarbons. Appl. Catal. B Environ. 2000, 25, 239–247. [Google Scholar] [CrossRef]
- She, X.; Flytzani-Stephanopoulos, M. The role of Ag–O–Al species in silver–alumina catalysts for the selective catalytic reduction of NOx with methane. J. Catal. 2006, 237, 79–93. [Google Scholar] [CrossRef]
- Luo, Y.; Hao, J.; Hou, Z.; Fu, L.; Li, R.; Ning, P.; Zheng, X. Influence of preparation methods on selective catalytic reduction of nitric oxides by propene over silver–alumina catalyst. Catal. Today 2004, 93–95, 797–803. [Google Scholar] [CrossRef]
- Chaieb, T.; Delannoy, L.; Costentin, G.; Louis, C.; Casale, S.; Chantry, R.L.; Li, Z.Y.; Thomas, C. Insights into the influence of the Ag loading on Al2O3 in the H2-assisted C3H6-SCR of NOx. Appl. Catal. B Environ. 2014, 156–157, 192–201. [Google Scholar] [CrossRef]
- Tamm, S.; Vallim, N.; Skoglundh, M.; Olsson, L. The influence of hydrogen on the stability of nitrates during H2-assisted SCR over Ag/Al2O3 catalysts—A DRIFT study. J. Catal. 2013, 307, 153–161. [Google Scholar] [CrossRef]
- Azis, M.M.; Härelind, H.; Creaser, D. On the role of H2 to modify surface NOx species, over Ag–Al2O3 as lean NOx reduction catalyst: TPD and DRIFTS studies. Catal. Sci. Technol. 2015, 5, 296–309. [Google Scholar] [CrossRef] [Green Version]
- Azis, M.M.; Härelind, H.; Creaser, D. Kinetic modeling of H2-assisted C3H6 selective catalytic reduction of NO over silver alumina catalyst. Chem. Eng. J. 2015, 278, 394–406. [Google Scholar] [CrossRef] [Green Version]
- Singh, P.; Yadav, D.; Thakur, P.; Pandey, J.; Prasad, R. Studies on H2-Assisted Liquefied Petroleum Gas Reduction of NO over Ag/Al2O3 Catalyst. Bull. Chem. React. Eng. Catal. 2018, 13, 227–235. [Google Scholar]
- Hernández-Terán, M.E.; Fuentes, G.A. Enhancement by H2 of C3H8-SCR of NOx using Ag/γ-Al2O3. Fuel 2014, 138, 91–97. [Google Scholar] [CrossRef]
- Ström, L.; Carlsson, P.-A.; Skoglundh, M.; Härelind, H. Surface Species and Metal Oxidation State during H2-Assisted NH3-SCR of NOx over Alumina-Supported Silver and Indium. Catalysts 2018, 8, 38. [Google Scholar] [CrossRef] [Green Version]
- Xu, G.; Ma, J.; Wang, L.; Lv, Z.; Wang, S.; Yu, Y.; He, H. Mechanism of the H2 Effect on NH3-Selective Catalytic Reduction over Ag/Al2O3: Kinetic and Diffuse Reflectance Infrared Fourier Transform Spectroscopy Studies. ACS Catal. 2019, 9, 10489–10498. [Google Scholar] [CrossRef]
- Barreau, M.; Tarot, M.-L.; Duprez, D.; Courtois, X.; Can, F. Remarkable enhancement of the selective catalytic reduction of NO at low temperature by collaborative effect of ethanol and NH3 over silver supported catalyst. Appl. Catal. B Environ. 2018, 220, 19–30. [Google Scholar] [CrossRef]
- Pihl, J.A.; Toops, T.J.; Fisher, G.B.; West, B.H. Selective catalytic reduction of nitric oxide with ethanol/gasoline blends over a silver/alumina catalyst. Catal. Today 2014, 231, 46–55. [Google Scholar] [CrossRef]
- Wu, S.; Li, X.; Fang, X.; Sun, Y.; Sun, J.; Zhou, M.; Zang, S. NO reduction by CO over TiO2-γ-Al2O3 supported In/Ag catalyst under lean burn conditions. Chin. J. Catal. 2016, 37, 2018–2024. [Google Scholar] [CrossRef]
- Shang, Z.; Cao, J.; Wang, L.; Guo, Y.; Lu, G.; Guo, Y. The study of C3H8-SCR on Ag/Al2O3 catalysts with the presence of CO. Catal. Today 2017, 281, 605–660. [Google Scholar] [CrossRef]
- Gunnarsson, F.; Kannisto, H.; Skoglundh, M.; Härelind, H. Improved low-temperature activity of silver–alumina for lean NOx reduction—Effects of Ag loading and low-level Pt doping. Appl. Catal. B Environ. 2014, 152–153, 218–225. [Google Scholar] [CrossRef]
- Tamm, S.; Andonova, S.; Olsson, L. The Effect of Hydrogen on the Storage of NOx over Silver, Platinum and Barium Containing NSR Catalysts. Catal. Lett. 2014, 144, 1101–1112. [Google Scholar] [CrossRef]
- Bonet, F.; Grugeon, S.; Herrera Urbina, R.H.; Tekaia-Elhsissen, K.; Tarascon, J.-M. In situ deposition of silver and palladium nanoparticles prepared by the polyol process and their performance as catalytic converters of automobile exhaust gases. Solid State Sci. 2002, 4, 665–670. [Google Scholar] [CrossRef]
- He, H.; Wang, J.; Feng, Q.; Yu, Y.; Yoshida, K. Novel Pd, promoted Ag/Al2O3 catalyst for the selective reduction of NOx. Appl. Catal. B Environ. 2003, 46, 365–370. [Google Scholar] [CrossRef]
- Inderwildi, O.R.; Jenkins, S.J.; King, D.A. When adding an unreactive metal enhances catalytic activity: NOx decomposition over silver–rhodium bimetallic surfaces. Surf. Sci. 2007, 601, L103–L108. [Google Scholar] [CrossRef]
- Lanza, R.; Eriksson, E.; Pettersson, L.J. NOx selective catalytic reduction over supported metallic catalysts. Catal. Today 2009, 147S, S279–S284. [Google Scholar] [CrossRef]
- Schott, F.J.P.; Balle, P.; Adler, J.; Kureti, S. Reduction of NOx by H2 on Pt/WO3/ZrO2 catalysts in oxygen-rich exhaust. Appl. Catal. B Environ. 2009, 87, 18–29. [Google Scholar] [CrossRef]
- Contreras, J.L.; Fuentes, G.A.; García, L.A.; Salmones, J.; Zeifert, B. WOx effect on the catalytic properties of Pt particles on Al2O3. J. Alloy Compd. 2009, 483, 450–452. [Google Scholar] [CrossRef]
- Contreras, J.L.; Fuentes, G.A.; Zeifert, B.; García, L.A.; Salmones, J. Stabilization of Supported Platinum Nanoparticles on γ-Alumina Catalysts by Addition of Tungsten. J. Alloy Compd. 2009, 483, 371–373. [Google Scholar] [CrossRef]
- Muñoz, H.P.; Delmás, R.D. Alumina synthesis from AlCl3 acid solution and XRD characterization. J. Per. Quim. Ing. Química 2001, 4, 68–71. [Google Scholar]
- Aguado, J.; Escola, J.M.; Castro, M.C. Influence of the thermal treatment upon the textural properties of sol–gel mesoporous γ-alumina synthesized with cationic surfactants. Microporous Mesoporous Mater. 2010, 128, 48–55. [Google Scholar] [CrossRef]
- Richter, M.; Bentrup, U.; Eckelt, R.; Schneider, M.; Pohl, M.M.; Fricke, R. The effect of hydrogen on the selective catalytic reduction of NO in excess oxygen over Ag/Al2O3. Appl. Catal. B Environ. 2004, 51, 261–274. [Google Scholar] [CrossRef]
- Vojvodic, A.; Calle-Vallejo, F.; Guo, W.; Wang, S.; Toftelund, A.; Studt, F.; Martínez, J.I.; Shen, J.; Man, J.; Rossmeisl, I.C.; et al. On the behavior of Brønsted-Evans-Polanyi relations for transition metal oxides. J. Chem. Phys. 2011, 134, 244–509. [Google Scholar] [CrossRef] [Green Version]
- Hernández-Terán, M.E. Development of Ag/γ-Al2O3 and Ag/η-Al2O3 Catalytic Systems for H2-Assisted NO C3H8-SCR under Oxidizing Operation for Emission Control Systems for Diesel Engines or Stationary Sources. Ph.D. Thesis, Universidad Autónoma Metropolitana-Iztapalapa, Ciudad de México, Mexico, 2020. [Google Scholar]
- Zhang, Q.; Li, J.; Liu, X.; Zhu, Q. Synergetic effect of Pd and Ag dispersed on Al2O3 in the selective hydrogenation of acetylene. Appl. Catal. A General 2000, 197, 221–228. [Google Scholar] [CrossRef]
- Lieske, H.; Lietz, G.; Spindler, H.; Volter, J. Reactions of Platinum in Oxygen- and Hydrogen-Treated Pt/A1203 Catalysts. Temperature-Programmed Reduction, Adsorption, and Redispersion of Platinum. J. Catal. 1983, 81, 8–16. [Google Scholar]
- Gauthard, F.; Epron, F.; Barbier, J. Palladium and platinum-based catalysts in the catalytic reduction of nitrate in water: Effect of cooper, silver or gold addition. J. Catal. 2003, 220, 182–191. [Google Scholar] [CrossRef]
- De Jong, K.P.; Bongenaar-Schlenter, B.E.; Meima, G.R.; Verkerk, R.C.; Lammers, M.J.J.; Geus, J.W. Investigations on silica-supported platinum-silver alloy particles by infrared spectra of adsorbed CO and N2. J. Catal. 1983, 81, 67–76. [Google Scholar] [CrossRef]
- Prasad, J.; Murthy, K.R.; Menon, P.G. The stoichiometry of hydrogen-oxygen titrations on supported platinum catalysts. J. Catal. 1978, 52, 515–520. [Google Scholar] [CrossRef]
- Arve, K.; Svennerberg, K.; Klingstedt, F.; Eranen, K.; Wallenberg, L.R.; Bovin, J.O.; Capek, L.; Murzin, D.Y. Structure-Activity Relationship in HC-SCR of NOx by TEM, O2-Chemisorption, and EDXS Study of Ag/Al2O3. J. Phys. Chem. B 2006, 110, 420–427. [Google Scholar] [CrossRef]
- Bligaard, T.; Nørskov, J.K.; Dahl, S.; Matthiesen, J.; Christensen, C.H.; Sehested, J. The Brønsted–Evans–Polanyi relation and the volcano curve in heterogeneous catalysis. J. Catal. 2004, 224, 206–217. [Google Scholar] [CrossRef]
- Lietz, G.; Lieske, H.; Spindler, H.; Hanke, W.; Völter, J. Reactions of Platinum in Oxygen- and Hydrogen-Treated Pt/γ-Al2O3 catalysts, II. Ultraviolet-Visible Studies, Sintering of Platinum, and Soluble Platinum. J. Catal. 1983, 81, 17–25. [Google Scholar] [CrossRef]
- Barton, D.G.; Soled, S.L.; Meitzner, G.D.; Fuentes, G.A.; Iglesia, E. Structural and Catalytic Characterization of Solid Acids Based on Zirconia Modified by Tungsten Oxide. J. Catal. 1999, 181, 57–72. [Google Scholar] [CrossRef] [Green Version]
- Satokawa, S. Enhancing the NO/C3H8/O2 Reaction by Using H2 over Ag/Al2O3 Catalysts under Lean-Exhaust Conditions. Chem. Lett. 2000, 29, 294–295. [Google Scholar] [CrossRef]
- Wang, J.; He, H.; Feng, Q.; Yu, Y.; Yoshida, K. Selective catalytic reduction of NOx with C3H6 over Ag/Al2O3 catalyst with a small quantity of noble metal. Catal. Today 2004, 93–95, 783–789. [Google Scholar] [CrossRef]
- Bordley, J.A.; El-Sayed, M.A. Enhanced Electrocatalytic Activity toward the Oxygen Reduction Reaction through Alloy Formation: Platinum-Silver Alloy Nano cages. J. Phys. Chem. 2016, 120, 14643–14651. [Google Scholar] [CrossRef]
- Fogler, S.H. Elements of Chemical Reaction Engineering, 3rd ed.; Prentice Hall Inc.: Upper Saddle River, NJ, USA, 1999; pp. 738–758. [Google Scholar]
- Smith, J.F. Chemical Engineering Kinetics, 3rd ed.; McGraw-Hill Inc.: New York, NY, USA, 1986; pp. 524–534. [Google Scholar]
- Hirshfelder, J.O.; Curtis, C.F.; Bird, R.B. Molecular Theory of Gases and Liquids, 1st ed.; Wiley: Hoboken, NJ, USA, 1964; ISBN 0-471-40065-3. [Google Scholar]
- David, G.C. Some comments on the repeatability of measurements. Ringing Migr. 1994, 15, 84–90. [Google Scholar]
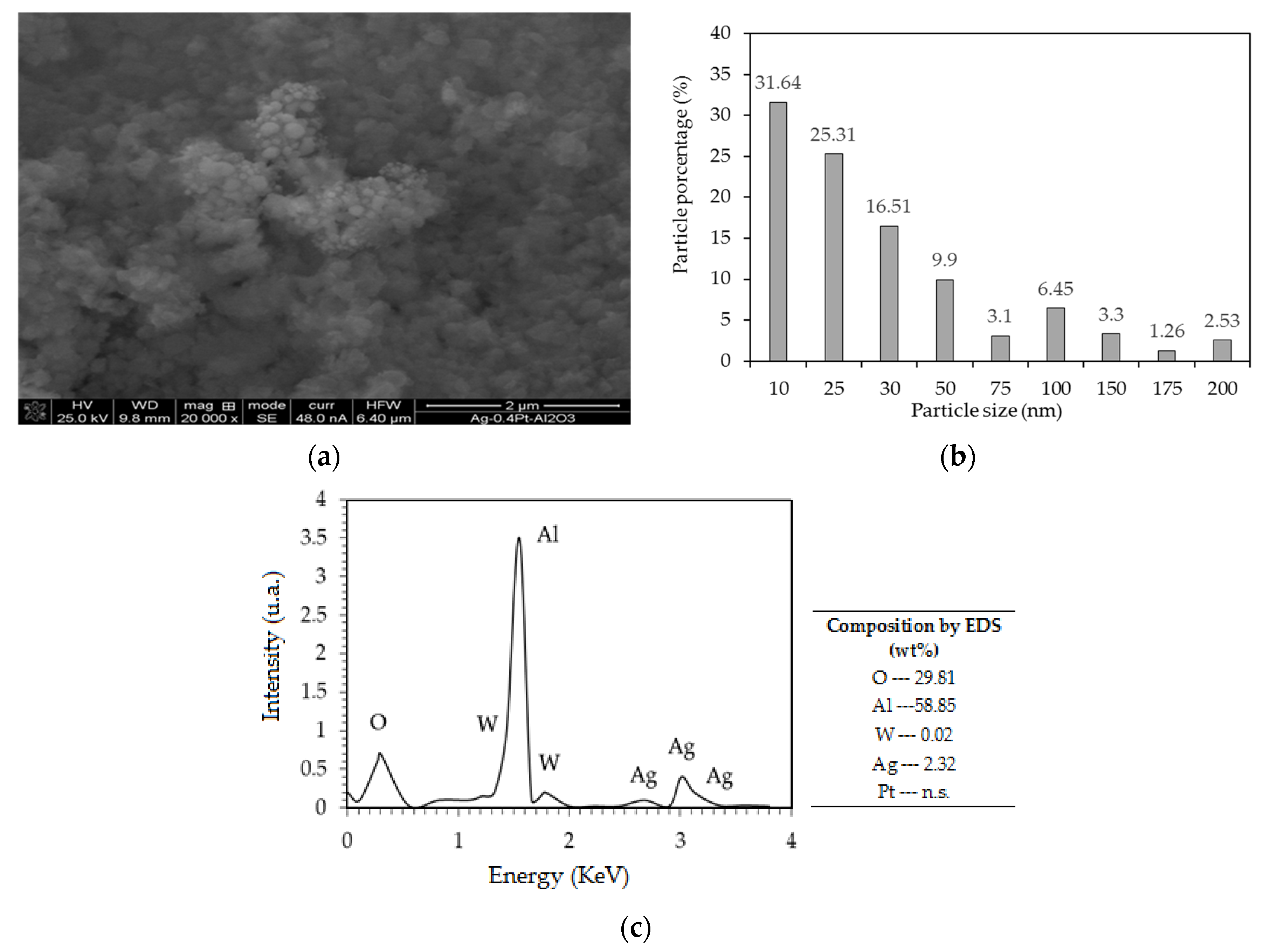
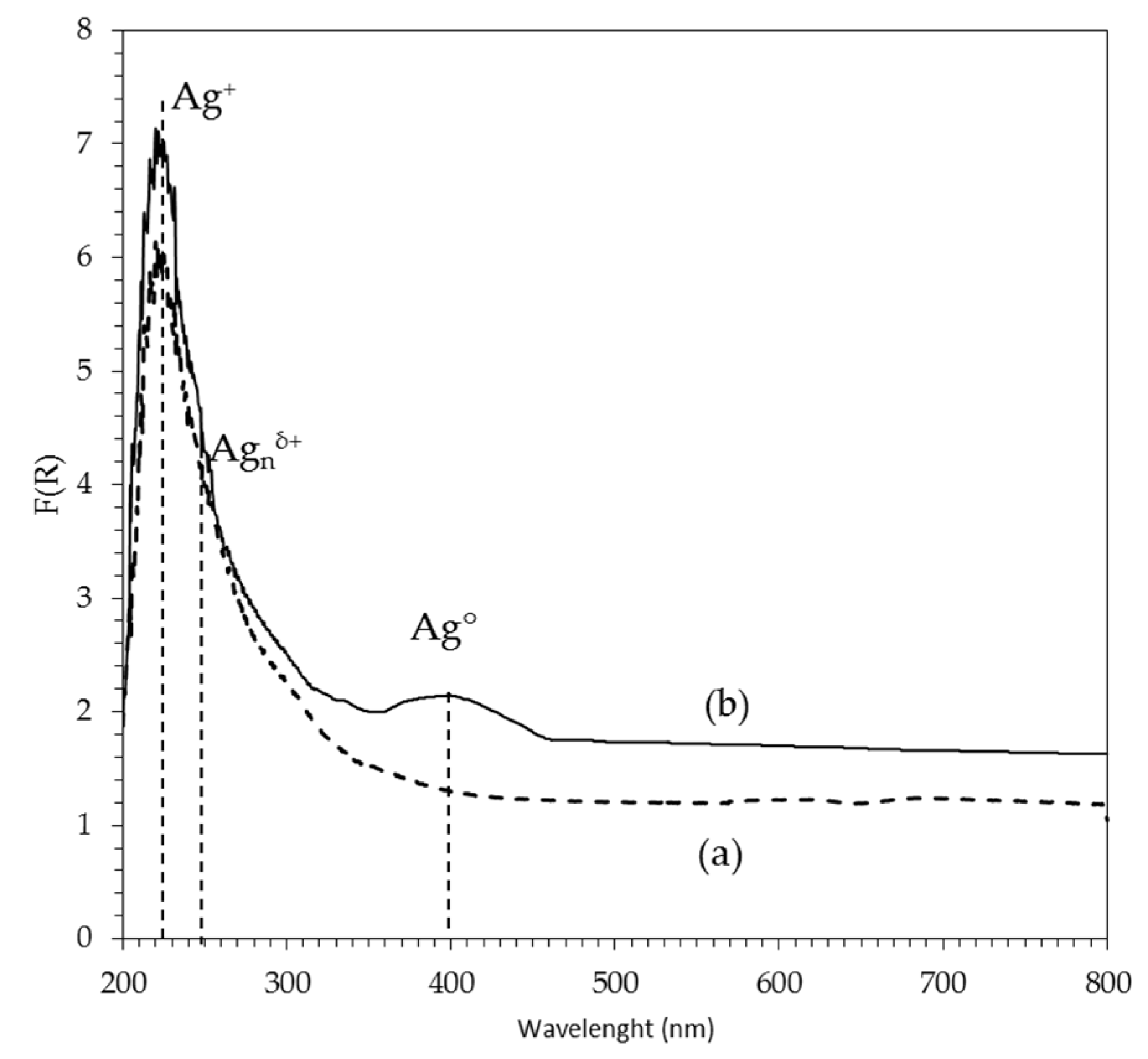
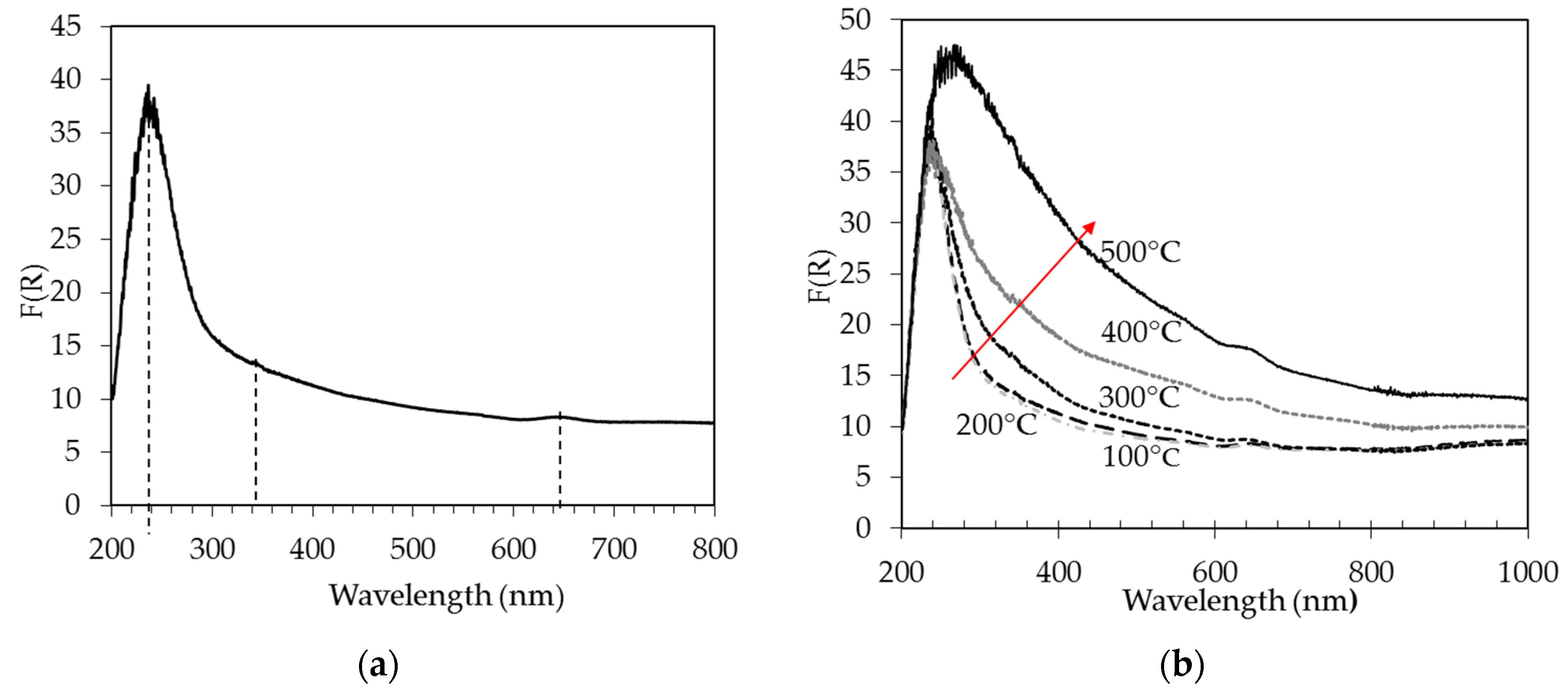
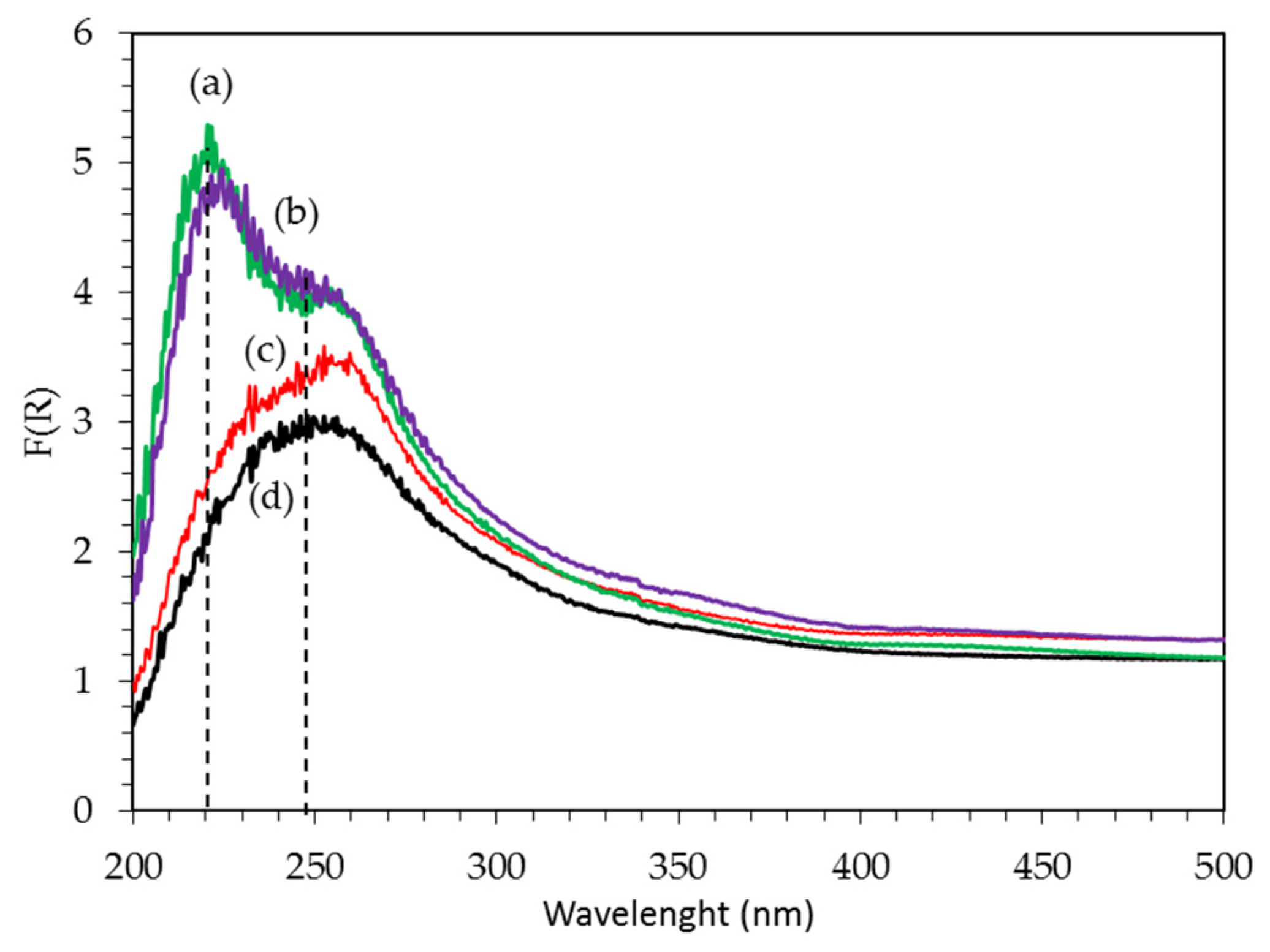
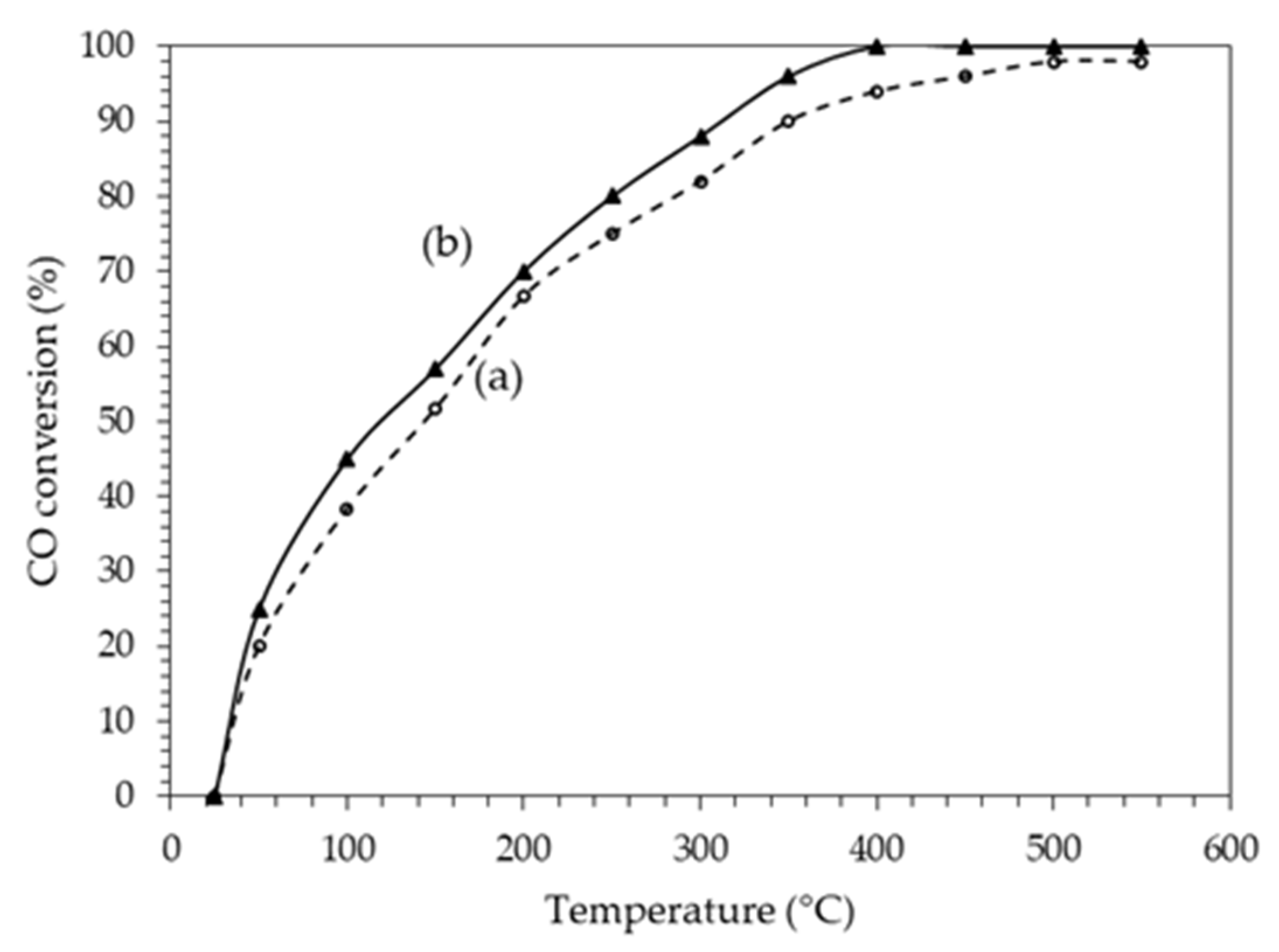

| Catalyst Name | Pt (%) | Ag (%) | Pt/Ag Atomic Ratio | BET Area (m2/g) | Pore Vol. (cm3/g) | Pore Diam. (Å) | H2 Consumption (μmol/gc) | Pt Dispersion (%) |
|---|---|---|---|---|---|---|---|---|
| A | 0 | 0 | 0 | 267 | 0.36 | 54 | 0 | 0 |
| 0.4Pt/A | 0.4 | 0 | - | 256 | 0.36 | 55 | 45.1 | 61 |
| 2Ag/AW | 0 | 2 | - | 264 | 0.38 | 56 | 67 | 0 |
| 0.4Pt/AW | 0.4 | 0 | - | 230 | 0.39 | 68 | 43 | 57 |
| 0.1PtAg/AW | 0.1 | 2 | 0.027 | 226 | 0.37 | 66 | 2.5 | 60 |
| 0.25PtAg/AW | 0.25 | 2 | 0.069 | 218 | 0.36 | 67 | 26 | 46 |
| 0.4PtAg/AW | 0.4 | 2 | 0.110 | 225 | 0.37 | 66 | 41 | 38 |
| 1PtAg/AW | 1 | 2 | 0.270 | 228 | 0.37 | 65 | 112 | 21 |
| Catalyst | Pt/W Atomic Ratio | YN2O(%) at T(°C) | |
|---|---|---|---|
| 200 | 400 | ||
| Ag/Al2O3 | 0 | 7 | 5 |
| 0.1PtAg/AW | 0.027 | 8.5 | 6.5 |
| 0.25PtAg/AW | 0.069 | 9 | 6.5 |
| 0.4PtAg/AW | 0.11 | 12 | 7 |
| 1PtAg/AW | 0.27 | 14 | 6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
González Hernández, N.N.; Contreras, J.L.; Pinto, M.; Zeifert, B.; Flores Moreno, J.L.; Fuentes, G.A.; Hernández-Terán, M.E.; Vázquez, T.; Salmones, J.; Jurado, J.M. Improved NOx Reduction Using C3H8 and H2 with Ag/Al2O3 Catalysts Promoted with Pt and WOx. Catalysts 2020, 10, 1212. https://doi.org/10.3390/catal10101212
González Hernández NN, Contreras JL, Pinto M, Zeifert B, Flores Moreno JL, Fuentes GA, Hernández-Terán ME, Vázquez T, Salmones J, Jurado JM. Improved NOx Reduction Using C3H8 and H2 with Ag/Al2O3 Catalysts Promoted with Pt and WOx. Catalysts. 2020; 10(10):1212. https://doi.org/10.3390/catal10101212
Chicago/Turabian StyleGonzález Hernández, Naomi N., José Luis Contreras, Marcos Pinto, Beatriz Zeifert, Jorge L. Flores Moreno, Gustavo A. Fuentes, María E. Hernández-Terán, Tamara Vázquez, José Salmones, and José M. Jurado. 2020. "Improved NOx Reduction Using C3H8 and H2 with Ag/Al2O3 Catalysts Promoted with Pt and WOx" Catalysts 10, no. 10: 1212. https://doi.org/10.3390/catal10101212