Optimizing Management of Patients with Adult T Cell Leukemia-Lymphoma
Abstract
:1. Introduction
2. Recognizing ATL
3. Shimoyama Classification
| Acute ^ (Leukemic) | ATL Lymphoma | Chronic Unfav * | Chronic Fav | Smoldering | Pre-ATL | |
|---|---|---|---|---|---|---|
| Anti-HTLV-1 Ab | + | + | + | + | + | + |
| Circulating ATL cells | + | - | + | + | + | + |
| Lymphocytosis (ALC > 4000) | Variable | No | Yes | Yes | No | No |
| Circulating abnormal lymphocytes | Variable | ≤1% | Variable | Variable | ≥5% or <5% if ATL lesion(s) in the skin and/or lung | |
| LDH | Variable | Variable | <2× ULN, or * | normal | ≤1.5 ULN | normal |
| Calcium level | Variable | Variable | <11.0 | normal | normal | normal |
| Rash | Variable | Variable | Variable | Variable | Variable | No |
| Lymphadenopathy | Variable | >1.5 cm | Variable | Variable | No | No |
| Organomegaly | Variable | Variable | Mild | Mild | No | No |
| BUN | >ULN | NL | ||||
| Albumin | <LLN | NL | ||||
| CNS involvement | +/− | +/− | No | No | No | No |
| Bone lesions | +/− | +/− | No | No | No | No |
| Ascites | +/− | +/− | No | No | No | No |
| Pleural effusion | +/− | +/− | No | No | No | No |
| GI tract | +/− | +/− | No | No | No | No |
4. Baseline Laboratory Studies and Staging of ATL
| Exam: |
| Physical Examination including full skin exam and assessment of adenopathy and hepatosplenomegaly |
| Imaging: |
| PET/CT |
| Skeletal survey (for assessment of bone lytic lesions) |
| ECHO or MUGA (to rule-out cardiac involvement and before initiating anthracycline-based chemotherapy) |
| Laboratory: |
| CBC with differential |
| Chemistry panel including Calcium (assessment of hypercalcemia) |
| Peripheral blood smear (for assessment of circulating lymphocytes with “flower-like” nuclei) |
| LDH, uric acid |
| HTLV-1 quantitative DNA PCR |
| Troponin |
| HLA typing |
| CMV serology |
| G6PD |
| Other: |
| Bone marrow aspirate, biopsy, and cytogenetics |
| Transplantation evaluation |
5. Treatment
5.1. Smoldering and Chronic Favorable
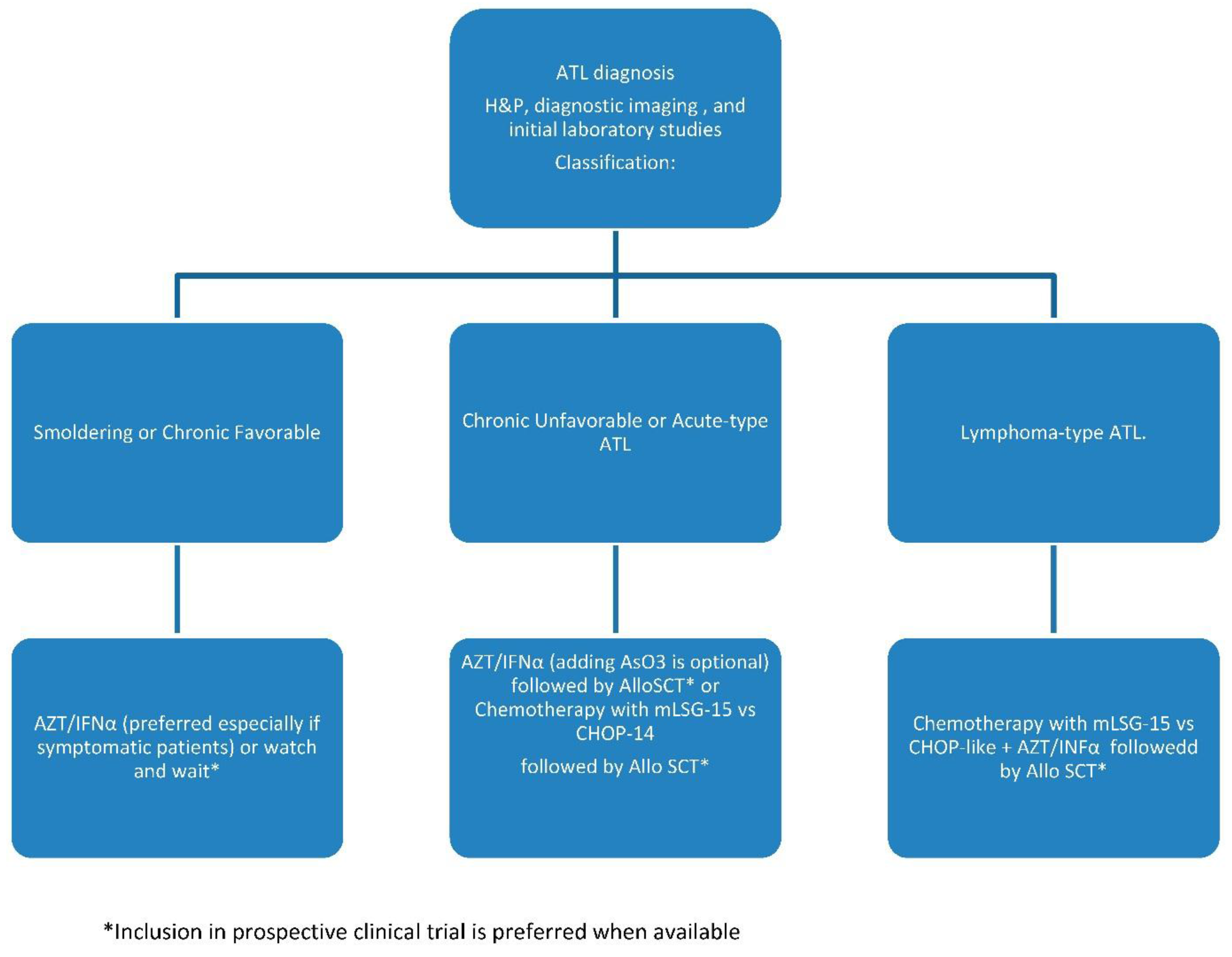
5.2. Chronic Unfavorable and Acute ATL
5.3. ATL Lymphoma
5.4. Single Site of Lymphomatous, or Bone, Disease
5.5. Pre-ATL
6. Response Assessment
7. Prophylaxis against Opportunistic Infection
8. Hematopoietic Cell Transplantation
9. Future Directions
Author Contributions
Conflicts of Interest
References
- Smith, M.R.; Greene, W.C. Molecular biology of the type I human T-cell leukemia virus (HTLV-I) and adult T-cell leukemia. J. Clin. Investig. 1991, 87, 761–766. [Google Scholar] [CrossRef] [PubMed]
- Matsuoka, M.; Watanabe, T.; Kannagi, M.; Bangham, C.; Grassmann, R.; Marriott, S.J.; Green, P.; Jeang, K.-T. Meeting report on the 13th International Conference on Human Retrovirology: Human T-cell leukemia virus research 30 years after adult T-cell leukemia. Cancer Res. 2007, 67, 10638–10641. [Google Scholar] [CrossRef] [PubMed]
- Murphy, E.L.; Hanchard, B.; Figueroa, J.P.; Gibbs, W.N.; Lofters, W.S.; Campbell, M.; Goedert, J.J.; Blattner, W.A. Modelling the risk of adult T-cell leukemia/lymphoma in persons infected with human T-lymphotropic virus type I. Int. J. Cancer 1989, 43, 250–253. [Google Scholar] [CrossRef] [PubMed]
- Umino, A.; Nakagawa, M.; Utsunomiya, A.; Tsukasaki, K.; Taira, N.; Katayama, N.; Seto, M. Clonal evolution of adult T-cell leukemia/lymphoma takes place in the lymph nodes. Blood 2011, 117, 5473–5478. [Google Scholar] [CrossRef] [PubMed]
- Chihara, D.; Ito, H.; Matsuda, T.; Shibata, A.; Katsumi, A.; Nakamura, S.; Tomotaka, S.; Morton, L.M.; Weisenburger, D.D.; Matsuo, K. Differences in incidence and trends of haematological malignancies in Japan and the United States. Br. J. Haematol. 2014, 164, 536–545. [Google Scholar] [CrossRef] [PubMed]
- Laurini, J.A.; Perry, A.M.; Boilesen, E.; Diebold, J.; Maclennan, K.A.; Muller-Hermelink, H.K.; Nathwani, B.N.; Armitage, J.A.; Weisenburger, D.D. Classification of non-Hodgkin lymphoma in Central and South America: A review of 1028 cases. Blood 2012, 120, 4795–4801. [Google Scholar] [CrossRef] [PubMed]
- De The, G.; Bomford, R. An HTLV-I vaccine: Why, how, for whom? AIDS Res. Hum. Retrovir. 1993, 9, 381–386. [Google Scholar] [CrossRef] [PubMed]
- Zell, M.I.; Assal, A.; Konda, B.; Braunschweig, I.; Derman, O.; Kornblum, N.; Battini, R.; Verma, A.; Janakiram, M. Analysis of Large Cohort Shows That Caribbean Adult T Cell Leukemia/Lymphoma Is a Chemotherapy Refractory Disease with Very Poor Prognosis That Behaves Distinctly from Japanese Subtypes. Blood 2014, 124, 1685–1685. [Google Scholar]
- Oshiro, A.; Tagawa, H.; Ohshima, K.; Karube, K.; Uike, N.; Tashiro, Y.; Utsunomiya, A.; Masuda, M.; Takasu, N.; Nakamura, S.; et al. Identification of subtype-specific genomic alterations in aggressive adult T-cell leukemia/lymphoma. Blood 2006, 107, 4500–4507. [Google Scholar] [CrossRef] [PubMed]
- Yamada, Y.; Tomonaga, M.; Fukuda, H.; Hanada, S.; Utsunomiya, A.; Tara, M.; Sano, M.; Ikeda, S.; Takatsuki, K.; Kozuru, M.; et al. A new G-CSF-supported combination chemotherapy, LSG15, for adult T-cell leukaemia-lymphoma: Japan Clinical Oncology Group Study 9303. Br. J. Haematol. 2001, 113, 375–382. [Google Scholar] [CrossRef] [PubMed]
- Bazarbachi, A.; Plumelle, Y.; Carlos Ramos, J.; Tortevoye, P.; Otrock, Z.; Taylor, G.; Gessain, A.; Harrington, W.; Panelatti, G.; Hermine, O. Meta-analysis on the use of zidovudine and interferon-alfa in adult T-cell leukemia/lymphoma showing improved survival in the leukemic subtypes. J. Clin. Oncol. 2010, 28, 4177–4183. [Google Scholar] [CrossRef] [PubMed]
- Ohshima, K.; Suzumiya, J.; Kikuchi, M. The World Health Organization classification of malignant lymphoma: Incidence and clinical prognosis in HTLV-1-endemic area of Fukuoka. Pathol. Int. 2002, 52, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Shimoyama, M. Diagnostic criteria and classification of clinical subtypes of adult T-cell leukaemia-lymphoma. A report from the Lymphoma Study Group (1984–87). Br. J. Haematol. 1991, 79, 428–437. [Google Scholar] [CrossRef] [PubMed]
- Swerdlow, S.H. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues; International Agency for Research on Cancer, World Health Organization: Lyon, France, 2008. [Google Scholar]
- Ishitsuka, K.; Tamura, K. Human T-cell leukaemia virus type I and adult T-cell leukaemia-lymphoma. Lancet Oncol. 2014, 15, e517–e526. [Google Scholar] [CrossRef]
- Barrington, S.F.; Mikhaeel, N.G.; Kostakoglu, L.; Meignan, M.; Hutchings, M.; Mueller, S.P.; Schwartz, L.H.; Zucca, E.; Fisher, R.I.; Trotman, J.; et al. Role of imaging in the staging and response assessment of lymphoma: Consensus of the International Conference on Malignant Lymphomas Imaging Working Group. J. Clin. Oncol. 2014, 32, 3048–3058. [Google Scholar] [CrossRef] [PubMed]
- Takasaki, Y.; Iwanaga, M.; Imaizumi, Y.; Tawara, M.; Joh, T.; Kohno, T.; Yamada, Y.; Kamihira, S.; Ikeda, S.; Miyazaki, Y.; et al. Long-term study of indolent adult T-cell leukemia-lymphoma. Blood 2010, 115, 4337–4343. [Google Scholar] [CrossRef] [PubMed]
- Kinpara, S.; Kijiyama, M.; Takamori, A.; Hasegawa, A.; Sasada, A.; Masuda, T.; Tanaka, Y.; Utsunomiya, A.; Kannag, M. Interferon-alpha (IFN-alpha) suppresses HTLV-1 gene expression and cell cycling, while IFN-alpha combined with zidovudine induces p53 signaling and apoptosis in HTLV-1-infected cells. Retrovirology 2013, 10, 52. [Google Scholar] [CrossRef] [PubMed]
- Tsukasaki, K.; Utsunomiya, A.; Fukuda, H.; Shibata, T.; Fukushima, T.; Takatsuka, Y.; Ikeda, S.; Masuda, M.; Nagoshi, H.; Ueda, R.; et al. VCAP-AMP-VECP compared with biweekly CHOP for adult T-cell leukemia-lymphoma: Japan Clinical Oncology Group Study JCOG9801. J. Clin. Oncol. 2007, 25, 5458–5464. [Google Scholar] [CrossRef] [PubMed]
- Matutes, E.; Taylor, G.P.; Cavenagh, J.; Pagliuca, A.; Bareford, D.; Domingo, A.; Hamblin, M.; Kelsey, S.; Mir, N.; Reilly, J.T. Interferon alpha and zidovudine therapy in adult T-cell leukaemia lymphoma: Response and outcome in 15 patients. Br. J. Haematol. 2001, 113, 779–784. [Google Scholar] [CrossRef] [PubMed]
- Kchour, G.; Tarhini, M.; Kooshyar, M.M.; El Hajj, H.; Wattel, E.; Mahmoudi, M.; Hatoum, H.; Rahimi, H.; Maleki, M.; Rafatpanah, H.; et al. Phase 2 study of the efficacy and safety of the combination of arsenic trioxide, interferon alpha, and zidovudine in newly diagnosed chronic adult T-cell leukemia/lymphoma (ATL). Blood 2009, 113, 6528–6532. [Google Scholar] [CrossRef] [PubMed]
- Gill, P.S.; Harrington, W., Jr.; Kaplan, M.H.; Ribeiro, R.C.; Bennett, J.M.; Liebman, H.A.; Bernstein-Singer, M.; Espina, B.M.; Cabral, L.; Allen, S.; et al. Treatment of adult T-cell leukemia-lymphoma with a combination of interferon alfa and zidovudine. N. Engl. J. Med. 1995, 332, 1744–1748. [Google Scholar] [CrossRef] [PubMed]
- Hermine, O.; Allard, I.; Levy, V.; Arnulf, B.; Gessain, A.; Bazarbachi, A. A prospective phase II clinical trial with the use of zidovudine and interferon-alpha in the acute and lymphoma forms of adult T-cell leukemia/lymphoma. Hematol. J. 2002, 3, 276–282. [Google Scholar] [CrossRef] [PubMed]
- Tsukasaki, K.; Tobinai, K.; Hotta, T.; Shimoyama, M. Lymphoma study group of JCOG. Jpn. J. Clin. Oncol. 2012, 42, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Hodson, A.; Crichton, S.; Montoto, S.; Mir, N.; Matutes, E.; Cwynarski, K.; Kumaran, T.; Ardeshna, K.M.; Pagliuca, A.; Taylor, G.P.; et al. Use of zidovudine and interferon alfa with chemotherapy improves survival in both acute and lymphoma subtypes of adult T-cell leukemia/lymphoma. J. Clin. Oncol. 2011, 29, 4696–4701. [Google Scholar] [CrossRef] [PubMed]
- Ratner, L.; Harrington, W.; Feng, X.; Grant, C.; Jacobson, S.; Noy, A.; Sparano, J.; Lee, J.; Ambinder, R.; Campbell, N.; et al. Human T cell leukemia virus reactivation with progression of adult T-cell leukemia-lymphoma. PLoS ONE 2009, 4, e4420. [Google Scholar] [CrossRef] [PubMed]
- Simone, C.B., 2nd; Morris, J.C.; Stewart, D.M.; Urquhart, N.E.; Janik, J.E.; Kreitman, R.J.; Lita, E.; Conlon, K.; Wharfe, G.; Waldmann, T.A.; et al. Radiation therapy for the management of patients with HTLV-1-associated adult T-cell leukemia/lymphoma. Blood 2012, 120, 1816–1819. [Google Scholar] [CrossRef] [PubMed]
- Tsukasaki, K.; Hermine, O.; Bazarbachi, A.; Ratner, L.; Ramos, J.C.; Harrington, W., Jr.; O’Mahony, D.; Janik, J.E.; Bittencourt, A.L.; Taylor, G.P.; et al. Definition, prognostic factors, treatment, and response criteria of adult T-cell leukemia-lymphoma: A proposal from an international consensus meeting. J. Clin. Oncol. 2009, 27, 453–459. [Google Scholar] [CrossRef] [PubMed]
- Chihara, D.; Ito, H.; Matsuda, T.; Katanoda, K.; Shibata, A.; Taniguchi, S.; Utsunomiya, A.; Sobue, T.; Matsuo, K. Association between decreasing trend in the mortality of adult T-cell leukemia/lymphoma and allogeneic hematopoietic stem cell transplants in Japan: Analysis of Japanese vital statistics and Japan Society for Hematopoietic Cell Transplantation (JSHCT). Blood Cancer J. 2013, 3, e159. [Google Scholar] [CrossRef] [PubMed]
- Phillips, A.A.; Willim, R.D.; Savage, D.G.; Horwitz, S.M.; Isola, L.; Zain, J.M.; O’Connor, O.A. A multi-institutional experience of autologous stem cell transplantation in North American patients with human T-cell lymphotropic virus type-1 adult T-cell leukemia/lymphoma suggests ineffective salvage of relapsed patients. Leuk. Lymphoma 2009, 50, 1039–1042. [Google Scholar] [CrossRef] [PubMed]
- Yonekura, K.; Utsunomiya, A.; Takatsuka, Y.; Takeuchi, S.; Tashiro, Y.; Kanzaki, T.; Kanekura, T. Graft-versus-adult T-cell leukemia/lymphoma effect following allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant. 2008, 41, 1029–1035. [Google Scholar] [CrossRef] [PubMed]
- Okamura, J.; Utsunomiya, A.; Tanosaki, R.; Uike, N.; Sonoda, S.; Kannagi, M.; Tomonaga, M.; Harada, M.; Kimura, N.; Masuda, M.; et al. Allogeneic stem-cell transplantation with reduced conditioning intensity as a novel immunotherapy and antiviral therapy for adult T-cell leukemia/lymphoma. Blood 2005, 105, 4143–4145. [Google Scholar] [CrossRef] [PubMed]
- Harashima, N.; Kurihara, K.; Utsunomiya, A.; Tanosaki, R.; Hanabuchi, S.; Masuda, M.; Ohashi, T.; Fukui, F.; Hasegawa, A.; Masuda, T.; et al. Graft-versus-Tax response in adult T-cell leukemia patients after hematopoietic stem cell transplantation. Cancer Res. 2004, 64, 391–399. [Google Scholar] [CrossRef] [PubMed]
- Shiratori, S.; Yasumoto, A.; Tanaka, J.; Shigematsu, A.; Yamamoto, S.; Nishio, M.; Hashino, S.; Morita, R.; Takahata, M.; Onozawa, M.; et al. A retrospective analysis of allogeneic hematopoietic stem cell transplantation for adult T cell leukemia/lymphoma (ATL): Clinical impact of graft-versus-leukemia/lymphoma effect. Biol. Blood Marrow Transplant. 2008, 14, 817–823. [Google Scholar] [CrossRef] [PubMed]
- Ishida, T.; Hishizawa, M.; Kato, K.; Tanosaki, R.; Fukuda, T.; Takatsuka, Y.; Eto, T.; Miyazaki, Y.; Hidaka, M.; Uike, N.; et al. Impact of graft-versus-host disease on allogeneic hematopoietic cell transplantation for adult T cell leukemia-lymphoma focusing on preconditioning regimens: Nationwide retrospective study. Biol. Blood Marrow Transplant. 2013, 19, 1731–1739. [Google Scholar] [CrossRef] [PubMed]
- Choi, I.; Tanosaki, R.; Uike, N.; Utsunomiya, A.; Tomonaga, M.; Harada, M.; Yamanaka, T.; Kannagi, M.; Okamura, J. Long-term outcomes after hematopoietic SCT for adult T-cell leukemia/lymphoma: Results of prospective trials. Bone Marrow Transplant. 2011, 46, 116–118. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Nakasone, H.; Yamazaki, R.; Wada, H.; Ishihara, Y.; Kawamura, K.; Sakamoto, K.; Ashizawa, M.; Machishima, T.; Sato, M.; et al. Long-term persistence of limited HTLV-I Tax-specific cytotoxic T cell clones in a patient with adult T cell leukemia/lymphoma after allogeneic stem cell transplantation. J. Clin. Immunol. 2012, 32, 1340–1352. [Google Scholar] [CrossRef] [PubMed]
- Hishizawa, M.; Kanda, J.; Utsunomiya, A.; Taniguchi, S.; Eto, T.; Moriuchi, Y.; Tanosaki, R.; Kawano, F.; Miyazaki, Y.; Masuda, M.; et al. Transplantation of allogeneic hematopoietic stem cells for adult T-cell leukemia: A nationwide retrospective study. Blood 2010, 116, 1369–1376. [Google Scholar] [CrossRef] [PubMed]
- Kawada, H.; Yoshimitsu, M.; Nakamura, D.; Arai, A.; Hayashida, M.; Kamada, Y.; Maekawa, K.; Fujino, S.; Arima, M.; Arima, N.; et al. A Retrospective Analysis of Treatment Outcomes in Adult T Cell Leukemia/Lymphoma Patients with Aggressive Disease Treated with or without Allogeneic Stem Cell Transplantation: A Single-Center Experience. Biol. Blood Marrow Transplant. 2015, 21, 696–700. [Google Scholar] [CrossRef] [PubMed]
- Kanda, J.; Hishizawa, M.; Utsunomiya, A.; Taniguchi, S.; Eto, T.; Moriuchi, Y.; Tanosaki, R.; Kawano, F.; Miyazaki, Y.; Masuda, M.; et al. Impact of graft-versus-host disease on outcomes after allogeneic hematopoietic cell transplantation for adult T-cell leukemia: A retrospective cohort study. Blood 2012, 119, 2141–2148. [Google Scholar] [CrossRef] [PubMed]
- Shigematsu, A.; Kobayashi, N.; Yasui, H.; Shindo, M.; Kakinoki, Y.; Koda, K.; Iyama, S.; Kuroda, H.; Tsutsumi, Y.; Imamura, M.; et al. High level of serum soluble interleukin-2 receptor at transplantation predicts poor outcome of allogeneic stem cell transplantation for adult T cell leukemia. Biol. Blood Marrow Transplant. 2014, 20, 801–805. [Google Scholar] [CrossRef] [PubMed]
- Ishida, T.; Hishizawa, M.; Kato, K.; Tanosaki, R.; Fukuda, T.; Taniguchi, S.; Eto, T.; Takatsuka, Y.; Miyazaki, Y.; Moriuchi, Y.; et al. Allogeneic hematopoietic stem cell transplantation for adult T-cell leukemia-lymphoma with special emphasis on preconditioning regimen: A nationwide retrospective study. Blood 2012, 120, 1734–1741. [Google Scholar] [CrossRef] [PubMed]
- Nakamizo, A.; Akagi, Y.; Amano, T.; Suzuki, S.O.; Otsuka, R.; Abe, Y.; Yoshimoto, K.; Iwaki, T.; Sasaki, T. Donor-derived adult T-cell leukaemia. Lancet 2011, 377, 1124. [Google Scholar] [CrossRef]
- Tamaki, H.; Matsuoka, M. Donor-derived T-cell leukemia after bone marrow transplantation. N. Engl. J. Med. 2006, 354, 1758–1759. [Google Scholar] [CrossRef] [PubMed]
- Kato, K.; Choi, I.; Wake, A.; Uike, N.; Taniguchi, S.; Moriuchi, Y.; Miyazaki, Y.; Nakamae, H.; Oku, E.; Murata, M.; et al. Treatment of patients with adult T cell leukemia/lymphoma with cord blood transplantation: A Japanese nationwide retrospective survey. Biol. Blood Marrow Transplant. 2014, 20, 1968–1974. [Google Scholar] [CrossRef] [PubMed]
- Cutler, C.; Li, S.; Ho, V.T.; Koreth, J.; Alyea, E.; Soiffer, R.J.; Antin, J.H. Extended follow-up of methotrexate-free immunosuppression using sirolimus and tacrolimus in related and unrelated donor peripheral blood stem cell transplantation. Blood 2007, 109, 3108–3114. [Google Scholar] [CrossRef] [PubMed]
- Darwiche, N.; Sinjab, A.; Abou-Lteif, G.; Chedid, M.B.; Hermine, O.; Dbaibo, G.; Bazarbachi, A. Inhibition of mammalian target of rapamycin signaling by everolimus induces senescence in adult T-cell leukemia/lymphoma and apoptosis in peripheral T-cell lymphomas. Int. J. Cancer 2011, 129, 993–1004. [Google Scholar] [CrossRef] [PubMed]
- Itonaga, H.; Tsushima, H.; Taguchi, J.; Fukushima, T.; Taniguchi, H.; Sato, S.; Ando, K.; Sawayama, Y.; Matsuo, E.; Yamasaki, R.; et al. Treatment of relapsed adult T-cell leukemia/lymphoma after allogeneic hematopoietic stem cell transplantation: The Nagasaki Transplant Group experience. Blood 2013, 121, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Kamimura, T.; Miyamoto, T.; Kawano, N.; Numata, A.; Ito, Y.; Chong, Y.; Nagafuji, K.; Teshima, T.; Hayashi, S.; Akashi, K. Successful treatment by donor lymphocyte infusion of adult T-cell leukemia/lymphoma relapse following allogeneic hematopoietic stem cell transplantation. Int. J. Hematol. 2012, 95, 725–730. [Google Scholar] [CrossRef] [PubMed]
- Subramaniam, J.M.; Whiteside, G.; McKeage, K.; Croxtall, J.C. Mogamulizumab: First global approval. Drugs 2012, 72, 1293–1298. [Google Scholar] [CrossRef] [PubMed]
- Ishida, T.; Joh, T.; Uike, N.; Yamamoto, K.; Utsunomiya, A.; Yoshida, S.; Saburi, Y.; Miyamoto, T.; Takemoto, S.; Suzushima, H.; et al. Defucosylated anti-CCR4 monoclonal antibody (KW-0761) for relapsed adult T-cell leukemia-lymphoma: A multicenter phase II study. J. Clin. Oncol. 2012, 30, 837–842. [Google Scholar] [CrossRef] [PubMed]
- Fanale, M.A.; Shustov, A.R.; Forero-Torres, A.; Bartlett, N.L.; Advani, R.H.; Pro, B.; Chen, R.W.; Davies, A.; Illidge, T.; Kennedy, D.A.; et al. Brentuximab vedotin administered concurrently with multi-agent chemotherapy as frontline treatment of ALCL and other CD30-positive mature T-cell and NK-cell lymphomas. ASH Annu. Meet. Abstr. 2012, 120, 60. [Google Scholar]
- Satou, Y.; Nosaka, K.; Koya, Y.; Yasunaga, J.I.; Toyokuni, S.; Matsuoka, M. Proteasome inhibitor, bortezomib, potently inhibits the growth of adult T-cell leukemia cells both in vivo and in vitro. Leukemia 2004, 18, 1357–1363. [Google Scholar] [CrossRef] [PubMed]
- Uike, N.; Ogura, M.; Imaizumi, Y.; Asou, N.; Utsunomiya, A.; Uchida, T.; Aoki, T.; Tsukasaki, K.; Taguchi, J.; Choi, I.; et al. Multicenter Phase I Dose-Escalation Study of Lenalidomide in Patients with Relapsed Adult T-Cell Leukemia-Lymphoma (ATL) or Peripheral T-Cell Lymphoma (PTCL). ASH Annu. Meet. Abstr. 2012, 120, 2737. [Google Scholar]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yared, J.A.; Kimball, A.S. Optimizing Management of Patients with Adult T Cell Leukemia-Lymphoma. Cancers 2015, 7, 2318-2329. https://doi.org/10.3390/cancers7040893
Yared JA, Kimball AS. Optimizing Management of Patients with Adult T Cell Leukemia-Lymphoma. Cancers. 2015; 7(4):2318-2329. https://doi.org/10.3390/cancers7040893
Chicago/Turabian StyleYared, Jean A., and Amy S. Kimball. 2015. "Optimizing Management of Patients with Adult T Cell Leukemia-Lymphoma" Cancers 7, no. 4: 2318-2329. https://doi.org/10.3390/cancers7040893





