Cancers 2011, 3(1), 872-882; https://doi.org/10.3390/cancers3010872 - 24 Feb 2011
Cited by 7 | Viewed by 13907
Abstract
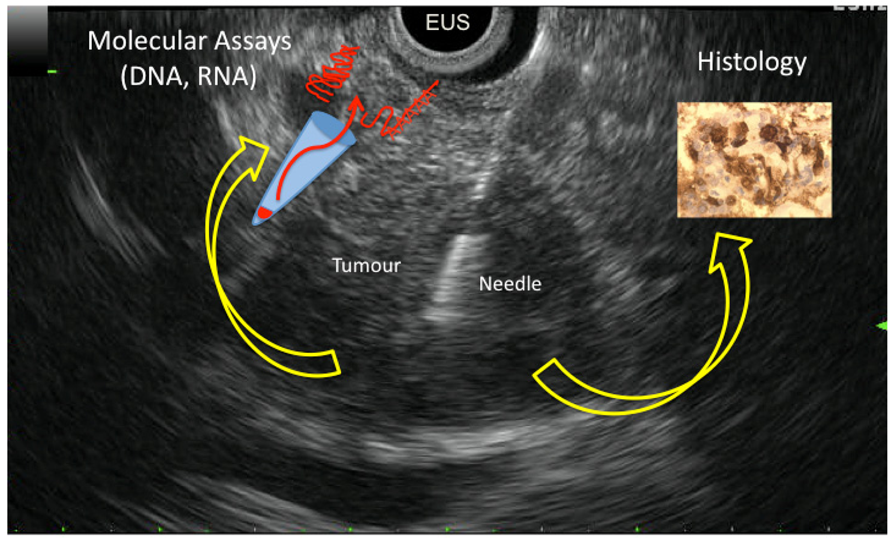
Endoscopic ultrasound-guided fine needle aspiration-biopsy is a safe and effective technique in diagnosing and staging of pancreatic ductal adenocarcinoma. However its predictive negative value does not exceed 50% to 60%. Unfortunately, the majority of pancreatic cancer patients have a metastatic and/or a locally
[...] Read more.
Endoscopic ultrasound-guided fine needle aspiration-biopsy is a safe and effective technique in diagnosing and staging of pancreatic ductal adenocarcinoma. However its predictive negative value does not exceed 50% to 60%. Unfortunately, the majority of pancreatic cancer patients have a metastatic and/or a locally advanced disease (i.e., not eligible for curative resection) which explains the limited access to pancreatic tissue specimens. Endoscopic ultrasound-guided fine needle aspiration-biopsy is the most widely used approach for cytological and histological material sampling in these situations used in up to two thirds of patients with pancreatic cancer. Based on this unique material, we and others developed strategies to improve the differential diagnosis between carcinoma and inflammatory pancreatic lesions by analysis of KRAS oncogene mutation, microRNA expression and methylation, as well as mRNA expression using both qRT-PCR and Low Density Array Taqman analysis. Indeed, differentiating pancreatic cancer from pseudotumoral chronic pancreatitis remains very difficult in current clinical practice, and endoscopic ultrasound-guided fine needle aspiration-biopsy analysis proved to be very helpful. In this review, we will compile the clinical and molecular advantages of using endoscopic ultrasound-guided fine needle aspiration-biopsy in managing pancreatic cancer.
Full article
(This article belongs to the Special Issue Pancreatic Cancer)
►
Show Figures