Advances in the Pathophysiology and Management of Cancer Pain: A Scoping Review
Simple Summary
Abstract
1. Introduction
1.1. Epidemiology and Clinical Burden of Cancer Pain
1.2. Pathophysiological Mechanisms
1.3. Current Management Approaches
1.4. Digital Health and Remote Monitoring
1.5. Rationale and Scope of This Review
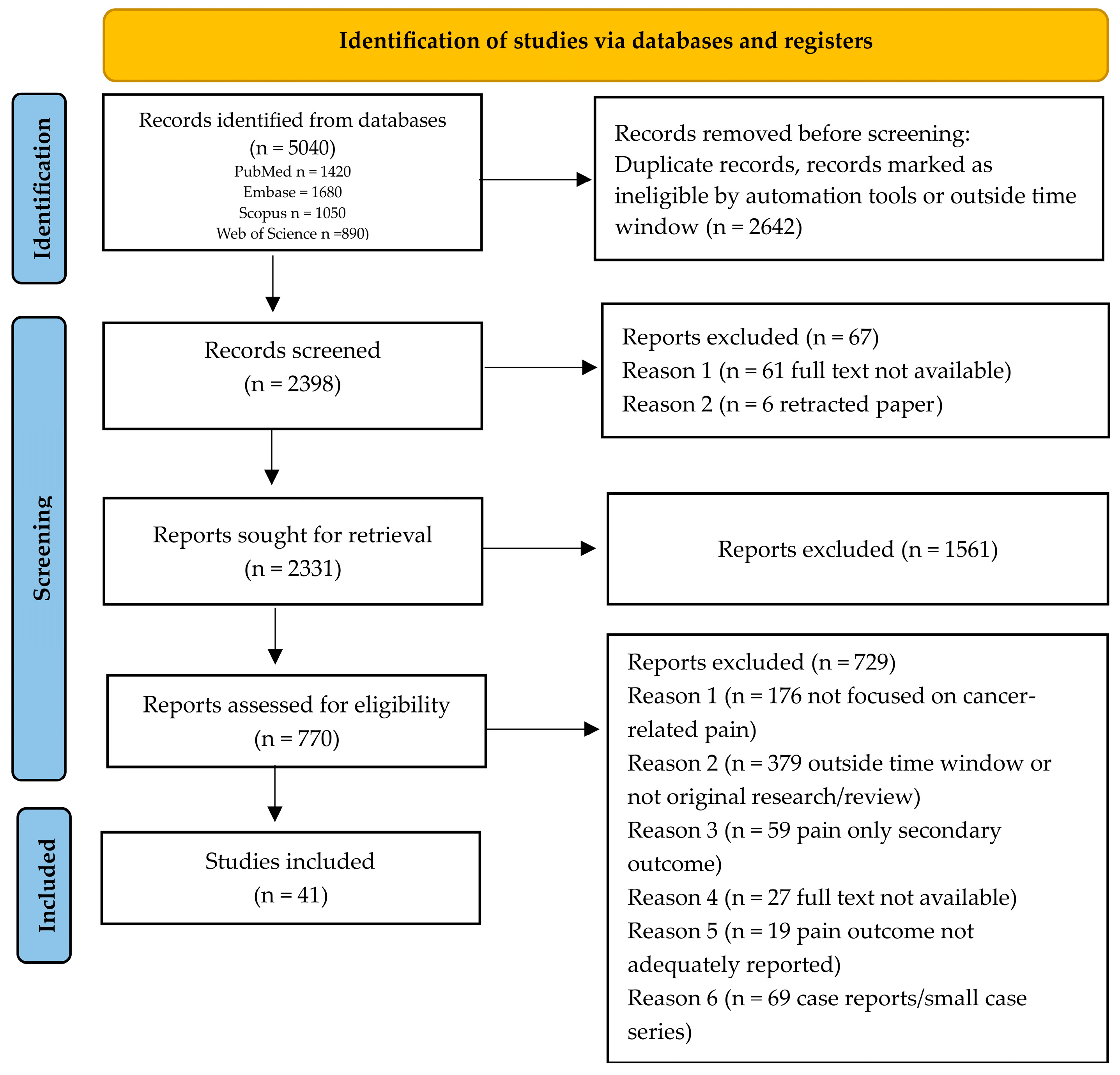
2. Materials and Methods
2.1. Study Design
2.2. Research Question
2.3. Eligibility Criteria
2.4. Search Strategy
3. Results
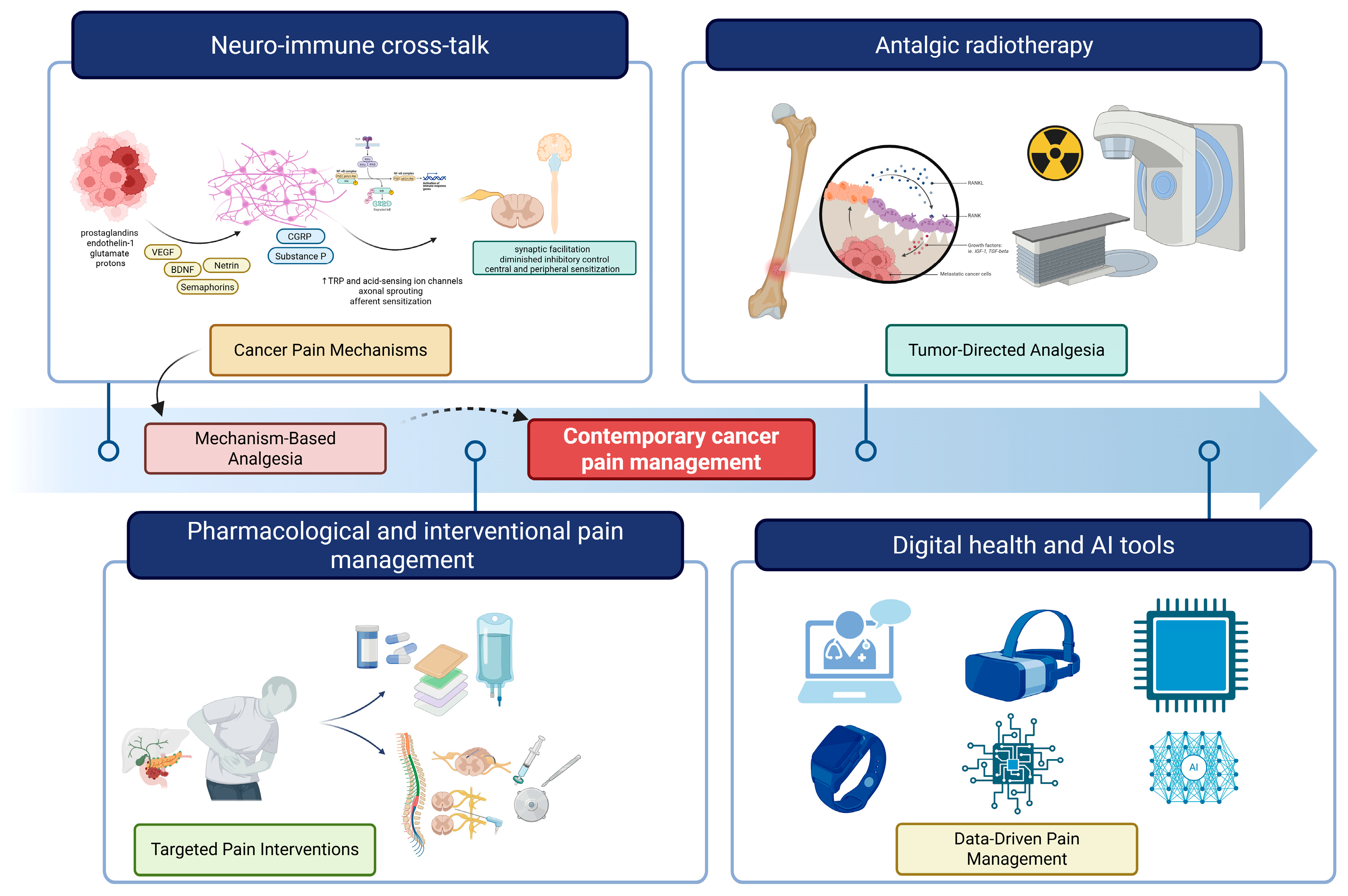
3.1. Advances in Biological Mechanisms
3.2. Pharmacological Management
3.2.1. Re-Evaluation of the WHO Analgesic Ladder
3.2.2. NSAIDs Show Limited High-Quality Evidence but Retain Clinical Utility
3.2.3. Targeted Biological Therapies: Anti-NGF Monoclonal Antibodies
3.2.4. Adjuvant Analgesics: Mechanism-Based Selection
- Neuropathic cancer pain (nerve compression, chemotherapy-induced peripheral neuropathy): Gabapentinoids (gabapentin, pregabalin) demonstrated efficacy [75,76]. Serotonin–norepinephrine reuptake inhibitors (duloxetine and venlafaxine) have demonstrated clinical benefit in alleviating refractory neuropathic pain in patients with cancer [77].
- Bone metastasis pain: Bisphosphonates (zoledronic acid, pamidronate) and RANKL inhibitors (denosumab) reduce osteoclast-mediated bone resorption, decreasing local acidosis and ATP release that activate periosteal nociceptors [78] demonstrate skeletal-related event reduction and modest analgesic benefit (number needed to treat 11 for pain reduction at 4 weeks) [79].
- Corticosteroids: Dexamethasone reduces peritumoral edema and inflammatory mediator release (prostaglandins, cytokines), providing analgesic effects particularly for visceral pain, bone pain, and neuropathic pain from nerve compression. However, long-term use requires careful risk-benefit assessment given immunosuppression, hyperglycemia, and myopathy concerns in cancer populations [68].
3.2.5. Opioid Optimization Strategies
3.2.6. Cannabinoids
3.3. Neuromodulatory Approaches
3.4. Intrathecal Drug Delivery Systems
3.5. Radiotherapy Developments
3.6. Digital Health, Remote Monitoring, and AI-Enabled Tools
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AI | Artificial Intelligence |
| ASCO | American Society of Clinical Oncology |
| ASIC | Acid-Sensing Ion Channel |
| ATX | Autotaxin |
| BDNF | Brain-Derived Neurotrophic Factor |
| CB1 | Cannabinoid Receptor 1 |
| CB2 | Cannabinoid Receptor 2 |
| CBD | Cannabidiol |
| cEBRT | Conventional External Beam Radiotherapy |
| CGRP | Calcitonin Gene-Related Peptide |
| CIPN | Chemotherapy-Induced Peripheral Neuropathy |
| COMT | Catechol-O-Methyltransferase |
| COVID-19 | Coronavirus Disease 2019 |
| COX | Cyclooxygenase |
| DNA | Deoxyribonucleic acid |
| DREZ | Dorsal Root Entry Zone |
| DRGS | Dorsal Root Ganglion Stimulation |
| EBRT | External Beam Radiotherapy |
| FDA | Food and Drug Administration |
| GAD1 | Glutamic Acid Decarboxylase 1 |
| GAD2 | Glutamic Acid Decarboxylase 2 |
| HDAC | Histone Deacetylase |
| IDDS | Intrathecal Drug Delivery Systems |
| IL | Interleukin |
| JBI | Joanna Briggs Institute |
| LPA | Lysophosphatidic Acid |
| MDSC | Myeloid-Derived Suppressor Cell |
| MEDD | Morphine Equivalent Daily Dose |
| MeSH | Medical Subject Headings |
| mHealth | Mobile Health |
| miRNA | MicroRNA |
| ML | Machine Learning |
| mRNA | Messenger RNA |
| MSK | Memorial Sloan Kettering |
| NGF | Nerve Growth Factor |
| NLP | Natural Language Processing |
| NNH | Number Needed to Harm |
| NNT | Number Needed to Treat |
| NRS | Numeric Rating Scale |
| NSAID | Non-Steroidal Anti-Inflammatory Drug |
| OME | Oral Morphine Equivalent |
| OPRD1 | Delta-Opioid Receptor Gene |
| OPRK1 | Kappa-Opioid Receptor Gene |
| OPRM1 | Mu-Opioid Receptor Gene |
| PAR2 | Protease-Activated Receptor 2 |
| PCC | Population–Concept–Context |
| PENK | Proenkephalin Gene |
| PNS | Peripheral Nerve Stimulation |
| PRAIS | Palliative Radiotherapy and Inflammation Study |
| PRISMA-ScR | Preferred Reporting Items for Systematic Reviews and Meta-Analyses Extension for Scoping Reviews |
| QOL | Quality of Life |
| RANKL | Receptor Activator of Nuclear Factor Kappa-B Ligand |
| RCT | Randomized Controlled Trial |
| RIPN | Radiation-Induced Peripheral Neuropathy |
| RNA | Ribonucleic Acid |
| RUA | Rational Use of Analgesics |
| SBRT | Stereotactic Body Radiotherapy |
| SCN9A | Voltage-Gated Sodium Channel 9A Gene |
| SCS | Spinal Cord Stimulation |
| sEVs | Small Extracellular Vesicles |
| SNP | Single Nucleotide Polymorphism |
| TAM | Tumor-Associated Macrophage |
| THC | Tetrahydrocannabinol |
| TME | Tumor Microenvironment |
| TNF | Tumor Necrosis Factor |
| TrkA | Tropomyosin Receptor Kinase A |
| TRP | Transient Receptor Potential |
| TRPV1 | Transient Receptor Potential Vanilloid 1 |
| TRPV4 | Transient Receptor Potential Vanilloid 4 |
| WHO | World Health Organization |
References
- Getie, A.; Ayalneh, M.; Bimerew, M. Global Prevalence and Determinant Factors of Pain, Depression, and Anxiety among Cancer Patients: An Umbrella Review of Systematic Reviews and Meta-Analyses. BMC Psychiatry 2025, 25, 156. [Google Scholar] [CrossRef] [PubMed]
- Copenhaver, D.J.; Huang, M.; Singh, J.; Fishman, S.M. History and Epidemiology of Cancer Pain. Cancer Treat. Res. 2021, 182, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Snijders, R.A.H.; Brom, L.; Theunissen, M.; van den Beuken-van Everdingen, M.H.J. Update on Prevalence of Pain in Patients with Cancer 2022: A Systematic Literature Review and Meta-Analysis. Cancers 2023, 15, 591. [Google Scholar] [CrossRef] [PubMed]
- Haier, J.; Schaefers, J. Economic Perspective of Cancer Care and Its Consequences for Vulnerable Groups. Cancers 2022, 14, 3158. [Google Scholar] [CrossRef]
- Varrassi, G.; Farì, G.; Narvaez Tamayo, M.A.; Gomez, M.P.; Guerrero Liñeiro, A.M.; Pereira, C.L.; Samy Aziz, E.; Gharibo, C.; Kaye, A.D.; Garcia-Larrea, L.; et al. Mixed Pain: Clinical Practice Recommendations. Front. Med. 2025, 12, 1659490. [Google Scholar] [CrossRef]
- Huang, Ǫ.; Hu, B.; Zhang, P.; Yuan, Y.; Yue, S.; Chen, X.; Liang, J.; Tang, Z.; Zhang, B. Neuroscience of Cancer: Unraveling the Complex Interplay between the Nervous System, the Tumor and the Tumor Immune Microenvironment. Mol. Cancer 2025, 24, 24. [Google Scholar] [CrossRef]
- Ma, H.; Pan, Z.; Lai, B.; Li, M.; Wang, J. Contribution of Immune Cells to Cancer-Related Neuropathic Pain: An Updated Review. Mol. Pain 2023, 19, 17448069231182235. [Google Scholar] [CrossRef]
- Mardelle, U.; Bretaud, N.; Daher, C.; Feuillet, V. From Pain to Tumor Immunity: Influence of Peripheral Sensory Neurons in Cancer. Front. Immunol. 2024, 15, 1335387. [Google Scholar] [CrossRef]
- Huang, S.; Zhu, J.; Yu, L.; Huang, Y.; Hu, Y. Cancer-Nervous System Crosstalk: From Biological Mechanism to Therapeutic Opportunities. Mol. Cancer 2025, 24, 133. [Google Scholar] [CrossRef]
- Yang, J.-X.; Wang, H.-F.; Chen, J.-Z.; Li, H.-Y.; Hu, J.-C.; Yu, A.-A.; Wen, J.-J.; Chen, S.-J.; Lai, W.-D.; Wang, S.; et al. Potential Neuroimmune Interaction in Chronic Pain: A Review on Immune Cells in Peripheral and Central Sensitization. Front. Pain Res. 2022, 3, 946846. [Google Scholar] [CrossRef]
- Xiong, H.-Y.; Hendrix, J.; Schabrun, S.; Wyns, A.; Campenhout, J.V.; Nijs, J.; Polli, A. The Role of the Brain-Derived Neurotrophic Factor in Chronic Pain: Links to Central Sensitization and Neuroinflammation. Biomolecules 2024, 14, 71. [Google Scholar] [CrossRef] [PubMed]
- WHO Guidelines for the Pharmacological and Radiotherapeutic Management of Cancer Pain in Adults and Adolescents; WHO Guidelines Approved by the Guidelines Review Committee; World Health Organization: Geneva, Switzerland, 2018; ISBN 978-92-4-155039-0.
- Daud, M.L.; Simone, G.G.D. Management of Pain in Cancer Patients—An Update. Ecancermedicalscience 2024, 18, 1821. [Google Scholar] [CrossRef] [PubMed]
- Paice, J.A.; Bohlke, K.; Barton, D.; Craig, D.S.; El-Jawahri, A.; Hershman, D.L.; Kong, L.R.; Kurita, G.P.; LeBlanc, T.W.; Mercadante, S.; et al. Use of Opioids for Adults With Pain From Cancer or Cancer Treatment: ASCO Guideline. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2023, 41, 914–930. [Google Scholar] [CrossRef] [PubMed]
- Knegtmans, M.F.; Wauben, L.S.G.L.; Wagemans, M.F.M.; Oldenmenger, W.H. Home Telemonitoring Improved Pain Registration in Patients With Cancer. Pain Pract. Off. J. World Inst. Pain 2020, 20, 122–128. [Google Scholar] [CrossRef]
- Levac, D.; Colquhoun, H.; O’Brien, K.K. Scoping Studies: Advancing the Methodology. Implement. Sci. IS 2010, 5, 69. [Google Scholar] [CrossRef]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef]
- Fan, H.-Y.; Liang, X.-H.; Tang, Y.-L. Neuroscience in Peripheral Cancers: Tumors Hijacking Nerves and Neuroimmune Crosstalk. MedComm 2024, 5, e784. [Google Scholar] [CrossRef]
- Yoneda, T.; Hiasa, M.; Okui, T.; Hata, K. Cancer-Nerve Interplay in Cancer Progression and Cancer-Induced Bone Pain. J. Bone Miner. Metab. 2023, 41, 415–427. [Google Scholar] [CrossRef]
- Shaikh, M.; Shirodkar, S.; Doshi, G. Unraveling the Role of Perineural Invasion in Cancer Progression across Multiple Tumor Types. Med. Oncol. Northwood Lond. Engl. 2025, 42, 283. [Google Scholar] [CrossRef]
- Santoni, A.; Santoni, M.; Arcuri, E. Chronic Cancer Pain: Opioids within Tumor Microenvironment Affect Neuroinflammation, Tumor and Pain Evolution. Cancers 2022, 14, 2253. [Google Scholar] [CrossRef]
- Martel Matos, A.A.; Scheff, N.N. Sensory Neurotransmission and Pain in Solid Tumor Progression. Trends Cancer 2025, 11, 309–320. [Google Scholar] [CrossRef] [PubMed]
- Haroun, R.; Wood, J.N.; Sikandar, S. Mechanisms of Cancer Pain. Front. Pain Res. 2022, 3, 1030899. [Google Scholar] [CrossRef] [PubMed]
- Ruivo, J.; Tavares, I.; Pozza, D.H. Molecular Targets in Bone Cancer Pain: A Systematic Review of Inflammatory Cytokines. J. Mol. Med. 2024, 102, 1063–1088. [Google Scholar] [CrossRef] [PubMed]
- Varrassi, G.; Leoni, M.L.G.; Farì, G.; Al-Alwany, A.A.; Al-Sharie, S.; Fornasari, D. Neuromodulatory Signaling in Chronic Pain Patients: A Narrative Review. Cells 2025, 14, 1320. [Google Scholar] [CrossRef]
- Santoni, A.; Mercadante, S.; Arcuri, E. Chronic Cancer and Non-Cancer Pain and Opioid-Induced Hyperalgesia Share Common Mechanisms: Neuroinflammation and Central Sensitization. Minerva Anestesiol. 2021, 87, 210–222. [Google Scholar] [CrossRef]
- Ji, R.-R.; Berta, T.; Nedergaard, M. Glia and Pain: Is Chronic Pain a Gliopathy? Pain 2013, 154, S10–S28. [Google Scholar] [CrossRef]
- Matsuda, M.; Huh, Y.; Ji, R.-R. Roles of Inflammation, Neurogenic Inflammation, and Neuroinflammation in Pain. J. Anesth. 2019, 33, 131–139. [Google Scholar] [CrossRef]
- Ferrini, F.; De Koninck, Y. Microglia Control Neuronal Network Excitability via BDNF Signalling. Neural Plast. 2013, 2013, 429815. [Google Scholar] [CrossRef]
- Ji, R.-R.; Donnelly, C.R.; Nedergaard, M. Astrocytes in Chronic Pain and Itch. Nat. Rev. Neurosci. 2019, 20, 667–685. [Google Scholar] [CrossRef]
- Chiang, C.-Y.; Wang, J.; Xie, Y.-F.; Zhang, S.; Hu, J.W.; Dostrovsky, J.O.; Sessle, B.J. Astroglial Glutamate-Glutamine Shuttle Is Involved in Central Sensitization of Nociceptive Neurons in Rat Medullary Dorsal Horn. J. Neurosci. Off. J. Soc. Neurosci. 2007, 27, 9068–9076. [Google Scholar] [CrossRef]
- Eroglu, C.; Barres, B.A. Regulation of Synaptic Connectivity by Glia. Nature 2010, 468, 223–231. [Google Scholar] [CrossRef]
- Ji, R.-R.; Nackley, A.; Huh, Y.; Terrando, N.; Maixner, W. Neuroinflammation and Central Sensitization in Chronic and Widespread Pain. Anesthesiology 2018, 129, 343–366. [Google Scholar] [CrossRef]
- Wang, W.-L.; Hao, Y.-H.; Pang, X.; Tang, Y.-L. Cancer Pain: Molecular Mechanisms and Management. Mol. Biomed. 2025, 6, 45. [Google Scholar] [CrossRef] [PubMed]
- Vanderwall, A.G.; Milligan, E.D. Cytokines in Pain: Harnessing Endogenous Anti-Inflammatory Signaling for Improved Pain Management. Front. Immunol. 2019, 10, 3009. [Google Scholar] [CrossRef] [PubMed]
- Bugada, D.; Lorini, L.F.; Fumagalli, R.; Allegri, M. Genetics and Opioids: Towards More Appropriate Prescription in Cancer Pain. Cancers 2020, 12, 1951. [Google Scholar] [CrossRef] [PubMed]
- Ho, K.W.D.; Wallace, M.R.; Staud, R.; Fillingim, R.B. OPRM1, OPRK1 and COMT Genetic Polymorphisms Associated with Opioid Effects on Experimental Pain: A Randomized, Double-Blind, Placebo-Controlled Study. Pharmacogenom. J. 2020, 20, 471–481. [Google Scholar] [CrossRef]
- Rembiałkowska, N.; Rekiel, K.; Urbanowicz, P.; Mamala, M.; Marczuk, K.; Wojtaszek, M.; Żywica, M.; Radzevičiūtė-Valčiukė, E.; Novickij, V.; Kulbacka, J. Epigenetic Dysregulation in Cancer: Implications for Gene Expression and DNA Repair-Associated Pathways. Int. J. Mol. Sci. 2025, 26, 6531. [Google Scholar] [CrossRef]
- Cao, B.; Xu, Ǫ.; Shi, Y.; Zhao, R.; Li, H.; Zheng, J.; Liu, F.; Wan, Y.; Wei, B. Pathology of Pain and Its Implications for Therapeutic Interventions. Signal Transduct. Target. Ther. 2024, 9, 155. [Google Scholar] [CrossRef]
- Crist, R.C.; Berrettini, W.H. Pharmacogenetics of OPRM1. Pharmacol. Biochem. Behav. 2014, 123, 25–33. [Google Scholar] [CrossRef]
- Gerra, M.C.; Dallabona, C.; Arendt-Nielsen, L. Epigenetic Alterations in Prescription Opioid Misuse: New Strategies for Precision Pain Management. Genes 2021, 12, 1226. [Google Scholar] [CrossRef]
- Hwang, C.K.; Wagley, Y.; Law, P.-Y.; Wei, L.-N.; Loh, H.H. Analysis of Epigenetic Mechanisms Regulating Opioid Receptor Gene Transcription. Methods Mol. Biol. 2015, 1230, 39–51. [Google Scholar] [CrossRef] [PubMed]
- García-Domínguez, M. Enkephalins and Pain Modulation: Mechanisms of Action and Therapeutic Perspectives. Biomolecules 2024, 14, 926. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Cai, Y.-Ǫ.; Zou, F.; Bie, B.; Pan, Z.Z. Epigenetic Suppression of GAD65 Expression Mediates Persistent Pain. Nat. Med. 2011, 17, 1448–1455. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Lei, Y.; Tian, Y.; Xu, S.; Shen, X.; Wu, H.; Bao, S.; Wang, F. The Etiological Contribution of GABAergic Plasticity to the Pathogenesis of Neuropathic Pain. Mol. Pain 2019, 15, 1744806919847366. [Google Scholar] [CrossRef]
- Imran, K.; Iqbal, M.J.; Ahmed, M.M.; Khalid, A.; Cortés, H.; Reyes-Hernández, O.D.; Figueroa-González, G.; Leyva-Gómez, G.; Falzone, L.; Libra, M.; et al. Epigenetic Dysregulation in Cancer: Mechanisms, Diagnostic Biomarkers and Therapeutic Strategies. Med. Oncol. 2025, 42, 359. [Google Scholar] [CrossRef]
- Khangura, R.K.; Bali, A.; Jaggi, A.S.; Singh, N. Histone Acetylation and Histone Deacetylation in Neuropathic Pain: An Unresolved Puzzle? Eur. J. Pharmacol. 2017, 795, 36–42. [Google Scholar] [CrossRef]
- Jiang, W.; Zhang, L.-X.; Tan, X.-Y.; Yu, P.; Dong, M. Inflammation and Histone Modification in Chronic Pain. Front. Immunol. 2022, 13, 1087648. [Google Scholar] [CrossRef]
- Jackson, S.; Gigliobianco, M.R.; Casadidio, C.; Di Martino, P.; Censi, R. MicroRNA-Based Delivery Systems for Chronic Neuropathic Pain Treatment in Dorsal Root Ganglion. Pharmaceutics 2025, 17, 930. [Google Scholar] [CrossRef]
- Frankel, L.B.; Christoffersen, N.R.; Jacobsen, A.; Lindow, M.; Krogh, A.; Lund, A.H. Programmed Cell Death 4 (PDCD4) Is an Important Functional Target of the microRNA miR-21 in Breast Cancer Cells. J. Biol. Chem. 2008, 283, 1026–1033. [Google Scholar] [CrossRef]
- Li, X.; Fan, Ǫ.; Li, J.; Song, J.; Gu, Y. MiR-124 down-Regulation Is Critical for Cancer Associated Fibroblasts-Enhanced Tumor Growth of Oral Carcinoma. Exp. Cell Res. 2017, 351, 100–108. [Google Scholar] [CrossRef]
- Li, Y.; Jiang, Y.; Hu, Z.; Yang, S.; Li, L.; Xiong, C.; Gao, Y.; Sun, W.; Zhang, Y. Protein Kinase C Family: Structures, Biological Functions, Diseases, and Pharmaceutical Interventions. MedComm 2025, 6, e70474. [Google Scholar] [CrossRef] [PubMed]
- Pu, T.; Sun, J.; Ren, G.; Li, H. Neuro-Immune Crosstalk in Cancer: Mechanisms and Therapeutic Implications. Signal Transduct. Target. Ther. 2025, 10, 176. [Google Scholar] [CrossRef]
- Worsley, C.M.; Veale, R.B.; Mayne, E.S. The Acidic Tumour Microenvironment: Manipulating the Immune Response to Elicit Escape. Hum. Immunol. 2022, 83, 399–408. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A.; Sica, A.; Allavena, P.; Garlanda, C.; Locati, M. Tumor-Associated Macrophages and the Related Myeloid-Derived Suppressor Cells as a Paradigm of the Diversity of Macrophage Activation. Hum. Immunol. 2009, 70, 325–330. [Google Scholar] [CrossRef] [PubMed]
- Zhen, G.; Fu, Y.; Zhang, C.; Ford, N.C.; Wu, X.; Wu, Q.; Yan, D.; Chen, X.; Cao, X.; Guan, Y. Mechanisms of Bone Pain: Progress in Research from Bench to Bedside. Bone Res. 2022, 10, 44. [Google Scholar] [CrossRef]
- Ferraguti, G.; Terracina, S.; Tarani, L.; Fanfarillo, F.; Allushi, S.; Caronti, B.; Tirassa, P.; Polimeni, A.; Lucarelli, M.; Cavalcanti, L.; et al. Nerve Growth Factor and the Role of Inflammation in Tumor Development. Curr. Issues Mol. Biol. 2024, 46, 965–989. [Google Scholar] [CrossRef]
- Cata, J.P.; Uhelski, M.L.; Gorur, A.; Dougherty, P.M. Nociception and Pain: New Roles for Exosomes. Neurosci. Rev. J. Bringing Neurobiol. Neurol. Psychiatry 2022, 28, 349–363. [Google Scholar] [CrossRef]
- Schmidt, B.L. The Neurobiology of Cancer Pain. Neurosci. Rev. J. Bringing Neurobiol. Neurol. Psychiatry 2014, 20, 546–562. [Google Scholar] [CrossRef]
- Dubeykovskaya, Z.A.; Tu, N.H.; Garcia, P.D.R.; Schmidt, B.L.; Albertson, D.G. Oral Cancer Cells Release Vesicles That Cause Pain. Adv. Biol. 2022, 6, e2200073. [Google Scholar] [CrossRef]
- Bhansali, D.; Tu, N.H.; Inoue, K.; Teng, S.; Li, T.; Tran, H.D.; Kim, D.H.; Dong, J.; Peach, C.J.; Sokrat, B.; et al. PAR2 on Oral Cancer Cells and Nociceptors Contributes to Oral Cancer Pain That Can Be Relieved by Nanoparticle-Encapsulated AZ3451. Biomaterials 2025, 314, 122874. [Google Scholar] [CrossRef]
- Khasabova, I.A.; Khasabov, S.G.; Johns, M.; Juliette, J.; Zheng, A.; Morgan, H.; Flippen, A.; Allen, K.; Golovko, M.Y.; Golovko, S.A.; et al. Exosome-Associated Lysophosphatidic Acid Signaling Contributes to Cancer Pain. Pain 2023, 164, 2684–2695. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Su, Y.; Zhong, S.; Cong, L.; Liu, B.; Yang, J.; Tao, Y.; He, Z.; Chen, C.; Jiang, Y. Exosomes: Key Players in Cancer and Potential Therapeutic Strategy. Signal Transduct. Target. Ther. 2020, 5, 145. [Google Scholar] [CrossRef] [PubMed]
- Pudło, K.; Żylicz, Z. Potential Role of Microbiota in Oncology and Palliative Care. Palliat. Med. Pract. 2024, 18, 95–102. [Google Scholar] [CrossRef]
- Fernandez, E.; Wargo, J.A.; Helmink, B.A. The Microbiome and Cancer: A Translational Science Review. JAMA 2025, 333, 2188–2196. [Google Scholar] [CrossRef]
- Fallon, M.; Dierberger, K.; Leng, M.; Hall, P.S.; Allende, S.; Sabar, R.; Verastegui, E.; Gordon, D.; Grant, L.; Lee, R.; et al. An International, Open-Label, Randomised Trial Comparing a Two-Step Approach versus the Standard Three-Step Approach of the WHO Analgesic Ladder in Patients with Cancer. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2022, 33, 1296–1303. [Google Scholar] [CrossRef]
- Page, A.J.; Mulvey, M.R.; Bennett, M.I. Designing a Clinical Trial of Non-Steroidal Anti-Inflammatory Drugs for Cancer Pain: A Survey of UK Palliative Care Physicians. BMJ Support. Palliat. Care 2023, 13, e55–e58. [Google Scholar] [CrossRef]
- Shapoo, N.; Rehman, A.; Izaguirre-Rojas, C.; Gotlieb, V.; Boma, N. Cancer Pain Is Not One-Size-Fits-All: Evolving from Tradition to Precision. Clin. Pract. 2025, 15, 173. [Google Scholar] [CrossRef]
- Senthilnathan, V.; Priyadharshini, V.; Jenifer Selin Asha, J. From Pain Relief to Cancer Defense: The Promise of NSAIDs. J. Adv. Med. Pharm. Sci. 2023, 25, 59–67. [Google Scholar] [CrossRef]
- Abdel Shaheed, C.; Hayes, C.; Maher, C.G.; Ballantyne, J.C.; Underwood, M.; McLachlan, A.J.; Martin, J.H.; Narayan, S.W.; Sidhom, M.A. Opioid Analgesics for Nociceptive Cancer Pain: A Comprehensive Review. CA. Cancer J. Clin. 2024, 74, 286–313. [Google Scholar] [CrossRef]
- Flausino, L.E.; Ferreira, I.N.; Tuan, W.-J.; Estevez-Diz, M.D.P.; Chammas, R. Association of COX-Inhibitors with Cancer Patients’ Survival under Chemotherapy and Radiotherapy Regimens: A Real-World Data Retrospective Cohort Analysis. Front. Oncol. 2024, 14, 1433497. [Google Scholar] [CrossRef]
- Fallon, M.; Sopata, M.; Dragon, E.; Brown, M.T.; Viktrup, L.; West, C.R.; Bao, W.; Agyemang, A. A Randomized Placebo-Controlled Trial of the Anti-Nerve Growth Factor Antibody Tanezumab in Subjects with Cancer Pain Due to Bone Metastasis. Oncologist 2023, 28, e1268–e1278. [Google Scholar] [CrossRef]
- Nwosu, A.D.G.; Chukwu, L.C.; Onwuasoigwe, O.; Nweze, S.O.; Nwadike, K. Redefining the Role of Analgesic Adjuvants in Pain Management: A Narrative Review. Indian J. Pain 2023, 37, 65. [Google Scholar] [CrossRef]
- Wong, A.K.; Hawke, J.; Eastman, P.; Buizen, L.; Le, B. Does Cancer Type and Adjuvant Analgesic Prescribing Influence Opioid Dose?-A Retrospective Cross-Sectional Study. Ann. Palliat. Med. 2023, 12, 783–790. [Google Scholar] [CrossRef] [PubMed]
- Chang, T.W.; Yang, F.-Y.; Liu, Y.-C.; Hung, C.-H. Gabapentinoids for Chemotherapy-Induced Peripheral Neuropathy: Systematic Review and Meta-Analysis. BMJ Support. Palliat. Care 2024, 14, 269–278. [Google Scholar] [CrossRef] [PubMed]
- Bao, H.; Wu, Z.; Wang, Q.; Wang, J.; Zhang, L.; Meng, L.; Han, F. The Efficacy of Gabapentin Combined with Opioids for Neuropathic Cancer Pain: A Meta-Analysis. Transl. Cancer Res. 2021, 10, 637–644. [Google Scholar] [CrossRef]
- Matsuoka, H.; Iwase, S.; Miyaji, T.; Kawaguchi, T.; Ariyoshi, K.; Oyamada, S.; Satomi, E.; Ishiki, H.; Hasuo, H.; Sakuma, H.; et al. Additive Duloxetine for Cancer-Related Neuropathic Pain Nonresponsive or Intolerant to Opioid-Pregabalin Therapy: A Randomized Controlled Trial (JORTC-PAL08). J. Pain Symptom Manag. 2019, 58, 645–653. [Google Scholar] [CrossRef]
- Huang, X.-L.; Liu, C.; Shi, X.-M.; Cheng, Y.-T.; Zhou, Ǫ.; Li, J.-P.; Liao, J. Zoledronic Acid Inhibits Osteoclastogenesis and Bone Resorptive Function by Suppressing RANKL-mediated NF-κB and JNK and Their Downstream Signalling Pathways. Mol. Med. Rep. 2022, 25, 59. [Google Scholar] [CrossRef]
- Coleman, R.; Hadji, P.; Body, J.-J.; Santini, D.; Chow, E.; Terpos, E.; Oudard, S.; Bruland, Ø.; Flamen, P.; Kurth, A.; et al. Bone Health in Cancer: ESMO Clinical Practice Guidelines. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2020, 31, 1650–1663. [Google Scholar] [CrossRef]
- Kelleher, D.C.; Kirksey, M.A.; Wu, C.L.; Cheng, S.I. Integrating Complementary Medicine in the Perioperative Period: A Simple, Opioid-Sparing Addition to Your Multimodal Analgesia Strategy? Reg. Anesth. Pain Med. 2020, 45, 468–473. [Google Scholar] [CrossRef]
- Mohiuddin, A.L.; Ahmed, Z. Suzetrigine—A Novel FDA-Approved Analgesic—Opportunities, Challenges and Future Perspectives: A Perspective Review. Health Sci. Rep. 2025, 8, e71545. [Google Scholar] [CrossRef]
- Bertoch, T.; D’Aunno, D.; McCoun, J.; Solanki, D.; Taber, L.; Urban, J.; Oswald, J.; Swisher, M.W.; Tian, S.; Miao, X.; et al. Suzetrigine, a Nonopioid Na V 1.8 Inhibitor for Treatment of Moderate-to-Severe Acute Pain: Two Phase 3 Randomized Clinical Trials. Anesthesiology 2025, 142, 1085–1099. [Google Scholar] [CrossRef] [PubMed]
- Al-Husinat, L.; Obeidat, S.; Azzam, S.; Al-Gwairy, Y.; Obeidat, F.; Al Sharie, S.; Haddad, D.; Haddad, F.; Rekatsina, M.; Leoni, M.L.G.; et al. Role of Cannabis in the Management of Chronic Non-Cancer Pain: A Narrative Review. Clin. Pract. 2025, 15, 16. [Google Scholar] [CrossRef] [PubMed]
- Gorzo, A.; Havași, A.; Spînu, S.; Oprea, A.; Burz, C.; Sur, D. Practical Considerations for the Use of Cannabis in Cancer Pain Management-What a Medical Oncologist Should Know. J. Clin. Med. 2022, 11, 5036. [Google Scholar] [CrossRef] [PubMed]
- Lichtman, A.H.; Lux, E.A.; McǪuade, R.; Rossetti, S.; Sanchez, R.; Sun, W.; Wright, S.; Kornyeyeva, E.; Fallon, M.T. Results of a Double-Blind, Randomized, Placebo-Controlled Study of Nabiximols Oromucosal Spray as an Adjunctive Therapy in Advanced Cancer Patients with Chronic Uncontrolled Pain. J. Pain Symptom Manag. 2018, 55, 179–188.e1. [Google Scholar] [CrossRef]
- Häuser, W.; Welsch, P.; Klose, P.; Radbruch, L.; Fitzcharles, M.-A. Efficacy, Tolerability and Safety of Cannabis-Based Medicines for Cancer Pain: A Systematic Review with Meta-Analysis of Randomised Controlled Trials. Schmerz Berl. Ger. 2019, 33, 424–436. [Google Scholar] [CrossRef]
- Yanes, J.A.; McKinnell, Z.E.; Reid, M.A.; Busler, J.N.; Michel, J.S.; Pangelinan, M.M.; Sutherland, M.T.; Younger, J.W.; Gonzalez, R.; Robinson, J.L. Effects of Cannabinoid Administration for Pain: A Meta-Analysis and Meta-Regression. Exp. Clin. Psychopharmacol. 2019, 27, 370–382. [Google Scholar] [CrossRef]
- Bar-Sela, G.; Zalman, D.; Semenysty, V.; Ballan, E. The Effects of Dosage-Controlled Cannabis Capsules on Cancer-Related Cachexia and Anorexia Syndrome in Advanced Cancer Patients: Pilot Study. Integr. Cancer Ther. 2019, 18, 1534735419881498. [Google Scholar] [CrossRef]
- Häuser, W.; Welsch, P.; Radbruch, L.; Fisher, E.; Bell, R.F.; Moore, R.A. Cannabis-Based Medicines and Medical Cannabis for Adults with Cancer Pain. Cochrane Database Syst. Rev. 2023, CD014915. [Google Scholar] [CrossRef]
- Schmidt, A.P. The Role of Cannabinoids in Chronic Pain Management: Clinical Insights and Challenges. Braz. J. Anesthesiol. 2024, 74, 844523. [Google Scholar] [CrossRef]
- Good, P.; Haywood, A.; Gogna, G.; Martin, J.; Yates, P.; Greer, R.; Hardy, J. Oral Medicinal Cannabinoids to Relieve Symptom Burden in the Palliative Care of Patients with Advanced Cancer: A Double-Blind, Placebo Controlled, Randomised Clinical Trial of Efficacy and Safety of Cannabidiol (CBD). BMC Palliat. Care 2019, 18, 110. [Google Scholar] [CrossRef]
- Christensen, C.; Allesø, M.; Rose, M.; Cornett, C. Clinical Research Evidence Supporting Administration and Dosing Recommendations of Medicinal Cannabis as Analgesic in Cancer Patients. J. Clin. Med. 2022, 12, 307. [Google Scholar] [CrossRef] [PubMed]
- Habib, M.H.; Schlögl, M.; Raza, S.; Chwistek, M.; Gulati, A. Interventional Pain Management in Cancer Patients-a Scoping Review. Ann. Palliat. Med. 2023, 12, 1198–1214. [Google Scholar] [CrossRef] [PubMed]
- Deo, S.V.S.; Kumar, N.; Rajendra, V.K.J.; Kumar, S.; Bhoriwal, S.K.; Ray, M.; Bhatnagar, S.; Mishra, S. Palliative Surgery for Advanced Cancer: Clinical Profile, Spectrum of Surgery and Outcomes from a Tertiary Care Cancer Centre in Low-Middle-Income Country. Indian J. Palliat. Care 2021, 27, 281–285. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, N.; Sindt, J.E.; Whittle, J.; Anderson, J.S.; Odell, D.W.; Mahan, M.; Brogan, S.E. Impact of Neuromodulation on Opioid Use, Adjunct Medication Use, and Pain Control in Cancer-Related Pain: A Retrospective Case Series. Pain Med. Malden Mass 2023, 24, 903–906. [Google Scholar] [CrossRef]
- Grenouillet, S.; Balayssac, D.; Moisset, X.; Peyron, R.; Fauchon, C. Analgesic Efficacy of Non-Invasive Neuromodulation Techniques in Chronic Cancer Pain: A Systematic Review. Support. Care Cancer Off. J. Multinatl. Assoc. Support. Care Cancer 2025, 33, 346. [Google Scholar] [CrossRef]
- Vu, P.D.; Mach, S.; Javed, S. Neurostimulation for the Treatment of Cancer-Induced Pain: A Scoping Review. Neuromodul. J. Int. Neuromodul. Soc. 2025, 28, 191–203. [Google Scholar] [CrossRef]
- Crowther, J.E.; Chen, G.H.; Legler, A.; Gulati, A. Spinal Cord Stimulation in the Treatment of Cancer Pain: A Retrospective Review. Neuromodul. J. Int. Neuromodul. Soc. 2022, 25, 693–699. [Google Scholar] [CrossRef]
- Bulat, E.; Chakravarthy, V.; Crowther, J.; Rakesh, N.; Barzilai, O.; Gulati, A. Exceptional Cases of Spinal Cord Stimulation for the Treatment of Refractory Cancer-Related Pain. Neuromodul. J. Int. Neuromodul. Soc. 2023, 26, 1051–1058. [Google Scholar] [CrossRef]
- Chung, M.; Abd-Elsayed, A. Comparative Efficacy of Closed-Loop Spinal Cord Stimulation and Dorsal Root Ganglion Stimulation through Combination Trialing for Cancer Pain—A Retrospective Case Series. Pain Pract. Off. J. World Inst. Pain 2025, 25, e70010. [Google Scholar] [CrossRef]
- Doyle, A.; Sharma, M.L.; Gupta, M.; Goebel, A.; Marley, K. Percutaneous Cervical Cordotomy for Cancer-Related Pain: Prospective Multimodal Outcomes Evaluation. BMJ Support. Palliat. Care 2022, 12, e21–e27. [Google Scholar] [CrossRef]
- Viswanathan, A.; Vedantam, A.; Hess, K.R.; Ochoa, J.; Dougherty, P.M.; Reddy, A.S.; Koyyalagunta, D.; Reddy, S.; Bruera, E. Minimally Invasive Cordotomy for Refractory Cancer Pain: A Randomized Controlled Trial. Oncologist 2019, 24, e590–e596. [Google Scholar] [CrossRef]
- Morais, M.V.; Lopes, R.A.; Oliveira Júnior, J.O. Cordotomy for Pain Control and Opioid Reduction in Cancer Patients: A Cancer Center 11-Year Experience. Eur. J. Surg. Oncol. J. Eur. Soc. Surg. Oncol. Br. Assoc. Surg. Oncol. 2024, 50, 108571. [Google Scholar] [CrossRef] [PubMed]
- Shepherd, T.M.; Hoch, M.J.; Cohen, B.A.; Bruno, M.T.; Fieremans, E.; Rosen, G.; Pacione, D.; Mogilner, A.Y. Palliative CT-Guided Cordotomy for Medically Intractable Pain in Patients with Cancer. AJNR Am. J. Neuroradiol. 2017, 38, 387–390. [Google Scholar] [CrossRef] [PubMed]
- Adams, J.L.; Goble, G.; Johnson, A. Multidisciplinary Approaches: Cingulotomy in an Adult With Refractory Neuropathic Cancer-Related Pain. J. Palliat. Med. 2023, 26, 1297–1301. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, H.; Sandhu, H.; Tombazzi, C.; Paulo, D.; Tillman, S.; Misra, S.; Shah, H.; Karlekar, M. Cingulotomy for Refractory Metastatic Cancer Pain: A Case Series (Sci215). J. Pain Symptom Manag. 2023, 65, e647–e648. [Google Scholar] [CrossRef]
- Strauss, I.; Berger, A.; Ben Moshe, S.; Arad, M.; Hochberg, U.; Gonen, T.; Tellem, R. Double Anterior Stereotactic Cingulotomy for Intractable Oncological Pain. Stereotact. Funct. Neurosurg. 2017, 95, 400–408. [Google Scholar] [CrossRef]
- McBenedict, B.; Hauwanga, W.N.; Pires, M.P.; Netto, J.G.M.; Petrus, D.; Kanchwala, J.A.; Joshi, R.; Alurkar, S.R.A.; Chankseliani, O.; Mansoor, Z.; et al. Cingulotomy for Intractable Pain: A Systematic Review of an Underutilized Procedure. Cureus 2024, 16, e56746. [Google Scholar] [CrossRef]
- Mongardi, L.; Visani, J.; Mantovani, G.; Vitali, C.; Ricciardi, L.; Giordano, F.; Cavallo, M.A.; Lofrese, G.; D’andrea, M.; Roblot, P.; et al. Long Term Results of Dorsal Root Entry Zone (DREZ) Lesions for the Treatment of Intractable Pain: A Systematic Review of the Literature on 1242 Cases. Clin. Neurol. Neurosurg. 2021, 210, 107004. [Google Scholar] [CrossRef]
- Ferraresi, S.; Basso, E.; Maistrello, L.; Scerrati, A.; Di Pasquale, P. Dorsal Root Entry Zone Lesion: Nuances of the Technique and Long-Term Results. Neurosurg. Focus Video 2020, 3, V13. [Google Scholar] [CrossRef]
- Gadgil, N.; Viswanathan, A. DREZotomy in the Treatment of Cancer Pain: A Review. Stereotact. Funct. Neurosurg. 2012, 90, 356–360. [Google Scholar] [CrossRef]
- Chivukula, S.; Tempel, Z.J.; Chen, C.-J.; Shin, S.S.; Gande, A.V.; Moossy, J.J. Spinal and Nucleus Caudalis Dorsal Root Entry Zone Lesioning for Chronic Pain: Efficacy and Outcomes. World Neurosurg. 2015, 84, 494–504. [Google Scholar] [CrossRef]
- Altamirano, J.M.; Khalid, S.I.; Slavin, K.V. Neurosurgical Techniques for Chronic Pain in Adult Cancer Survivors. Stereotact. Funct. Neurosurg. 2025, 103, 566–575. [Google Scholar] [CrossRef] [PubMed]
- Potocnik, I.; Strazisar, B.; Lenasi, H.; Zupanc, T. Breaking the Pain Barrier: Implantable Intrathecal Pump Therapy as a Game-Changer in Cancer Pain Management. Radiol. Oncol. 2025, 59, 477–487. [Google Scholar] [CrossRef] [PubMed]
- De Andres, J.; Hayek, S.; Perruchoud, C.; Lawrence, M.M.; Reina, M.A.; De Andres-Serrano, C.; Rubio-Haro, R.; Hunt, M.; Yaksh, T.L. Intrathecal Drug Delivery: Advances and Applications in the Management of Chronic Pain Patient. Front. Pain Res. 2022, 3, 900566. [Google Scholar] [CrossRef] [PubMed]
- Smith, T.J.; Coyne, P.J.; Staats, P.S.; Deer, T.; Stearns, L.J.; Rauck, R.L.; Boortz-Marx, R.L.; Buchser, E.; Català, E.; Bryce, D.A.; et al. An Implantable Drug Delivery System (IDDS) for Refractory Cancer Pain Provides Sustained Pain Control, Less Drug-Related Toxicity, and Possibly Better Survival Compared with Comprehensive Medical Management (CMM). Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2005, 16, 825–833. [Google Scholar] [CrossRef]
- Goel, V.; Kumar, V.; Blaes, A.; Gulati, A. Intrathecal Drug Delivery Systems for Cancer Pain Control: Insights on Current Contemporary Practices in the US. Neuromodul. J. Int. Neuromodul. Soc. 2023, 26, 1256–1262. [Google Scholar] [CrossRef]
- Ontario Health. Intrathecal Drug Delivery Systems for Cancer Pain: A Health Technology Assessment. Ont. Health Technol. Assess. Ser. 2024, 24, 1–162. [Google Scholar]
- Wang, W.; Shi, Ǫ.; Cao, Y.; Fan, B.; Yang, Y. Intrathecal Drug Delivery Systems for Cancer Pain: A Retrospective Analysis at a Single Tertiary Medical Center in China. Heliyon 2024, 10, e34522. [Google Scholar] [CrossRef]
- Stearns, L.M.; Abd-Elsayed, A.; Perruchoud, C.; Spencer, R.; Hammond, K.; Stromberg, K.; Weaver, T. Intrathecal Drug Delivery Systems for Cancer Pain: An Analysis of a Prospective, Multicenter Product Surveillance Registry. Anesth. Analg. 2020, 130, 289–297. [Google Scholar] [CrossRef]
- Sindt, J.E.; Odell, D.W.; Dalley, A.P.; Brogan, S.E. Initiation of Intrathecal Drug Delivery Dramatically Reduces Systemic Opioid Use in Patients With Advanced Cancer. Neuromodul. J. Int. Neuromodul. Soc. 2020, 23, 978–983. [Google Scholar] [CrossRef]
- Deer, T.R.; Hayek, S.M.; Grider, J.S.; Pope, J.E.; Brogan, S.E.; Gulati, A.; Hagedorn, J.M.; Strand, N.; Hah, J.; Yaksh, T.L.; et al. The Polyanalgesic Consensus Conference (PACC)®: Updates on Clinical Pharmacology and Comorbidity Management in Intrathecal Drug Delivery for Cancer Pain. Neuromodul. J. Int. Neuromodul. Soc. 2025, 28, 1029–1053. [Google Scholar] [CrossRef]
- Goel, V.; Yang, Y.; Kanwar, S.; Banik, R.K.; Patwardhan, A.M.; Ibrahim, M.; Sivanesan, E.; Shankar, H. Adverse Events and Complications Associated With Intrathecal Drug Delivery Systems: Insights From the Manufacturer and User Facility Device Experience (MAUDE) Database. Neuromodul. J. Int. Neuromodul. Soc. 2021, 24, 1181–1189. [Google Scholar] [CrossRef]
- Zheng, S.; He, L.; Yang, X.; Li, X.; Yang, Z. Evaluation of Intrathecal Drug Delivery System for Intractable Pain in Advanced Malignancies: A Prospective Cohort Study. Medicine 2017, 96, e6354. [Google Scholar] [CrossRef]
- Cilla, S.; Rossi, R.; Donati, C.M.; Habberstad, R.; Klepstad, P.; Dall’Agata, M.; Valenti, V.; Kaasa, S.; Medici, F.; Morganti, A.G.; et al. Pain Management Adequacy in Patients With Bone Metastases: A Secondary Analysis From the Palliative Radiotherapy and Inflammation Study Trial. Clin. Med. Insights Oncol. 2025, 19, 11795549241297054. [Google Scholar] [CrossRef]
- Murakami, S.; Kitani, A.; Kubota, T.; Uezono, Y. Increased Pain after Palliative Radiotherapy: Not Only Due to Cancer Progression. Ann. Palliat. Med. 2024, 13, 18–21. [Google Scholar] [CrossRef]
- Grosinger, A.J.; Alcorn, S.R. An Update on the Management of Bone Metastases. Curr. Oncol. Rep. 2024, 26, 400–408. [Google Scholar] [CrossRef] [PubMed]
- Bindels, B.J.J.; Mercier, C.; Gal, R.; Verlaan, J.-J.; Verhoeff, J.J.C.; Dirix, P.; Ost, P.; Kasperts, N.; van der Linden, Y.M.; Verkooijen, H.M.; et al. Stereotactic Body and Conventional Radiotherapy for Painful Bone Metastases: A Systematic Review and Meta-Analysis. JAMA Netw. Open 2024, 7, e2355409. [Google Scholar] [CrossRef] [PubMed]
- Rossi, R.; Medici, F.; Habberstad, R.; Klepstad, P.; Cilla, S.; Dall’Agata, M.; Kaasa, S.; Caraceni, A.T.; Morganti, A.G.; Maltoni, M. Development of a Predictive Model for Patients with Bone Metastases Referred to Palliative Radiotherapy: Secondary Analysis of a Multicenter Study (the PRAIS Trial). Cancer Med. 2024, 13, e70050. [Google Scholar] [CrossRef] [PubMed]
- Donati, C.M.; Galietta, E.; Cellini, F.; Di Rito, A.; Portaluri, M.; De Tommaso, C.; Santacaterina, A.; Tamburella, C.; Mammini, F.; Di Franco, R.; et al. Further Clarification of Pain Management Complexity in Radiotherapy: Insights from Modern Statistical Approaches. Cancers 2024, 16, 1407. [Google Scholar] [CrossRef]
- Konopka-Filippow, M.; Politynska, B.; Wojtukiewicz, A.M.; Wojtukiewicz, M.Z. Cancer Pain: Radiotherapy as a Double-Edged Sword. Int. J. Mol. Sci. 2025, 26, 5223. [Google Scholar] [CrossRef]
- Hamdoune, M.; Jounaidi, K.; Ammari, N.; Gantare, A. Digital Health for Cancer Symptom Management in Palliative Medicine: Systematic Review. BMJ Support. Palliat. Care 2024, 14, 392–402. [Google Scholar] [CrossRef]
- Paterson, C.; Bacon, R.; Dwyer, R.; Morrison, K.S.; Toohey, K.; O’Dea, A.; Slade, J.; Mortazavi, R.; Roberts, C.; Pranavan, G.; et al. The Role of Telehealth During the COVID-19 Pandemic Across the Interdisciplinary Cancer Team: Implications for Practice. Semin. Oncol. Nurs. 2020, 36, 151090. [Google Scholar] [CrossRef] [PubMed]
- Lo Bianco, G.; Papa, A.; Schatman, M.E.; Tinnirello, A.; Terranova, G.; Leoni, M.L.G.; Shapiro, H.; Mercadante, S. Practical Advices for Treating Chronic Pain in the Time of COVID-19: A Narrative Review Focusing on Interventional Techniques. J. Clin. Med. 2021, 10, 2303. [Google Scholar] [CrossRef] [PubMed]
- El-Tallawy, S.N.; Pergolizzi, J.V.; Vasiliu-Feltes, I.; Ahmed, R.S.; LeǪuang, J.K.; Alzahrani, T.; Varrassi, G.; Awaleh, F.I.; Alsubaie, A.T.; Nagiub, M.S. Innovative Applications of Telemedicine and Other Digital Health Solutions in Pain Management: A Literature Review. Pain Ther. 2024, 13, 791–812. [Google Scholar] [CrossRef] [PubMed]
- Salama, V.; Godinich, B.; Geng, Y.; Humbert-Vidan, L.; Maule, L.; Wahid, K.A.; Naser, M.A.; He, R.; Mohamed, A.S.R.; Fuller, C.D.; et al. Artificial Intelligence and Machine Learning in Cancer Pain: A Systematic Review. J. Pain Symptom Manag. 2024, 68, e462–e490. [Google Scholar] [CrossRef]
- Ortiz, B.L.; Gupta, V.; Kumar, R.; Jalin, A.; Cao, X.; Ziegenbein, C.; Singhal, A.; Tewari, M.; Choi, S.W. Data Preprocessing Techniques for AI and Machine Learning Readiness: Scoping Review of Wearable Sensor Data in Cancer Care. JMIR MHealth UHealth 2024, 12, e59587. [Google Scholar] [CrossRef]
- Innominato, P.F.; Macdonald, J.H.; Saxton, W.; Longshaw, L.; Granger, R.; Naja, I.; Allocca, C.; Edwards, R.; Rasheed, S.; Folkvord, F.; et al. Digital Remote Monitoring Using an mHealth Solution for Survivors of Cancer: Protocol for a Pilot Observational Study. JMIR Res. Protoc. 2024, 13, e52957. [Google Scholar] [CrossRef]
- Cloß, K.; Verket, M.; Müller-Wieland, D.; Marx, N.; Schuett, K.; Jost, E.; Crysandt, M.; Beier, F.; Brümmendorf, T.H.; Kobbe, G.; et al. Application of Wearables for Remote Monitoring of Oncology Patients: A Scoping Review. Digit. Health 2024, 10, 20552076241233998. [Google Scholar] [CrossRef]
- Cascella, M.; Leoni, M.L.G.; Shariff, M.N.; Varrassi, G. Towards Artificial Intelligence Application in Pain Medicine. Recenti Prog. Med. 2025, 116, 156–161. [Google Scholar] [CrossRef]
- Soltani, M.; Farahmand, M.; Pourghaderi, A.R. Machine Learning-Based Demand Forecasting in Cancer Palliative Care Home Hospitalization. J. Biomed. Inform. 2022, 130, 104075. [Google Scholar] [CrossRef]
- Bang, Y.H.; Choi, Y.H.; Park, M.; Shin, S.-Y.; Kim, S.J. Clinical Relevance of Deep Learning Models in Predicting the Onset Timing of Cancer Pain Exacerbation. Sci. Rep. 2023, 13, 11501. [Google Scholar] [CrossRef] [PubMed]
- Noe-Steinmüller, N.; Scherbakov, D.; Zhuravlyova, A.; Wager, T.D.; Goldstein, P.; Tesarz, J. Defining Suffering in Pain: A Systematic Review on Pain-Related Suffering Using Natural Language Processing. Pain 2024, 165, 1434–1449. [Google Scholar] [CrossRef] [PubMed]
- DiMartino, L.; Miano, T.; Wessell, K.; Bohac, B.; Hanson, L.C. Identification of Uncontrolled Symptoms in Cancer Patients Using Natural Language Processing. J. Pain Symptom Manag. 2022, 63, 610–617. [Google Scholar] [CrossRef] [PubMed]
- None, N. Towards an AI-Empowered Multimodal Pain Assessment Tool for Cancer-Related Pain. Master’s Thesis, TU Delft, Mechanical Engineering, Delft, The Netherlands, 2025. [Google Scholar]
- Cascella, M.; Shariff, M.N.; Viswanath, O.; Leoni, M.L.G.; Varrassi, G. Ethical Considerations in the Use of Artificial Intelligence in Pain Medicine. Curr. Pain Headache Rep. 2025, 29, 10. [Google Scholar] [CrossRef]
- Chou, K.-N.; Park, D.J.; Hori, Y.S.; Persad, A.R.; Chuang, C.F.; Emrich, S.C.; Ustrzynski, L.; Tayag, A.; Kumar, K.A.; Usoz, M.; et al. Stereotactic Body Radiotherapy for Painful Spinal Metastases: A Decade of Experience at a Single Institution. J. Neurosurg. Spine 2024, 41, 532–540. [Google Scholar] [CrossRef]
- Rivas, D.; de la Torre-Luque, A.; Suárez, V.; García, R.; Fernández, C.; Gonsalves, D.; Moreno-Olmedo, E.; Núñez, M.I.; López, E. Robotic Stereotactic Body Radiotherapy for Spine Metastasis Pain Relief. Appl. Sci. 2024, 14, 1775. [Google Scholar] [CrossRef]
- Desforges, A.D.; Hebert, C.M.; Spence, A.L.; Reid, B.; Dhaibar, H.A.; Cruz-Topete, D.; Cornett, E.M.; Kaye, A.D.; Urits, I.; Viswanath, O. Treatment and Diagnosis of Chemotherapy-Induced Peripheral Neuropathy: An Update. Biomed. Pharmacother. Biomed. Pharmacother. 2022, 147, 112671. [Google Scholar] [CrossRef]
- D’Souza, R.S.; Saini, C.; Hussain, N.; Javed, S.; Prokop, L.; Her, Y.F. Global Estimates of Prevalence of Chronic Painful Neuropathy and Moderate-to-Severe Neuropathy among Patients with Chemotherapy-Induced Peripheral Neuropathy: A Systematic Review and Meta-Analysis of Data from 29 Countries between 2000 and 2024. Reg. Anesth. Pain Med. 2025, 51, e1. [Google Scholar] [CrossRef]
- Cerrone, V.; Esposito, D.; Montedoro, M.; Andretta, V.; Leoni, M.L.G.; Cuomo, A.; Bimonte, S.; Cascella, M. Diagnosis of Chemotherapy-Induced Peripheral Neurotoxicity: A Scoping Review. In Vivo 2025, 39, 3041–3059. [Google Scholar] [CrossRef]
- Fonseca, M.M.; Gelblung, O.; Pennypacker, S.D.; Brooks, T.; Limia, M.; Morgan, J.W.; Zhu, X.; Tovias-Sanchez, L.C.; Pluma-Pluma, A.; Martinez, R.E.; et al. Leukocyte-Intrinsic ER Stress Responses Contribute to Chemotherapy-Induced Peripheral Neuropathy. Sci. Transl. Med. 2025, 17, eady5288. [Google Scholar] [CrossRef]
- Pham, H.H.; Newman, N.; Osmundson, E.C. Radiation-Induced Peripheral Neuropathy After Thoracic Stereotactic Ablative Radiotherapy: Case Report. JTO Clin. Res. Rep. 2022, 3, 100370. [Google Scholar] [CrossRef]
- Delanian, S.; Lefaix, J.-L.; Pradat, P.-F. Radiation-Induced Neuropathy in Cancer Survivors. Radiother. Oncol. J. Eur. Soc. Ther. Radiol. Oncol. 2012, 105, 273–282. [Google Scholar] [CrossRef]
- Mulvey, M.R.; Paley, C.A.; Schuberth, A.; King, N.; Page, A.; Neoh, K. Neuropathic Pain in Cancer: What Are the Current Guidelines? Curr. Treat. Options Oncol. 2024, 25, 1193–1202. [Google Scholar] [CrossRef] [PubMed]
- Pérez, C.; Ochoa, D.; Sánchez, N.; Ballesteros, A.I.; Santidrián, S.; López, I.; Mondéjar, R.; Carnaval, T.; Villoria, J.; Colomer, R. Pain in Long-Term Cancer Survivors: Prevalence and Impact in a Cohort Composed Mostly of Breast Cancer Survivors. Cancers 2024, 16, 1581. [Google Scholar] [CrossRef] [PubMed]
- Naseri, H.; Skamene, S.; Tolba, M.; Faye, M.D.; Ramia, P.; Khriguian, J.; David, M.; Kildea, J. A Scalable Radiomics- and Natural Language Processing-Based Machine Learning Pipeline to Distinguish Between Painful and Painless Thoracic Spinal Bone Metastases: Retrospective Algorithm Development and Validation Study. JMIR AI 2023, 2, e44779. [Google Scholar] [CrossRef] [PubMed]
- PDQ Supportive and Palliative Care Editorial Board Cancer Pain (PDǪ®): Health Professional Version. In PDQ Cancer Information Summaries; National Cancer Institute (US): Bethesda, MD, USA, 2002.
- Danés-López, F.; Diaz-Palominos, C.; Ortiz Domínguez, A.; Silva Rodriguez, A.; Astorga, C.; Martínez-Hernández, D.; Valenzuela-Fuenzalida, J.J.; Sanchis-Gimeno, J.; Nova-Baeza, P.; Suazo-Santibáñez, A.; et al. Clinical Characteristics of Neuropathic Pain and Its Relationship with Cancer in Different Corporal Areas-A Systematic Review. Diagnostics 2025, 15, 116. [Google Scholar] [CrossRef]
- Leoni, M.L.G.; Mercieri, M.; Viswanath, O.; Cascella, M.; Rekatsina, M.; Pasqualucci, A.; Caruso, A.; Varrassi, G. Neuropathic Pain: A Comprehensive Bibliometric Analysis of Research Trends, Contributions, and Future Directions. Curr. Pain Headache Rep. 2025, 29, 73. [Google Scholar] [CrossRef]
- Latapiat, V.; Saez, M.; Pedroso, I.; Martin, A.J.M. Unraveling Patient Heterogeneity in Complex Diseases through Individualized Co-Expression Networks: A Perspective. Front. Genet. 2023, 14, 1209416. [Google Scholar] [CrossRef]
- Shkodra, M.; Caraceni, A. Treatment of Neuropathic Pain Directly Due to Cancer: An Update. Cancers 2022, 14, 1992. [Google Scholar] [CrossRef]
| Domain | Study (Year) | Design/n | Intervention/Topic | Key Findings | Clinical Significance |
|---|---|---|---|---|---|
| Pharmacological interventions | WHO ladder re-evaluation | Multicenter RCT/153 | Two-step vs. three-step WHO ladder | No difference in time to pain control; >50% required escalation to strong opioids | Challenges necessity of weak-opioid step |
| Fallon et al., 2023 [72] | Phase III RCT/155 | Tanezumab (anti-NGF) | Greater NRS reduction vs. placebo; fracture risk near metastases | Proof-of-concept; safety limits use | |
| Bertoch et al., 2025 [82] | Phase 3 RCT/2191 | Suzetrigine (Nav1.8 inhibitor) for postoperative pain (abdominoplasty, bunionectomy) | Suzetrigine vs. placebo: 48.4 (abdominoplasty, p < 0.0001) and 29.3 (bunionectomy, p = 0.0002); comparable to hydrocodone/acetaminophen; AEs mild-moderate (pruritus, muscle spasms) | First FDA-approved selective Nav1.8 inhibitor for acute pain; non-opioid with peripheral mechanism, no addictive potential | |
| Neuromodulation | Crowther et al., 2022 [98] | Retrospective cohort/28 | Spinal cord stimulation (SCS) | NRS 8.0 → 2.2; opioid reduction | Viable option for refractory cancer pain |
| Bulat et al., 2023 [99] | Case series/8 | SCS | Rapid discharge; sustained opioid reduction | Feasible and safe in cancer patients | |
| Chung et al., 2025 [100] | Case series | DRGS vs. SCS | Greater pain reduction with DRGS | DRGS promising for selected syndromes | |
| Vu et al., 2025 [97] | Scoping review/24 studies | SCS, DRGS, PNS | Pain and functional improvement across cancers | Supports neuromodulation as multimodal care | |
| Intrathecal drug delivery systems (IDDS) | Wang et al., 2024 [119] | Retrospective cohort/96 | IDDS for refractory cancer pain at Chinese tertiary center | Mean NRS 7.5→3.0 (p < 0.001); median baseline OME 290 mg/day; 70.8% intrathecal trial; median survival 3 months; 75% family satisfaction | Real-world outcomes in advanced disease with high baseline opioid requirements |
| Stearns et al., 2020 [120] | Prospective multicenter registry/1403 | IDDS (US, Europe, Latin America) | Pain scores improved at 6 months and 12 months; EuroQol-5D improved; 87% followed through death; 4.3% explant; 3.2% infection | Largest real-world registry; sustained efficacy and high therapy retention through end of life | |
| Sindt et al., 2020 [121] | Retrospective cohort/173 | IDDS opioid-sparing effects in advanced cancer | Pre-implant median OME 240 mg/day→0 mg at 30 days; 72% discontinued systemic opioids; 85% reduced OME ≥ 80% | Dramatic systemic opioid-sparing effect; IDDS as replacement for high-dose systemic opioids | |
| Deer et al., 2025 (PACC) [122] | Consensus guidelines/Expert panel | PACC 2025 updates on IDDS for cancer pain | Evidence-based recommendations for patient selection, drug algorithms, comorbidity management; earlier implementation vs. salvage therapy | International standard for IDDS best practices; algorithmic approach to medication selection | |
| Neuroablative procedures | Doyle et al., 2022 [101] | RCT/16 | Cordotomy vs. standard care | Superior pain relief vs. control | Level-1 evidence for cordotomy |
| Adams et al., 2023 [105] | Systematic review | Cingulotomy | 32–83% meaningful pain relief | Option for diffuse/affective pain | |
| Radiotherapy | Bindels et al., 2024 [128] | Meta-analysis/1090 | SBRT vs. cEBRT | Similar response; SBRT faster and more durable | Precision RT for bone metastases |
| Rossi et al., 2024 (PRAIS) [129] | RCT secondary analysis | Palliative radiotherapy | Predictors of poor pain response identified | Enables risk stratification | |
| Chou et al., 2024 [147] | Retrospective cohort | Spine SBRT | Durable pain relief; favorable safety | Supports SBRT for spinal pain | |
| Digital health & AI | Salama et al., 2024 [136] | Systematic review/44 | AI/ML for cancer pain | Pain prediction AUC 0.75–0.92 | Feasible; needs validation |
| Bang et al., 2023 [142] | ML retrospective | Deep learning prediction | Accurate prediction of pain flares | Enables proactive pain management | |
| Hamdoune et al., 2024 [132] | Systematic review | Digital health tools | Reduced pain and distress | Supports telemedicine integration | |
| Mechanistic/translational | Martel Matos et al., 2022 [22] | Translational/preclinical | sEVs in head & neck cancer | sEVs necessary and sufficient for pain | New therapeutic targets |
| Fan et al., 2024 [18] | Preclinical study | Exosome–ATX–LPA axis | ATX inhibition reduces bone cancer pain | Druggable mechanism identified |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Varrassi, G.; Paladini, A.; Tran, Y.V.; Pham, V.P.; Alwany, A.A.A.; Farì, G.; Caruso, A.; Mercieri, M.; Pergolizzi, J.V.; Kaye, A.D.; et al. Advances in the Pathophysiology and Management of Cancer Pain: A Scoping Review. Cancers 2026, 18, 259. https://doi.org/10.3390/cancers18020259
Varrassi G, Paladini A, Tran YV, Pham VP, Alwany AAA, Farì G, Caruso A, Mercieri M, Pergolizzi JV, Kaye AD, et al. Advances in the Pathophysiology and Management of Cancer Pain: A Scoping Review. Cancers. 2026; 18(2):259. https://doi.org/10.3390/cancers18020259
Chicago/Turabian StyleVarrassi, Giustino, Antonella Paladini, Y Van Tran, Van Phong Pham, Ameen A. Al Alwany, Giacomo Farì, Annalisa Caruso, Marco Mercieri, Joseph V. Pergolizzi, Alan D. Kaye, and et al. 2026. "Advances in the Pathophysiology and Management of Cancer Pain: A Scoping Review" Cancers 18, no. 2: 259. https://doi.org/10.3390/cancers18020259
APA StyleVarrassi, G., Paladini, A., Tran, Y. V., Pham, V. P., Alwany, A. A. A., Farì, G., Caruso, A., Mercieri, M., Pergolizzi, J. V., Kaye, A. D., Breve, F., Corriero, A., Gharibo, C., & Leoni, M. L. G. (2026). Advances in the Pathophysiology and Management of Cancer Pain: A Scoping Review. Cancers, 18(2), 259. https://doi.org/10.3390/cancers18020259












