B7-H3 Chimeric Antigen Receptor Redirected T Cells Target Anaplastic Lymphoma Kinase-Positive Anaplastic Large Cell Lymphoma
Abstract
:Simple Summary
Abstract
1. Introduction
2. Results
2.1. B7-H3 Is Overexpressed in ALK-Induced ALCL
2.2. B7-H3-Redirected CAR-T Cells Have Similar Growth Rate as CD19-CAR-T Cells In Vitro
2.3. B7-H3 Redirected CAR-T Cells Show Their Potency in Controlling ALCLs In Vitro
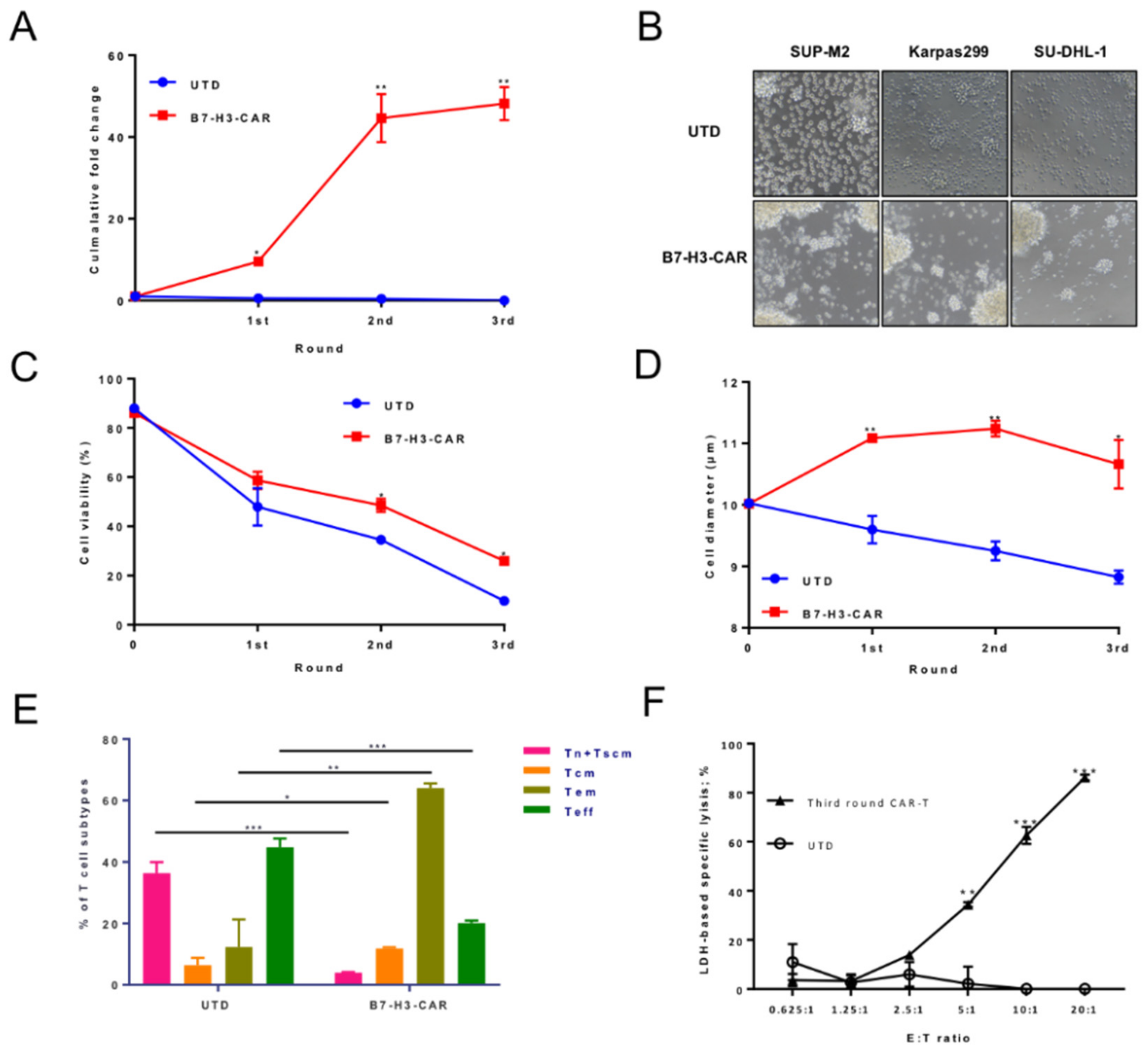
2.4. B7-H3 Redirected CAR-T Cells Can Be Expanded upon Repeat Antigen Stimulations
2.5. B7-H3 Redirected CAR-T Cells Show Potent Antitumor Effect In Vivo
3. Discussion
4. Materials and Methods
4.1. Cell Isolation, Culturing, Gene Transferring, and Expansion
4.2. Plasmids
4.3. Antibody Staining and Flow Cytometry
4.4. Quantitative Real Time PCR
4.5. ELISA
4.6. Cytotoxicity Assays
4.7. Repeat Antigen Stimulation Expansion Assay
4.8. Xenograft Assay
4.9. Multiple Cytokine Assay
4.10. RNA Sequencing, Bioinformatics and Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Johnson, L.A.; June, C.H. Driving gene-engineered T cell immunotherapy of cancer. Cell Res. 2017, 27, 38–58. [Google Scholar] [CrossRef] [Green Version]
- Perera, L.P.; Zhang, M.; Nakagawa, M.; Petrus, M.N.; Maeda, M.; Kadin, M.E.; Waldmann, T.A.; Perera, P.Y. Chimeric antigen receptor modified T cells that target chemokine receptor CCR4 as a therapeutic modality for T-cell malignancies. Am. J. Hematol. 2017, 92, 892–901. [Google Scholar] [CrossRef] [PubMed]
- Ramos, C.A.; Ballard, B.; Zhang, H.; Dakhova, O.; Gee, A.P.; Mei, Z.; Bilgi, M.; Wu, M.F.; Liu, H.; Grilley, B.; et al. Clinical and immunological responses after CD30-specific chimeric antigen receptor-redirected lymphocytes. J. Clin. Investig. 2017, 127, 3462–3471. [Google Scholar] [CrossRef] [PubMed]
- Stein, H.; Mason, D.Y.; Gerdes, J.; O’Connor, N.; Wainscoat, J.; Pallesen, G.; Gatter, K.; Falini, B.; Delsol, G.; Lemke, H.; et al. The expression of the Hodgkin’s disease associated antigen Ki-1 in reactive and neoplastic lymphoid tissue: Evidence that Reed-Sternberg cells and histiocytic malignancies are derived from activated lymphoid cells. Blood 1985, 66, 848–858. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kinney, M.C.; Higgins, R.A.; Medina, E.A. Anaplastic Large Cell Lymphoma Twenty-Five Years of Discovery. Arch. Pathol. Lab. Med. 2011, 135, 19–43. [Google Scholar]
- Mussolin, L.; Damm-Welk, C.; Pillon, M.; Zimmermann, M.; Franceschetto, G.; Pulford, K.; Reiter, A.; Rosolen, A.; Woessmann, W. Use of minimal disseminated disease and immunity to NPM-ALK antigen to stratify ALK-positive ALCL patients with different prognosis. Leukemia 2013, 27, 416–422. [Google Scholar] [CrossRef]
- Prokoph, N.; Larose, H.; Lim, M.S.; Burke, G.A.A.; Turner, S.D. Treatment Options for Paediatric Anaplastic Large Cell Lymphoma (ALCL): Current Standard and beyond. Cancers 2018, 10, 99. [Google Scholar] [CrossRef] [Green Version]
- Ma, H.; Marchi, E.; O’Connor, O.A. The peripheral T-cell lymphomas: An unusual path to cure. Lancet Haematol. 2020, 7, e765–e771. [Google Scholar] [CrossRef]
- Vose, J.; Armitage, J.; Weisenburger, D.; International, T.C.L.P. International peripheral T-cell and natural killer/T-cell lymphoma study: Pathology findings and clinical outcomes. J. Clin. Oncol. 2008, 26, 4124–4130. [Google Scholar] [CrossRef]
- Chen, Y.; Guan, S.Y.; Deng, J.; Yang, H.; Xu, W.; Xu, S.; Shao, M.; Gao, X.; Xu, S.; Shuai, Z.; et al. B7-H3: A promising therapeutic target for autoimmune diseases. Cell Immunol. 2020, 352, 104077. [Google Scholar] [CrossRef]
- Ling, V.; Wu, P.W.; Spaulding, V.; Kieleczawa, J.; Luxenberg, D.; Carreno, B.M.; Collins, M. Duplication of primate and rodent B7-H3 immunoglobulin V- and C-like domains: Divergent history of functional redundancy and exon loss. Genomics 2003, 82, 365–377. [Google Scholar] [CrossRef]
- Sun, M.; Richards, S.; Prasad, D.V.; Mai, X.M.; Rudensky, A.; Dong, C. Characterization of mouse and human B7-H3 genes. J. Immunol. 2002, 168, 6294–6297. [Google Scholar] [CrossRef] [PubMed]
- Flem-Karlsen, K.; Fodstad, O.; Tan, M.; Nunes-Xavier, C.E. B7-H3 in Cancer—Beyond Immune Regulation. Trends Cancer 2018, 4, 401–404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, S.; Wei, W.; Zhao, Q. B7-H3, a checkpoint molecule, as a target for cancer immunotherapy. Int. J. Biol. Sci. 2020, 16, 1767–1773. [Google Scholar] [CrossRef] [Green Version]
- Nehama, D.; Di Ianni, N.; Musio, S.; Du, H.; Patane, M.; Pollo, B.; Finocchiaro, G.; Park, J.J.H.; Dunn, D.E.; Edwards, D.S.; et al. B7-H3-redirected chimeric antigen receptor T cells target glioblastoma and neurospheres. EBioMedicine 2019, 47, 33–43. [Google Scholar] [CrossRef] [Green Version]
- Tang, X.; Zhao, S.; Zhang, Y.; Wang, Y.; Zhang, Z.; Yang, M.; Zhu, Y.; Zhang, G.; Guo, G.; Tong, A.; et al. B7-H3 as a Novel CAR-T Therapeutic Target for Glioblastoma. Mol. Ther. Oncolytics 2019, 14, 279–287. [Google Scholar] [CrossRef] [Green Version]
- Du, H.; Hirabayashi, K.; Ahn, S.; Kren, N.P.; Montgomery, S.A.; Wang, X.; Tiruthani, K.; Mirlekar, B.; Michaud, D.; Greene, K.; et al. Antitumor Responses in the Absence of Toxicity in Solid Tumors by Targeting B7-H3 via Chimeric Antigen Receptor T Cells. Cancer Cell 2019, 35, 221–237. [Google Scholar] [CrossRef] [Green Version]
- Zheng, M.; Yu, L.; Hu, J.; Zhang, Z.; Wang, H.; Lu, D.; Tang, X.; Huang, J.; Zhong, K.; Wang, Z.; et al. Efficacy of B7-H3-Redirected BiTE and CAR-T Immunotherapies Against Extranodal Nasal Natural Killer/T Cell Lymphoma. Transl. Oncol. 2020, 13, 100770. [Google Scholar] [CrossRef]
- Zhang, Z.; Jiang, C.; Liu, Z.; Yang, M.; Tang, X.; Wang, Y.; Zheng, M.; Huang, J.; Zhong, K.; Zhao, S.; et al. B7-H3-Targeted CAR-T Cells Exhibit Potent Antitumor Effects on Hematologic and Solid Tumors. Mol. Ther. Oncolytics 2020, 17, 180–189. [Google Scholar] [CrossRef]
- Zhang, Q.; Wei, F.; Wang, H.Y.; Liu, X.; Roy, D.; Xiong, Q.B.; Jiang, S.; Medvec, A.; Danet-Desnoyers, G.; Watt, C.; et al. The potent oncogene NPM-ALK mediates malignant transformation of normal human CD4(+) T lymphocytes. Am. J. Pathol. 2013, 183, 1971–1980. [Google Scholar] [CrossRef] [Green Version]
- Rhodes, D.R.; Yu, J.; Shanker, K.; Deshpande, N.; Varambally, R.; Ghosh, D.; Barrette, T.; Pandey, A.; Chinnaiyan, A.M. ONCOMINE: A cancer microarray database and integrated data-mining platform. Neoplasia 2004, 6, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Au-Yeung, B.B.; Smith, G.A.; Mueller, J.L.; Heyn, C.S.; Jaszczak, R.G.; Weiss, A.; Zikherman, J. IL-2 Modulates the TCR Signaling Threshold for CD8 but Not CD4 T Cell Proliferation on a Single-Cell Level. J. Immunol. 2017, 198, 2445–2456. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Golubovskaya, V.; Wu, L. Different Subsets of T Cells, Memory, Effector Functions, and CAR-T Immunotherapy. Cancers 2016, 8, 36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chapoval, A.I.; Ni, J.; Lau, J.S.; Wilcox, R.A.; Flies, D.B.; Liu, D.; Dong, H.; Sica, G.L.; Zhu, G.; Tamada, K.; et al. B7-H3: A costimulatory molecule for T cell activation and IFN-gamma production. Nat. Immunol. 2001, 2, 269–274. [Google Scholar] [CrossRef]
- Leitner, J.; Klauser, C.; Pickl, W.F.; Stockl, J.; Majdic, O.; Bardet, A.F.; Kreil, D.P.; Dong, C.; Yamazaki, T.; Zlabinger, G.; et al. B7-H3 is a potent inhibitor of human T-cell activation: No evidence for B7-H3 and TREML2 interaction. Eur. J. Immunol. 2009, 39, 1754–1764. [Google Scholar] [CrossRef]
- Ghorashian, S.; Kramer, A.M.; Onuoha, S.; Wright, G.; Bartram, J.; Richardson, R.; Albon, S.J.; Casanovas-Company, J.; Castro, F.; Popova, B.; et al. Enhanced CAR T cell expansion and prolonged persistence in pediatric patients with ALL treated with a low-affinity CD19 CAR. Nat. Med. 2019, 25, 1408–1414. [Google Scholar] [CrossRef]
- Nguyen, P.; Okeke, E.; Clay, M.; Haydar, D.; Justice, J.; O’Reilly, C.; Pruett-Miller, S.; Papizan, J.; Moore, J.; Zhou, S.; et al. Route of 41BB/41BBL Costimulation Determines Effector Function of B7-H3-CAR.CD28zeta T Cells. Mol. Ther. Oncolytics 2020, 18, 202–214. [Google Scholar] [CrossRef]
- Majzner, R.G.; Theruvath, J.L.; Nellan, A.; Heitzeneder, S.; Cui, Y.; Mount, C.W.; Rietberg, S.P.; Linde, M.H.; Xu, P.; Rota, C.; et al. CAR T Cells Targeting B7-H3, a Pan-Cancer Antigen, Demonstrate Potent Preclinical Activity Against Pediatric Solid Tumors and Brain Tumors. Clin. Cancer Res. 2019, 25, 2560–2574. [Google Scholar] [CrossRef]
- Theruvath, J.; Sotillo, E.; Mount, C.W.; Graef, C.M.; Delaidelli, A.; Heitzeneder, S.; Labanieh, L.; Dhingra, S.; Leruste, A.; Majzner, R.G.; et al. Locoregionally administered B7-H3-targeted CAR T cells for treatment of atypical teratoid/rhabdoid tumors. Nat. Med. 2020, 26, 712–719. [Google Scholar] [CrossRef]
- Brudno, J.N.; Kochenderfer, J.N. Recent advances in CAR T-cell toxicity: Mechanisms, manifestations and management. Blood Rev. 2019, 34, 45–55. [Google Scholar] [CrossRef]
- Wei, F.; Zhong, S.; Ma, Z.; Kong, H.; Medvec, A.; Ahmed, R.; Freeman, G.J.; Krogsgaard, M.; Riley, J.L. Strength of PD-1 signaling differentially affects T-cell effector functions. Proc. Natl. Acad. Sci. USA 2013, 110, E2480–E2489. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, E.L.; Mailankody, S.; Staehr, M.; Wang, X.; Senechal, B.; Purdon, T.J.; Daniyan, A.F.; Geyer, M.B.; Goldberg, A.D.; Mead, E.; et al. BCMA-Targeted CAR T-cell Therapy plus Radiotherapy for the Treatment of Refractory Myeloma Reveals Potential Synergy. Cancer Immunol. Res. 2019, 7, 1047–1053. [Google Scholar] [CrossRef] [PubMed]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zi, Z.; Zhao, H.; Wang, H.; Ma, X.; Wei, F. B7-H3 Chimeric Antigen Receptor Redirected T Cells Target Anaplastic Lymphoma Kinase-Positive Anaplastic Large Cell Lymphoma. Cancers 2020, 12, 3815. https://doi.org/10.3390/cancers12123815
Zi Z, Zhao H, Wang H, Ma X, Wei F. B7-H3 Chimeric Antigen Receptor Redirected T Cells Target Anaplastic Lymphoma Kinase-Positive Anaplastic Large Cell Lymphoma. Cancers. 2020; 12(12):3815. https://doi.org/10.3390/cancers12123815
Chicago/Turabian StyleZi, Zhenguo, Haihong Zhao, Huanyu Wang, Xiaojing Ma, and Fang Wei. 2020. "B7-H3 Chimeric Antigen Receptor Redirected T Cells Target Anaplastic Lymphoma Kinase-Positive Anaplastic Large Cell Lymphoma" Cancers 12, no. 12: 3815. https://doi.org/10.3390/cancers12123815
APA StyleZi, Z., Zhao, H., Wang, H., Ma, X., & Wei, F. (2020). B7-H3 Chimeric Antigen Receptor Redirected T Cells Target Anaplastic Lymphoma Kinase-Positive Anaplastic Large Cell Lymphoma. Cancers, 12(12), 3815. https://doi.org/10.3390/cancers12123815





