Diffusion-Weighted Imaging Can Differentiate between Malignant and Benign Pleural Diseases
Abstract
:1. Introduction
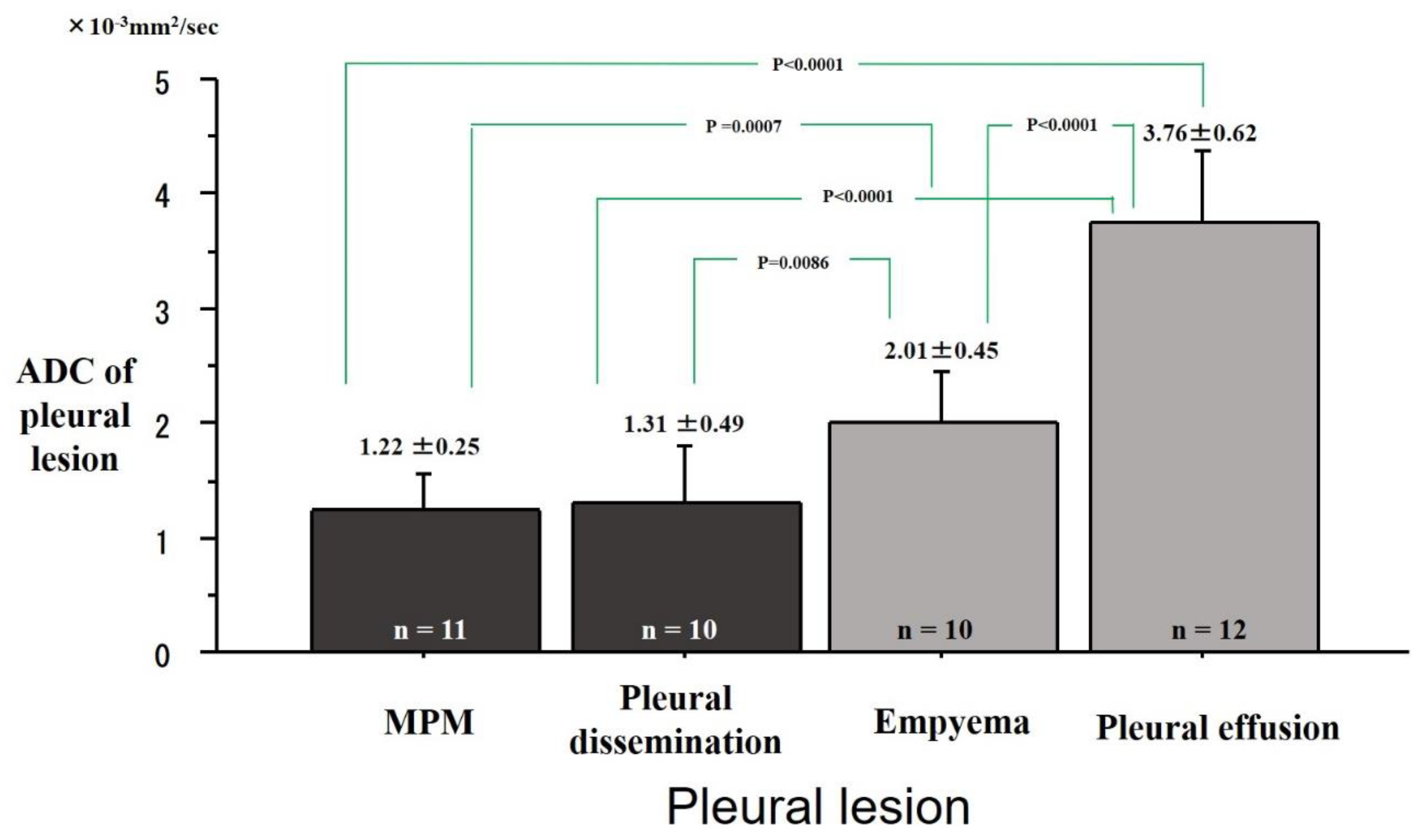
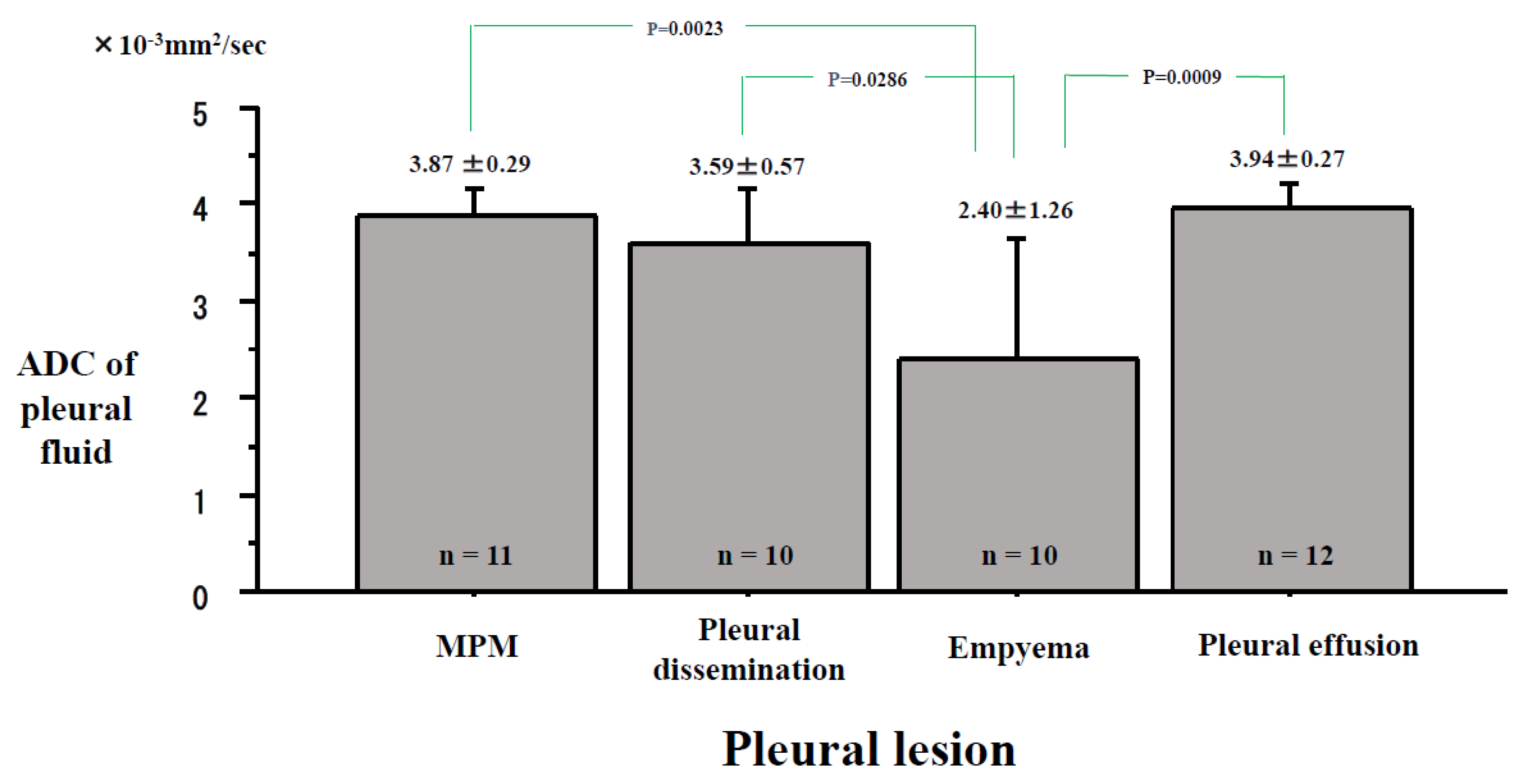
2. Results
3. Discussion
Limitations
4. Patients and Methods
4.1. Eligibility
4.2. Patients
4.3. Magnetic Resonance Imaging (MRI)
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nickell, L.T., Jr.; Lichtenberger, J.P., 3rd; Khorashadi, L.; Abbott, G.F.; Carter, B.W. Multimodality imaging for characterization, classification, and staging of malignant pleural mesothelioma. Radiographics 2014, 34, 1692–1706. [Google Scholar] [CrossRef] [PubMed]
- Pessôa, F.M.; de Melo, A.S.; Souza, A.S.; de Souza, L.S.; Hochhegger, B.; Zanetti, G.; Marchiori, E. Applications of magnetic resonance imaging of the thorax in pleural diseases: A state-of-the-art review. Lung 2016, 194, 501–509. [Google Scholar] [CrossRef] [PubMed]
- Heelan, R.T. Staging of malignant pleural mesothelioma: Comparison of CT and MR imaging. Am. J. Roentgenol 1999, 172, 1039–1047. [Google Scholar] [CrossRef] [PubMed]
- Coolen, J.; De Keyzer, F.; Nafteux, P.; De Wever, W.; Dooms, C.; Vansteenkiste, J.; Derweduwen, A.; Roebben, I.; Verbeken, E.; De Leyn, P.; et al. Malignant pleural mesothelioma: Visual assessment by using pleural pointillism at diffusion-weighted MR imaging. Radiology 2015, 274, 576–584. [Google Scholar] [CrossRef] [PubMed]
- Le Bihan, D.; Breton, E.; Lallemand, D.; Aubin, M.L.; Vignaud, J.; Laval-Jeantet, M. Separation of diffusion and perfusion in intravoxel incoherent motion MR imaging. Radiology 1988, 168, 497–505. [Google Scholar] [CrossRef] [PubMed]
- Tien, R.D.; Felsberg, G.J.; Friedman, H.; Brown, M.; MacFall, J. MR imaging of high-grade cerebral gliomas: Value of diffusion-weighted echoplanar plus sequences. Am. J. Roentgenol. 1994, 162, 671–676. [Google Scholar] [CrossRef] [PubMed]
- Kwee, T.C.; Takahara, T.; Ochiai, R.; Koh, D.M.; Ohno, Y.; Nakanishi, K.; Niwa, T.; Chenevert, T.L.; Luijten, P.R.; Alavi, A. Complementary roles of whole-body diffusion-weighted MRI and 18F-FDG PET: The state of the art and potential applications. J. Nucl. Med. 2010, 51, 1549–1558. [Google Scholar] [CrossRef] [PubMed]
- Usuda, K.; Sagawa, M.; Motono, N.; Ueno, M.; Tanaka, M.; Machida, Y.; Matoba, M.; Kuginuki, Y.; Taniguchi, M.; Ueda, Y.; et al. Advantages of diffusion-weighted imaging over positron emission tomography-computed tomography in assessment of hilar and mediastinal lymph node in lung cancer. Ann. Surg. Oncol. 2013, 20, 1676–1683. [Google Scholar] [CrossRef]
- Li, B.; Li, Q.; Chen, C.; Guan, Y.; Liu, S. A systematic review and meta-analysis of the accuracy of diffusion-weighted MRI in the detection of malignant pulmonary nodules and masses. Acad. Radiol. 2014, 21, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Shen, G.; Jia, Z.; Deng, H. Apparent diffusion coefficient values of diffusion-weighted imaging for distinguishing focal pulmonary lesions and characterizing the subtype of lung cancer: A meta-analysis. Eur. Radiol. 2016, 26, 556–566. [Google Scholar] [CrossRef]
- Usuda, K.; Zhao, X.T.; Sagawa, M.; Matoba, M.; Kuginuki, Y.; Ueda, Y.; Sakuma, T. Diffusion-weighted imaging is superior to positron emission tomography in the detection and nodal assessment of lung cancers. Ann. Thorac. Surg. 2011, 91, 1689–1695. [Google Scholar] [CrossRef] [PubMed]
- Peerlings, J.; Troost, E.G.; Nelemans, P.J.; Cobben, D.C.; de Boer, J.C.; Hoffmann, A.L.; Beets-Tan, R.G. The diagnostic value of MR Imaging in determining the lymph node status of patients with non-small cell lung cancer: A meta-analysis. Radiology 2016, 281, 86–98. [Google Scholar] [CrossRef] [PubMed]
- Shen, G.; Hu, S.; Deng, H.; Kuang, A. Performance of DWI in the nodal characterization and assessment of lung cancer: A meta-analysis. Am. J. Roentgenol. 2016, 206, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Hayes, S.A.; Plodkowski, A.J.; Ginsberg, M.S. Imaging of Thoracic Cavity Tumors. Surgical Oncology Clinics 2014, 23, 709–733. [Google Scholar] [CrossRef] [PubMed]
- Hierholzer, J.; Luo, L.; Bittner, R.C.; Stroszczynski, C.; Schröder, R.J.; Schoenfeld, N.; Dorow, P.; Loddenkemper, R.; Grassot, A. MRI and CT in the differential diagnosis of disease. Chest 2000, 118, 604–609. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Hierholzer, J.; Bittner, R.C.; Chen, J.; Huang, L. Magnetic resonance i pleural maging in distinguishing malignant from benign pleural disease. Chin. Med. J. 2001, 114, 645–649. [Google Scholar]
- Luna, A.; Sanchez-Gonzalez, J.; Caro, P. Diffusion weighted imaging of the chest. Magn. Reason. Imaging Clin. N. Am. 2011, 19, 69–94. [Google Scholar] [CrossRef] [PubMed]
- Coolen, J.; De Keyzer, F.; Nafteux, P.; De Wever, W.; Dooms, C.; Vansteenkiste, J.; Roebben, I.; Verbeken, E.; De Leyn, P.; Van Raemdonck, D.; et al. Malignant pleural disease: Diagnosis by using diffusion-weighted and dynamic contrast-enhanced MR imaging-initial experience. Radiology 2012, 263, 884–892. [Google Scholar] [CrossRef] [PubMed]
- Gill, R.R.; Umeoka, S.; Mamata, H.; Tilleman, T.R.; Stanwell, P.; Woodhams, R.; Padera, R.F.; Sugarbaker, D.J.; Hatabu, H. Diffusion-weighted MRI of malignant pleural mesothelioma: Preliminary assessment of apparent diffusion coefficient in histologic subtypes. Am. J. Roentgenol. 2010, 195, W125–W130. [Google Scholar] [CrossRef] [PubMed]
- Bonomi, M.; De Filippis, C.; Lopci, E.; Gianoncelli, L.; Rizzardi, G.; Cerchiaro, E.; Bortolotti, L.; Zanello, A.; Ceresoli, G.L. Clinical staging of malignant pleural mesothelioma: Current perspectives. Lung Cancer Target Ther. 2017, 8, 127–139. [Google Scholar] [CrossRef]
- Jaruskova, M.; Belohlavek, O. Role of FDG-PET and PET/CT in the diagnosis of prolonged febrile states. Eur. J. Nucl. Med. Mol. Imaging 2006, 33, 913–918. [Google Scholar] [CrossRef] [PubMed]
- Flores, R.M.; Akhurst, T.; Gonen, M.; Larson, S.M.; Rusch, V.W. Positron emission tomography defines metastatic disease but not locoregional disease in patients with malignant pleural mesothelioma. J. Thorac. Cardiovasc. Surg. 2003, 126, 11–16. [Google Scholar] [CrossRef]
- Otsuka, H.; Terazawa, K.; Morita, N.; Otomi, Y.; Yamashita, K.; Nishitani, H. Is FDG-PET/CT useful for managing malignant pleural mesothelioma? J. Med. Investig. 2009, 56, 16–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, S.M.; Kim, S.H.; Kang, B.J.; Song, B.J. Extramammary findings on breast MRI: Prevalence and imaging characteristics favoring malignancy detection: A retrospective analysis. World J. Surg. Oncol. 2016, 14, 119. [Google Scholar] [CrossRef] [PubMed]
- Desprechins, B.; Stadnik, T.; Koerts, G.; Shabana, W.; Breucq, C.; Osteaux, M. Use of diffusion-weighted MR imaging in differential diagnosis between intracerebral necrotic tumors and cerebral abscesses. Am. J. Neuroradiol. 1999, 20, 1252–1257. [Google Scholar] [PubMed]
- Qureshi, N.R.; Gleeson, F.V. Imaging of pleural disease. Clin. Chest Med. 2006, 27, 193–213. [Google Scholar] [CrossRef] [PubMed]
- Darge, K.; Jaramillo, D.; Siegel, M.J. Whole-body MRI in children: Current status and future applications. Eur. J. Radiol. 2008, 68, 289–298. [Google Scholar] [CrossRef] [PubMed]
- Usuda, K.; Sagawa, M.; Motono, N.; Ueno, M.; Tanaka, M.; Machida, Y.; Tonami, H. Diagnostic performance of diffusion weighted imaging of malignant and benign pulmonary nodules and masses: Comparison with positron emission tomography. Asian Pac. J. Cancer Prev. 2014, 15, 4629–4635. [Google Scholar] [CrossRef] [PubMed]
- Nasu, K.; Kuroki, Y.; Minami, M. Diffusion-weighted imaging findings of mucinous carcinoma arising in the ano-rectal region. Comparison of apparent diffusion coefficient with that of tubular adenocarcinoma. Jpn. J. Radiol. 2012, 30, 120–127. [Google Scholar] [CrossRef] [PubMed]
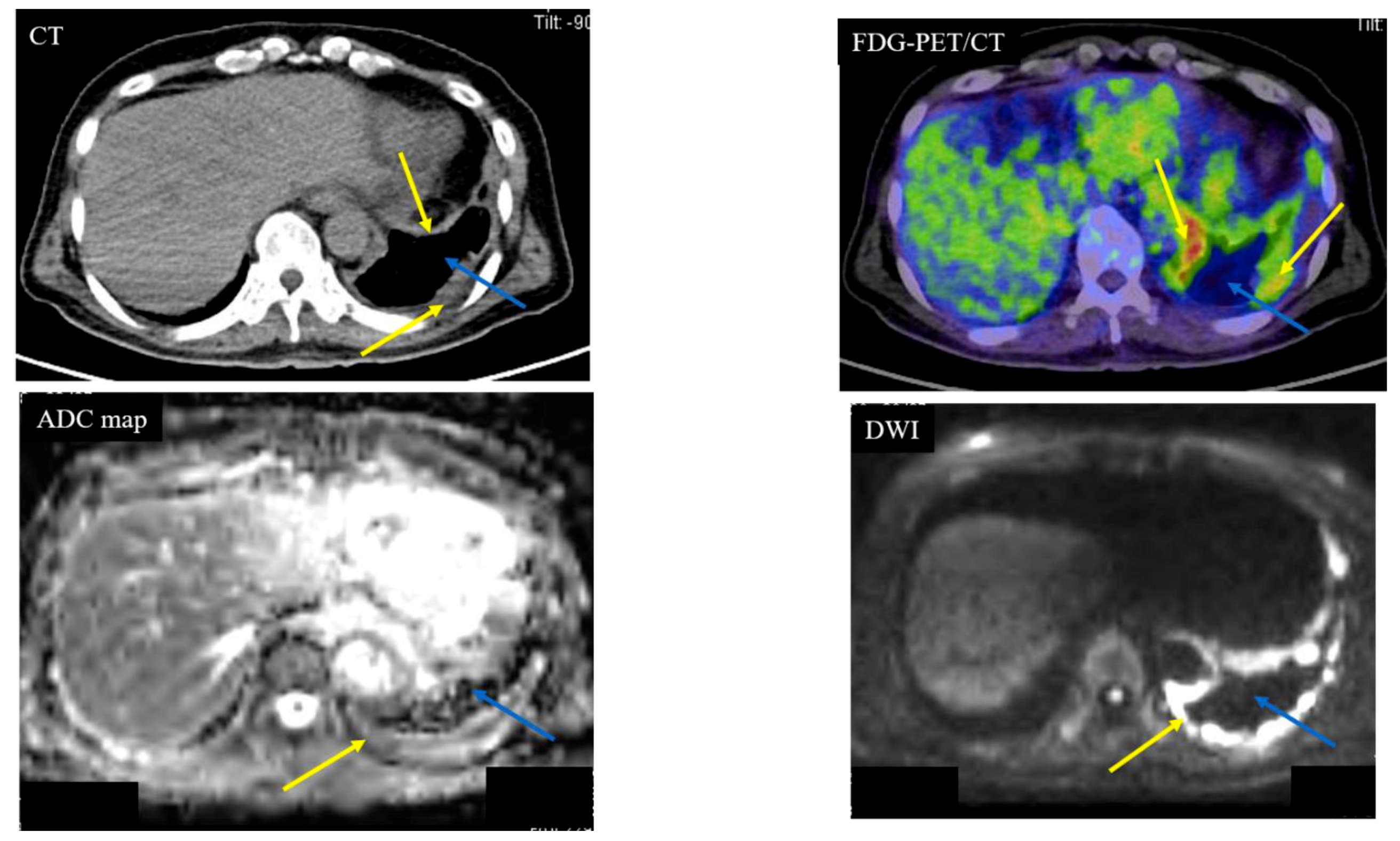
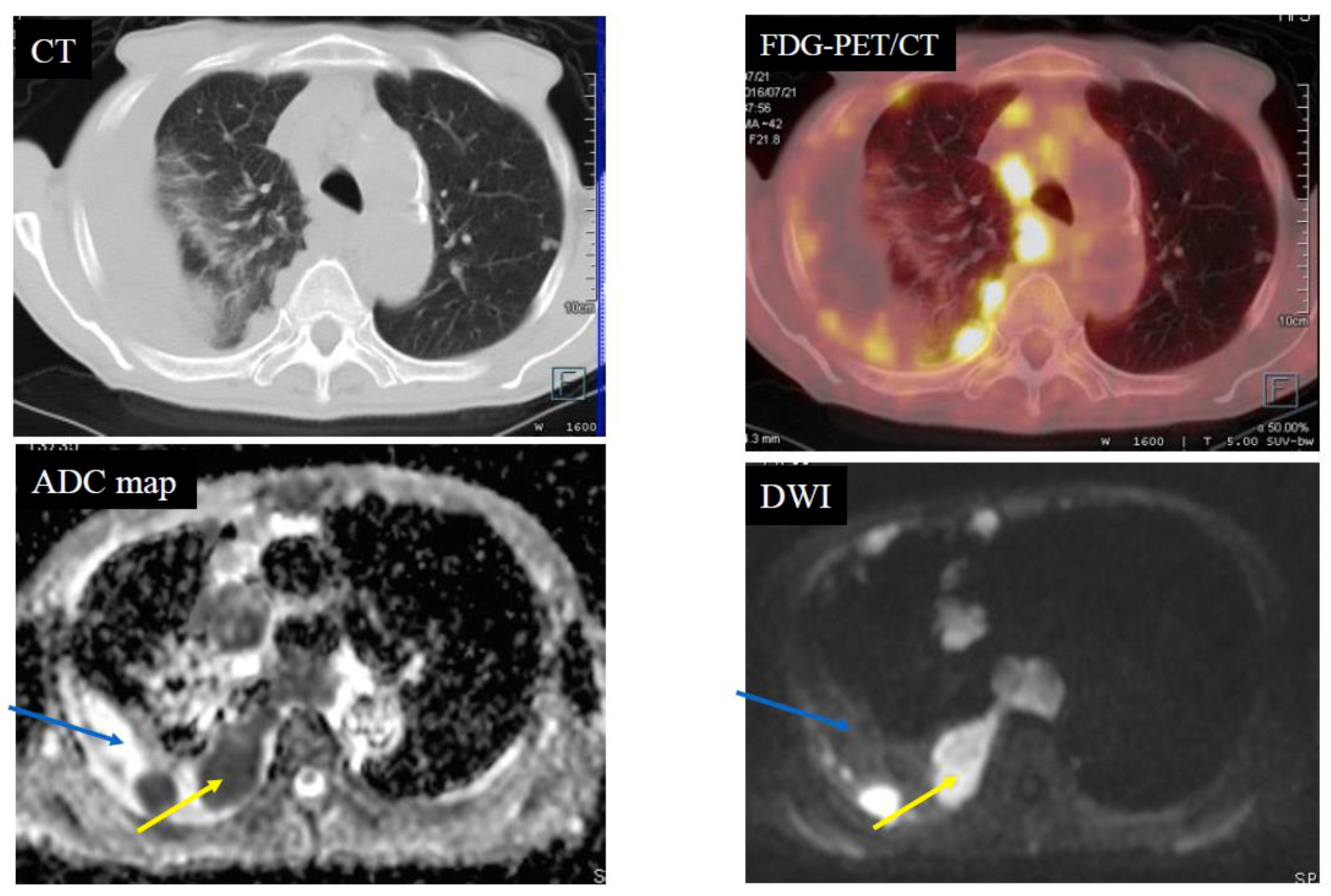
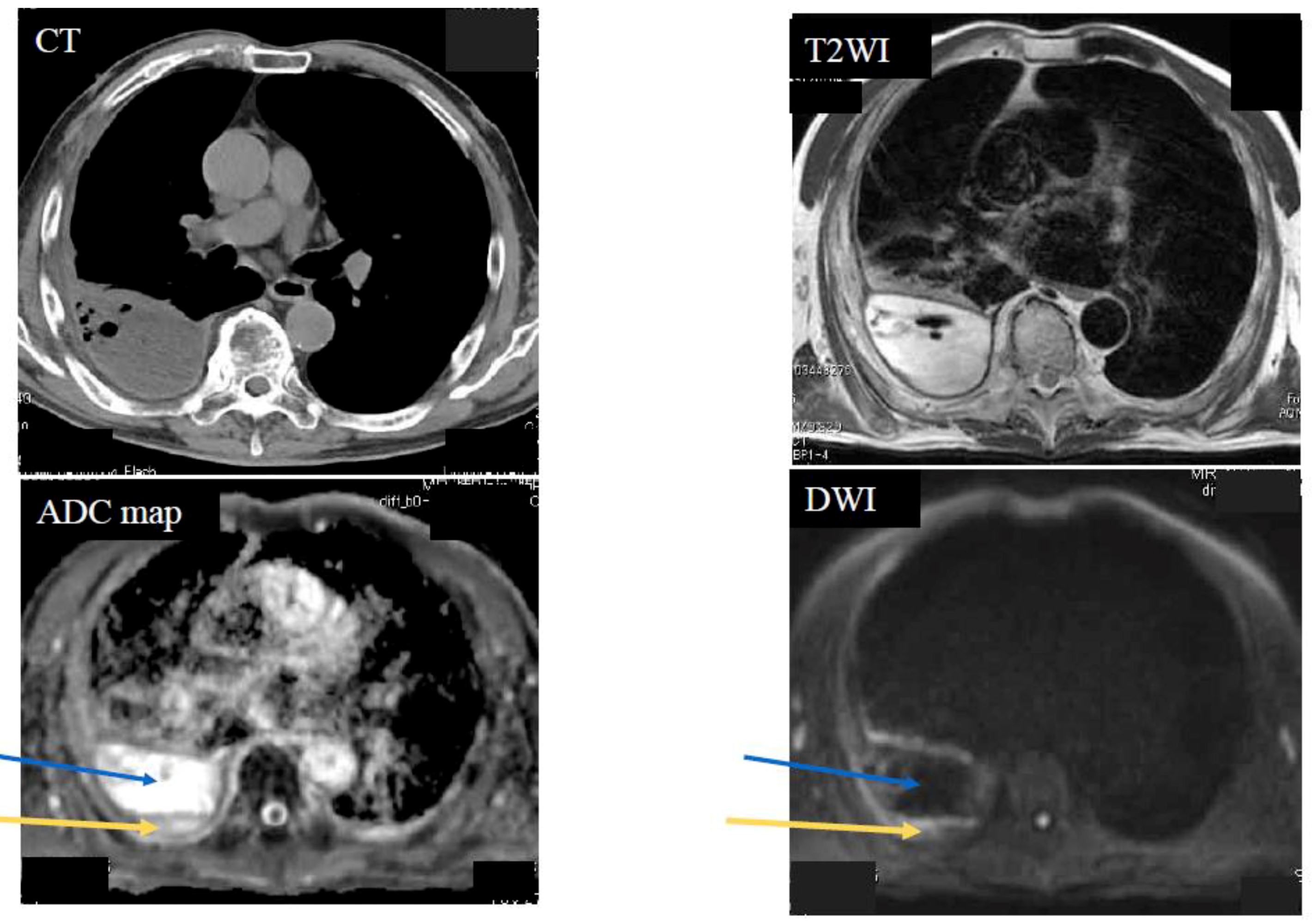
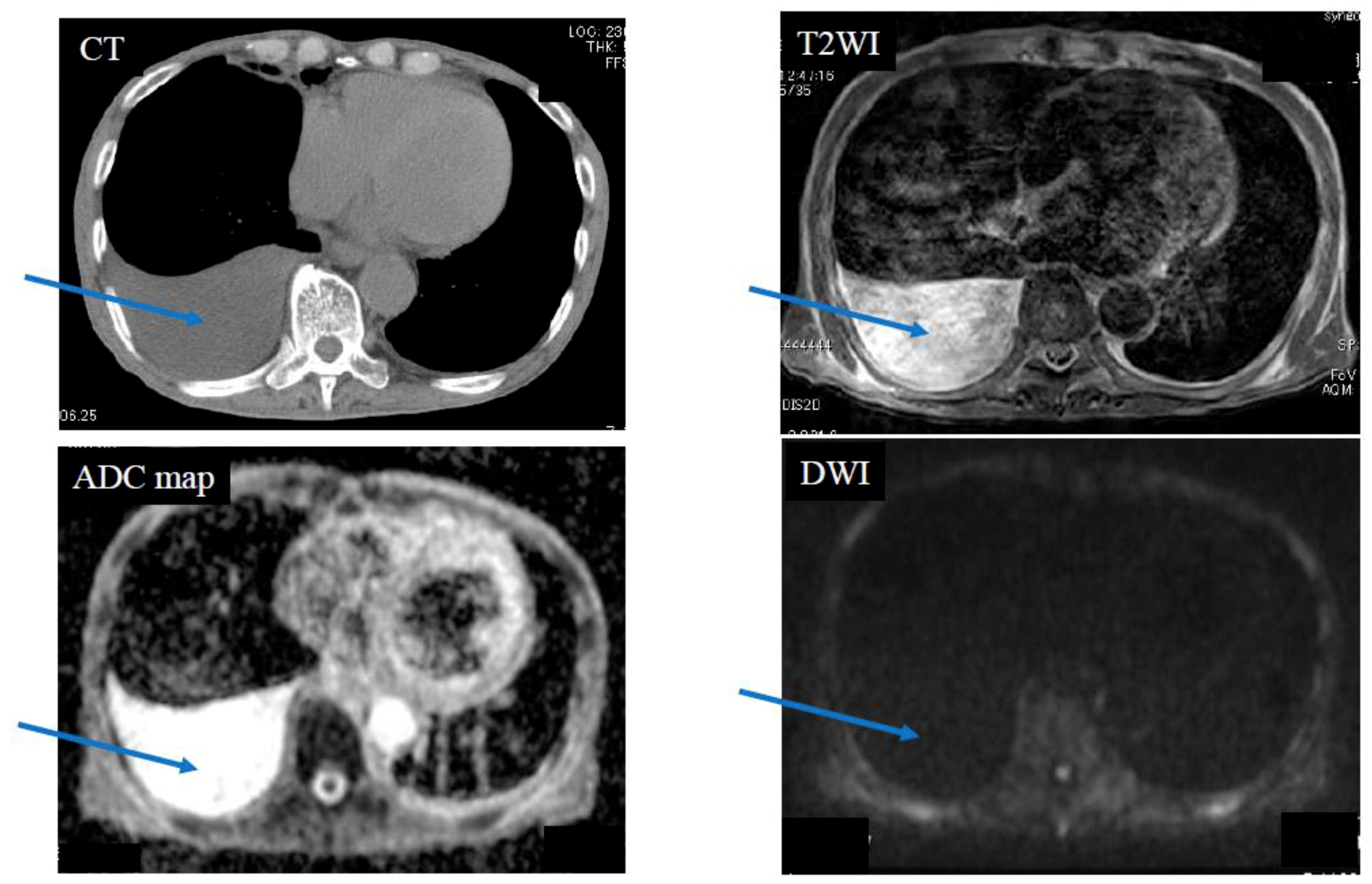
| Diffusion Pattern | Strong Continuous | Strong Scattered | Weak Continuous | No Decreased | No. of Cases | |
|---|---|---|---|---|---|---|
| Diagnosis | MPM | 11 | 0 | 0 | 0 | 11 |
| Pleural dissemination | 1 | 9 | 0 | 0 | 10 | |
| Empyema | 0 | 0 | 10 | 0 | 10 | |
| Pleural effusion | 0 | 0 | 0 | 12 | 12 | |
| No. of cases | 12 | 9 | 10 | 12 | 43 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Usuda, K.; Iwai, S.; Funasaki, A.; Sekimura, A.; Motono, N.; Matoba, M.; Doai, M.; Yamada, S.; Ueda, Y.; Uramoto, H. Diffusion-Weighted Imaging Can Differentiate between Malignant and Benign Pleural Diseases. Cancers 2019, 11, 811. https://doi.org/10.3390/cancers11060811
Usuda K, Iwai S, Funasaki A, Sekimura A, Motono N, Matoba M, Doai M, Yamada S, Ueda Y, Uramoto H. Diffusion-Weighted Imaging Can Differentiate between Malignant and Benign Pleural Diseases. Cancers. 2019; 11(6):811. https://doi.org/10.3390/cancers11060811
Chicago/Turabian StyleUsuda, Katsuo, Shun Iwai, Aika Funasaki, Atsushi Sekimura, Nozomu Motono, Munetaka Matoba, Mariko Doai, Sohsuke Yamada, Yoshimichi Ueda, and Hidetaka Uramoto. 2019. "Diffusion-Weighted Imaging Can Differentiate between Malignant and Benign Pleural Diseases" Cancers 11, no. 6: 811. https://doi.org/10.3390/cancers11060811






