Conversion Surgery with HIPEC for Peritoneal Oli-gometastatic Gastric Cancer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Inclusion Criteria
2.2. Evaluation of PM
2.3. CRS
2.4. HIPEC
2.5. Statistical Analysis
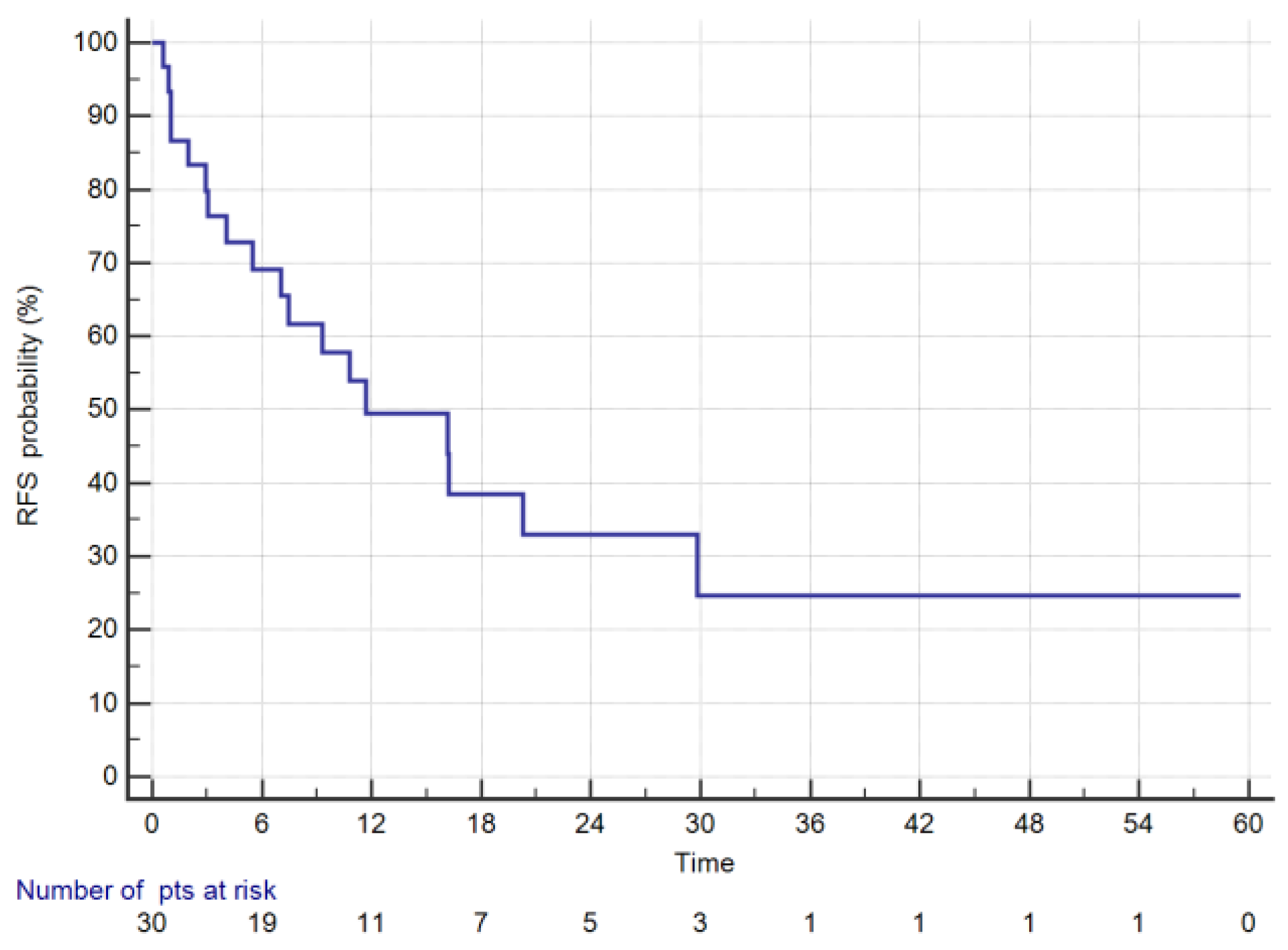
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Gill, R.S.; Al-Adra, D.P.; Nagendran, J.; Campbell, S.; Shi, X.; Haase, E.; Schiller, D. Treatment of gastric cancer with peritoneal carcinomatosis by cytoreductive surgery and HIPEC: A systematic review of survival, mortality, and morbidity. J. Surg. Oncol. 2011, 104, 692–698. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.L.; Chia, C.S.; Tan, G.H.; Choo, S.P.; Tai, D.W.; Chua, C.W.; Ng, M.C.; Soo, K.C.; Teo, M.C. Gastric peritoneal carcinomatosis—A retrospective review. World J. Gastrointest. Oncol. 2017, 9, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.-J.; Ito, S.; Yanagihara, K.; Mimori, K. Molecular mechanism of peritoneal dissemination in gastric cancer. J. Cancer Metastasis Treat. 2018, 4, 39. [Google Scholar] [CrossRef]
- Sitarz, R.; Skierucha, M.; Mielko, J.; Offerhaus, G.J.A.; Maciejewski, R.; Polkowski, W.P. Gastric cancer: Epidemiology, prevention, classification, and treatment. Cancer Manag. Res. 2018, 10, 239–248. [Google Scholar] [CrossRef] [PubMed]
- Sugarbaker, P.H. Gastric cancer: Prevention and treatment of peritoneal metastases. J. Cancer Metastasis Treat. 2018, 4, 7. [Google Scholar] [CrossRef]
- Bando, E.; Yonemura, Y.; Takeshita, Y.; Taniguchi, K.; Yasui, T.; Yoshimitsu, Y.; Fushida, S.; Fujimura, T.; Nishimura, G.; Miwa, K. Intraoperative lavage for cytological examination in 1297 patients with gastric carcinoma. Am. J. Surg. 1999, 178, 256–262. [Google Scholar] [CrossRef]
- Lee, J.H.; Son, S.Y.; Lee, C.M.; Ahn, S.H.; Park, D.J.; Kim, H.H. Factors predicting peritoneal recurrence in advanced gastric cancer: Implication for adjuvant intraperitoneal chemotherapy. Gastric Cancer 2014, 17, 529–536. [Google Scholar] [CrossRef]
- Yoshida, K.; Yamaguchi, K.; Okumura, N.; Tanahashi, T.; Kodera, Y. Is conversion therapy possible in stage IV gastric cancer: The proposal of new biological categories of classification. Gastric Cancer 2016, 19, 329–338. [Google Scholar] [CrossRef]
- Roviello, F.; Caruso, S.; Neri, A.; Marrelli, D. Treatment and prevention of peritoneal carcinomatosis from gastric cancer by cytoreductive surgery and hyperthermic intraperitoneal chemotherapy: Overview and rationale. Eur. J. Surg. Oncol. 2013, 39, 1309–1316. [Google Scholar] [CrossRef]
- Yang, X.J.; Huang, C.Q.; Suo, T.; Mei, L.J.; Yang, G.L.; Cheng, F.L.; Zhou, Y.F.; Xiong, B.; Yonemura, Y.; Li, Y. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy improves survival of patients with peritoneal carcinomatosis from gastric cancer: Final results of a phase III randomized clinical trial. Ann. Surg. Oncol. 2011, 18, 1575–1581. [Google Scholar] [CrossRef]
- Hotopp, T. HIPEC and CRS in peritoneal metastatic gastric cancer - who really benefits? Surg. Oncol. 2019, 28, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Rihuete Caro, C.; Manzanedo, I.; Pereira, F.; Carrion-Alvarez, L.; Serrano, A.; Perez-Viejo, E. Cytoreductive surgery combined with hyperthermic intraperitoneal chemotherapy (HIPEC) in patients with gastric cancer and peritoneal carcinomatosis. Eur. J. Surg. Oncol. 2018, 44, 1805–1810. [Google Scholar] [CrossRef] [PubMed]
- Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer 2011, 14, 101–112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wagner, P.L.; Austin, F.; Maduekwe, U.; Mavanur, A.; Ramalingam, L.; Jones, H.L.; Holtzman, M.P.; Ahrendt, S.A.; Zureikat, A.H.; Pingpank, J.F.; et al. Extensive cytoreductive surgery for appendiceal carcinomatosis: Morbidity, mortality, and survival. Ann. Surg. Oncol. 2013, 20, 1056–1062. [Google Scholar] [CrossRef] [PubMed]
- Clavien, P.A.; Barkun, J.; de Oliveira, M.L.; Vauthey, J.N.; Dindo, D.; Schulick, R.D.; de Santibanes, E.; Pekolj, J.; Slankamenac, K.; Bassi, C.; et al. The Clavien-Dindo classification of surgical complications: Five-year experience. Ann. Surg. 2009, 250, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Dindo, D.; Demartines, N.; Clavien, P.A. Classification of surgical complications: A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann. Surg. 2004, 240, 205–213. [Google Scholar] [CrossRef] [PubMed]
- AssesSurgery. CCI®-Calculator. Available online: https://www.assessurgery.com/index.php (accessed on 1 September 2019).
- Clavien, P.A.; Vetter, D.; Staiger, R.D.; Slankamenac, K.; Mehra, T.; Graf, R.; Puhan, M.A. The Comprehensive Complication Index (CCI(R)): Added Value and Clinical Perspectives 3 Years “Down the Line”. Ann. Surg. 2017, 265, 1045–1050. [Google Scholar] [CrossRef]
- Nakanishi, Y.; Tsuchikawa, T.; Okamura, K.; Nakamura, T.; Tamoto, E.; Noji, T.; Asano, T.; Amano, T.; Shichinohe, T.; Hirano, S. Risk factors for a high Comprehensive Complication Index score after major hepatectomy for biliary cancer: A study of 229 patients at a single institution. HPB (Oxford) 2016, 18, 735–741. [Google Scholar] [CrossRef]
- Coccolini, F.; Catena, F.; Glehen, O.; Yonemura, Y.; Sugarbaker, P.H.; Piso, P.; Montori, G.; Ansaloni, L. Complete versus incomplete cytoreduction in peritoneal carcinosis from gastric cancer, with consideration to PCI cut-off. Systematic review and meta-analysis. Eur. J. Surg. Oncol. 2015, 41, 911–919. [Google Scholar] [CrossRef]
- Fujitani, K.; Yang, H.K.; Mizusawa, J.; Kim, Y.W.; Terashima, M.; Han, S.U.; Iwasaki, Y.; Hyung, W.J.; Takagane, A.; Park, D.J.; et al. Gastrectomy plus chemotherapy versus chemotherapy alone for advanced gastric cancer with a single non-curable factor (REGATTA): A phase 3, randomised controlled trial. Lancet Oncol. 2016, 17, 309–318. [Google Scholar] [CrossRef]
- Al-Batran, S.E.; Homann, N.; Pauligk, C.; Illerhaus, G.; Martens, U.M.; Stoehlmacher, J.; Schmalenberg, H.; Luley, K.B.; Prasnikar, N.; Egger, M.; et al. Effect of Neoadjuvant Chemotherapy Followed by Surgical Resection on Survival in Patients With Limited Metastatic Gastric or Gastroesophageal Junction Cancer: The AIO-FLOT3 Trial. JAMA Oncol. 2017, 3, 1237–1244. [Google Scholar] [CrossRef] [PubMed]
- Al-Batran, S.E.; Goetze, T.O.; Mueller, D.W.; Vogel, A.; Winkler, M.; Lorenzen, S.; Novotny, A.; Pauligk, C.; Homann, N.; Jungbluth, T.; et al. The RENAISSANCE (AIO-FLOT5) trial: Effect of chemotherapy alone vs. chemotherapy followed by surgical resection on survival and quality of life in patients with limited-metastatic adenocarcinoma of the stomach or esophagogastric junction—A phase III trial of the German AIO/CAO-V/CAOGI. BMC Cancer 2017, 17, 893. [Google Scholar]
- Glehen, O.; Gilly, F.N.; Arvieux, C.; Cotte, E.; Boutitie, F.; Mansvelt, B.; Bereder, J.M.; Lorimier, G.; Quenet, F.; Elias, D.; et al. Peritoneal carcinomatosis from gastric cancer: A multi-institutional study of 159 patients treated by cytoreductive surgery combined with perioperative intraperitoneal chemotherapy. Ann. Surg. Oncol. 2010, 17, 2370–2377. [Google Scholar] [CrossRef] [PubMed]
- Rudloff, U.; Langan, R.C.; Mullinax, J.E.; Beane, J.D.; Steinberg, S.M.; Beresnev, T.; Webb, C.C.; Walker, M.; Toomey, M.A.; Schrump, D.; et al. Impact of maximal cytoreductive surgery plus regional heated intraperitoneal chemotherapy (HIPEC) on outcome of patients with peritoneal carcinomatosis of gastric origin: Results of the GYMSSA trial. J. Surg. Oncol. 2014, 110, 275–284. [Google Scholar] [CrossRef]
- Hall, J.J.; Loggie, B.W.; Shen, P.; Beamer, S.; Douglas Case, L.; McQuellon, R.; Geisinger, K.R.; Levine, E.A. Cytoreductive surgery with intraperitoneal hyperthermic chemotherapy for advanced gastric cancer. J. Gastrointest. Surg. 2004, 8, 454–463. [Google Scholar] [CrossRef]
- Magge, D.; Zenati, M.; Mavanur, A.; Winer, J.; Ramalingam, L.; Jones, H.; Zureikat, A.; Holtzman, M.; Lee, K.; Ahrendt, S.; et al. Aggressive locoregional surgical therapy for gastric peritoneal carcinomatosis. Ann. Surg. Oncol. 2014, 21, 1448–1455. [Google Scholar] [CrossRef]
- Boerner, T.; Graichen, A.; Jeiter, T.; Zemann, F.; Renner, P.; Marz, L.; Soeder, Y.; Schlitt, H.J.; Piso, P.; Dahlke, M.H. CRS-HIPEC Prolongs Survival but is Not Curative for Patients with Peritoneal Carcinomatosis of Gastric Cancer. Ann. Surg. Oncol. 2016, 23, 3972–3977. [Google Scholar] [CrossRef]


| Grade | Definition |
|---|---|
| Grade I | Any deviation from the normal postoperative course without the need for pharmacological treatment or surgical, endoscopic, and radiological interventions. Allowed therapeutic regimens are drugs as antiemetics, antipyretics, analgesics, diuretics and electrolytes and physiotherapy. This grade also includes wound infections opened at the bedside. |
| Grade II | Requiring pharmacological treatment with drugs other than such allowed for grade I complications. Blood transfusions and total parenteral nutrition are also included. |
| Grade III | Requiring surgical, endoscopic, or radiological intervention |
| - IIIa | Intervention not under general anaesthesia |
| - IIIb | Intervention under general anaesthesia |
| Grade IV | Life-threatening complication requiring IC/ICU-management |
| - IVa | single organ dysfunction |
| - IVb | multiorgan dysfunction |
| Grade V | Death of a patient |
| Clavien–Dindo Grade * | wC ** | CCI® Single Value |
|---|---|---|
| Grade I | 300 | 8.7 |
| Grade II | 1750 | 20.9 |
| Grade IIIa | 2750 | 26.2 |
| Grade IIIb | 4550 | 33.7 |
| Grade IVa | 7200 | 42.4 |
| Grade IVb | 8550 | 46.2 |
| Variables | No. of Patients = 30 (%) | |
|---|---|---|
| Age in years, mean ± SD; median, range | 51 ± 11.2; 55, 28–70 | |
| Gender: male/female | 20/10 (67/33 *) | |
| Neoadjuvant chemotherapy | ||
| EOX | 25 (83) | |
| FLOT | 5 (17) | |
| Pathological Assessment | ||
| Lauren type: intestinal/diffuse | 7/23 (23/77) | |
| ypT2/ypT3/ypT4 | 4/11/15 (13/37/50) | |
| pN1/pN2/pN3 | 17/8/5 (57/27/16) | |
| signet-ring cells | 23 (77) | |
| G2/G3 | 9/21 (30/70) | |
| P1/P2/P3 | 14/10/6 (47/33/20) | |
| PCI, mean ± SD; median, range | 5.06 ± 4.4; 6, 0–19 | |
| <6 * PCI | 23 (77) | |
| ≥6 PCI | 7 (23) | |
| CRS time (minutes) mean ± SD; median, range | 221.3 ± 58.7; 240, 90–300 | |
| Completeness Cytoreduction Score CC0/CC1, CC2 | 21/9 (70/30) | |
| Extensive cytoreduction | 22 (73) | |
| Surgical Gastric procedure | ||
| Total gastrectomy | 30 (100) | |
| Lymphadenectomy | ||
| D2 | 25 (83) | |
| D2+ | 3 (10) | |
| D3 | 2 (7) | |
| Visceral resections | ||
| Cholecystecomy | 7 (23) | |
| Appendectomy | 5 (17) | |
| Ovariectomy | 4 (13) | |
| Distal pancreatectomy | 3 (10) | |
| Small bowel resection | 2 (7) | |
| Transverse colon resection | 2 (7) | |
| Liver metastasectomy | 2 (7) | |
| Hysterectomy | 1 (3) | |
| Peritonectomies | ||
| Left diaphragm | 2 (7) | |
| Right diaphragm | 2 (7) | |
| Pelvic peritoneum | 3 (10) | |
| Mesenteric peritonectomy/electrovaporation | 21 (70) | |
| Intraperitoneal chemotherapy Mitomycin C/Oxaliplatin | 23/7 (77/23) | |
| HIPEC open/closed/laparoscopic | 22/7/1 (74/23/3) | |
| Postoperative complications (Clavien–Dindo Classification) | ||
| I | 1 (3) | |
| II | 7 (23) | |
| III | 3 (10) | |
| IV | 10 (33) | |
| V | 1 (3) | |
| Comprehensive Complication Index CCI, mean ± SD; median, range | 42.7 ± 22.7; 42.4, 8.7–100 | |
| Numbers of patients requiring ICU | 7 (23) | |
| ICU stay (days) mean ± SD; median, range | 3.9 ± 2.3; 4, 2–14 | |
| Hospital stay (days) mean ± SD; median, range | 15.2 ± 12; 11.5, 6–57 | |
| Postoperative mortality | ||
| 30 days | 1 (3.3) | |
| 90 days | 2 (6.7) | |
| Variables | Overall Survival Median | p Value Log-Rank Test | |
|---|---|---|---|
| Age (years) | <55 * | 16.5 | 0.91 |
| ≥55 | 19.3 | ||
| Gender | Male | 19.3 | 0.7 |
| Female | 16.5 | ||
| HIPEC protocol | MMC | 8.2 | 0.7 |
| Oxaliplatin | 16.5 | ||
| HIPEC technique | Closed | 16.5 | 0.79 |
| Open | 19.3 | ||
| CRS time (minutes) | <240 * | 19.3 | 0.33 |
| ≥240 | 16.5 | ||
| Laurén classification | Intestinal | # | 0.06 |
| Diffuse/mixed | 15.9 | ||
| Grade (histology) | G2 | 41.5 | 0.07 |
| G3 | 12.2 | ||
| ypT | pT2 | # | 0.03 |
| pT3 | 22.0 | ||
| pT4a/b | 8.2 | ||
| ypN | pN0 | 25.4 | 0.8 |
| pN+ | 18.1 | ||
| Peritoneal metastases classification (P) | P1 | 41.5 | 0.07 |
| P2 | 22.0 | ||
| P3 | 5.7 | ||
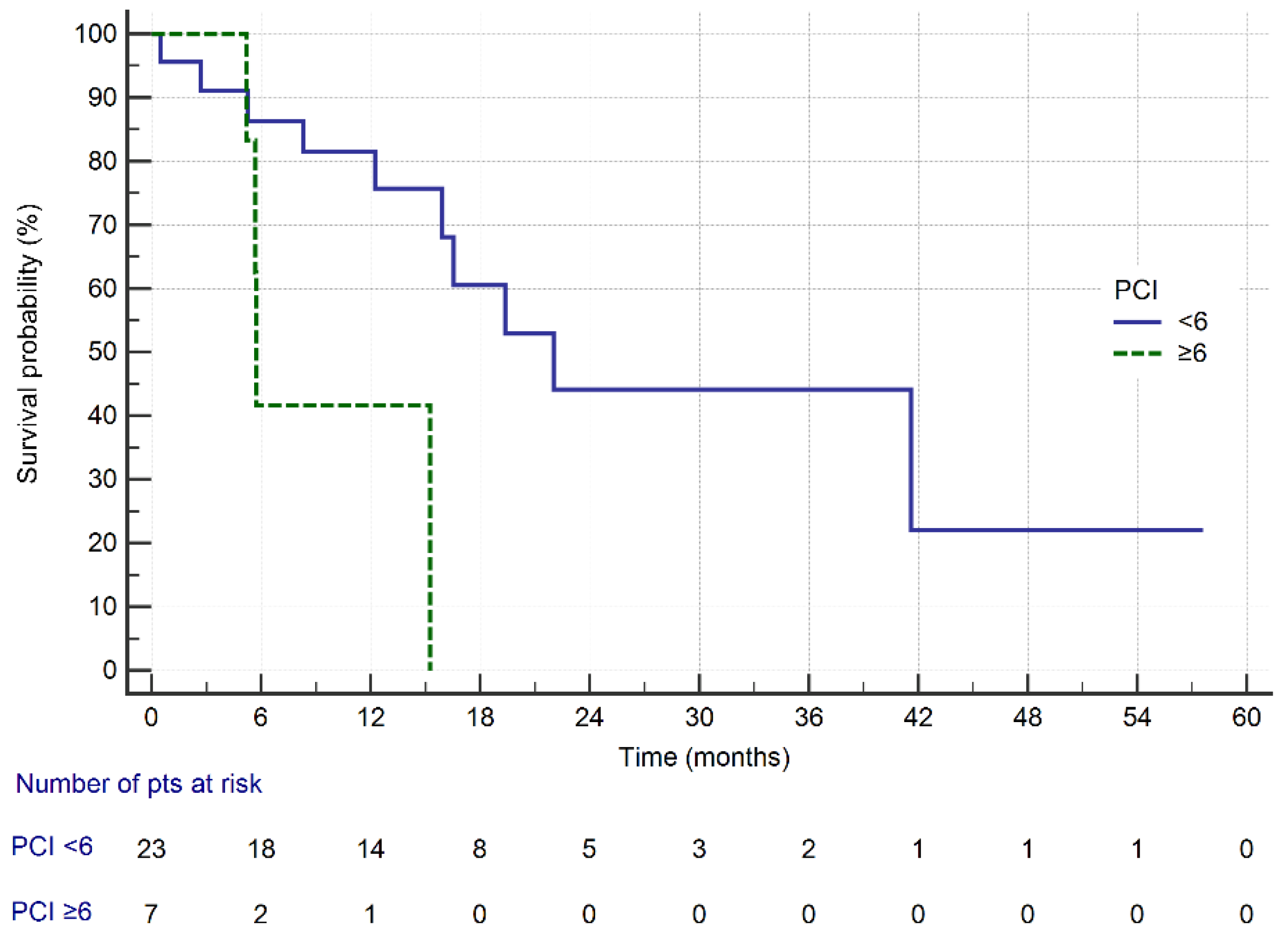
| PCI | <6 * PCI | 22.0 | 0.01 |
| ≥6 PCI | 5.7 | ||
| Completeness of cytoreduction score | CC0 | 16.5 | 0.1 |
| CC1/2 | # | ||
| Extensive cytoreduction | Normal | 30.2 | 0.2 |
| Extensive | 24.9 | ||
| Hospital stay (days) | <9 * | 19.3 | 0.4 |
| ≥9 | 15.9 | ||
| Postoperative complications (Clavien-Dindo Classification) | 0, I, II grade | 22.0 | 0.1 |
| III, IV grade | 15.2 | ||
| Comprehensive Complication Index CCI | <40 * | 19.3 | 0.28 |
| >40 | 15.2 | ||
| Author (Year) (Ref.#) | Number of Patients | Cytostatic for HIPEC | Mortality (%) | Morbidity (%) | 3-Year Survival (%) |
|---|---|---|---|---|---|
| Hall (2004) [26] | 34 | MMC | 0 | 35 | 45 |
| Glehen (2010) [24] | 139 | MMC ± Cisplatine Oxaliplatine ± Irinotecan | 6.5 | 28 | 18 |
| Yang (2011) [10] | 34 | MMC + Cisplatine | - | 15 | 15 |
| Magge (2014) [27] | 23 | MMC | 4.3 | 52 | 18 |
| Boerner (2016) [28] | 38 | Cisplatine + Doxorubicin | - | - | 43 |
| present study | 30 | MMC Oxaliplatine | 3 | 43 | 37 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mielko, J.; Rawicz-Pruszyński, K.; Skórzewska, M.; Ciseł, B.; Pikuła, A.; Kwietniewska, M.; Gęca, K.; Sędłak, K.; Kurylcio, A.; Polkowski, W.P. Conversion Surgery with HIPEC for Peritoneal Oli-gometastatic Gastric Cancer. Cancers 2019, 11, 1715. https://doi.org/10.3390/cancers11111715
Mielko J, Rawicz-Pruszyński K, Skórzewska M, Ciseł B, Pikuła A, Kwietniewska M, Gęca K, Sędłak K, Kurylcio A, Polkowski WP. Conversion Surgery with HIPEC for Peritoneal Oli-gometastatic Gastric Cancer. Cancers. 2019; 11(11):1715. https://doi.org/10.3390/cancers11111715
Chicago/Turabian StyleMielko, Jerzy, Karol Rawicz-Pruszyński, Magdalena Skórzewska, Bogumiła Ciseł, Agnieszka Pikuła, Magdalena Kwietniewska, Katarzyna Gęca, Katarzyna Sędłak, Andrzej Kurylcio, and Wojciech P. Polkowski. 2019. "Conversion Surgery with HIPEC for Peritoneal Oli-gometastatic Gastric Cancer" Cancers 11, no. 11: 1715. https://doi.org/10.3390/cancers11111715





