Should All Patients With HR-Positive HER2-Negative Metastatic Breast Cancer Receive CDK 4/6 Inhibitor As First-Line Based Therapy? A Network Meta-Analysis of Data from the PALOMA 2, MONALEESA 2, MONALEESA 7, MONARCH 3, FALCON, SWOG and FACT Trials
Abstract
:1. Introduction
2. Materials and Methods
2.1. Search Strategy and Study Selection
2.2. Eligibility Criteria
2.3. Outcomes
2.4. Data Collection and Statistical Analysis
3. Results
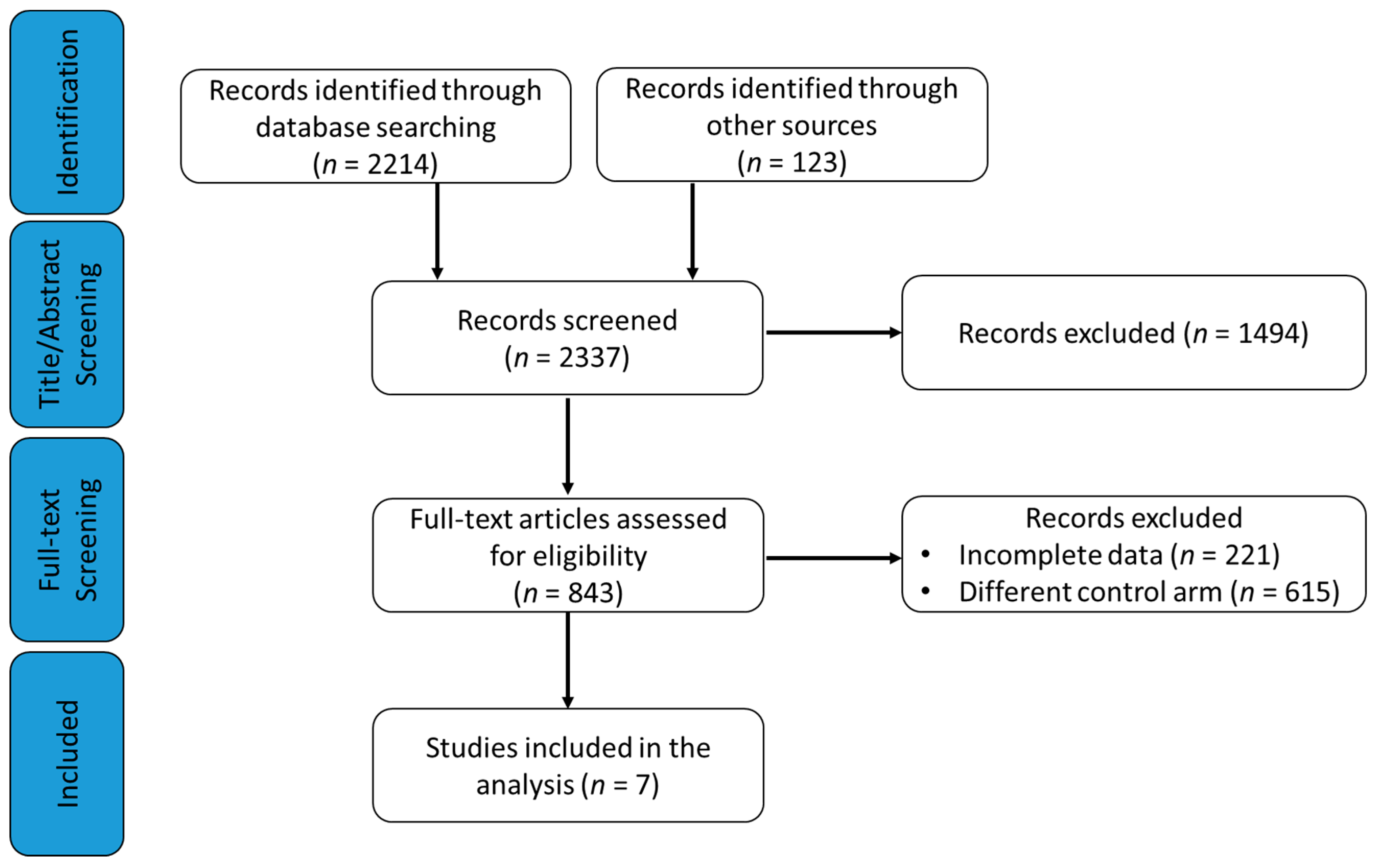
3.1. Study Selection
3.2. Description of Studies and Patients
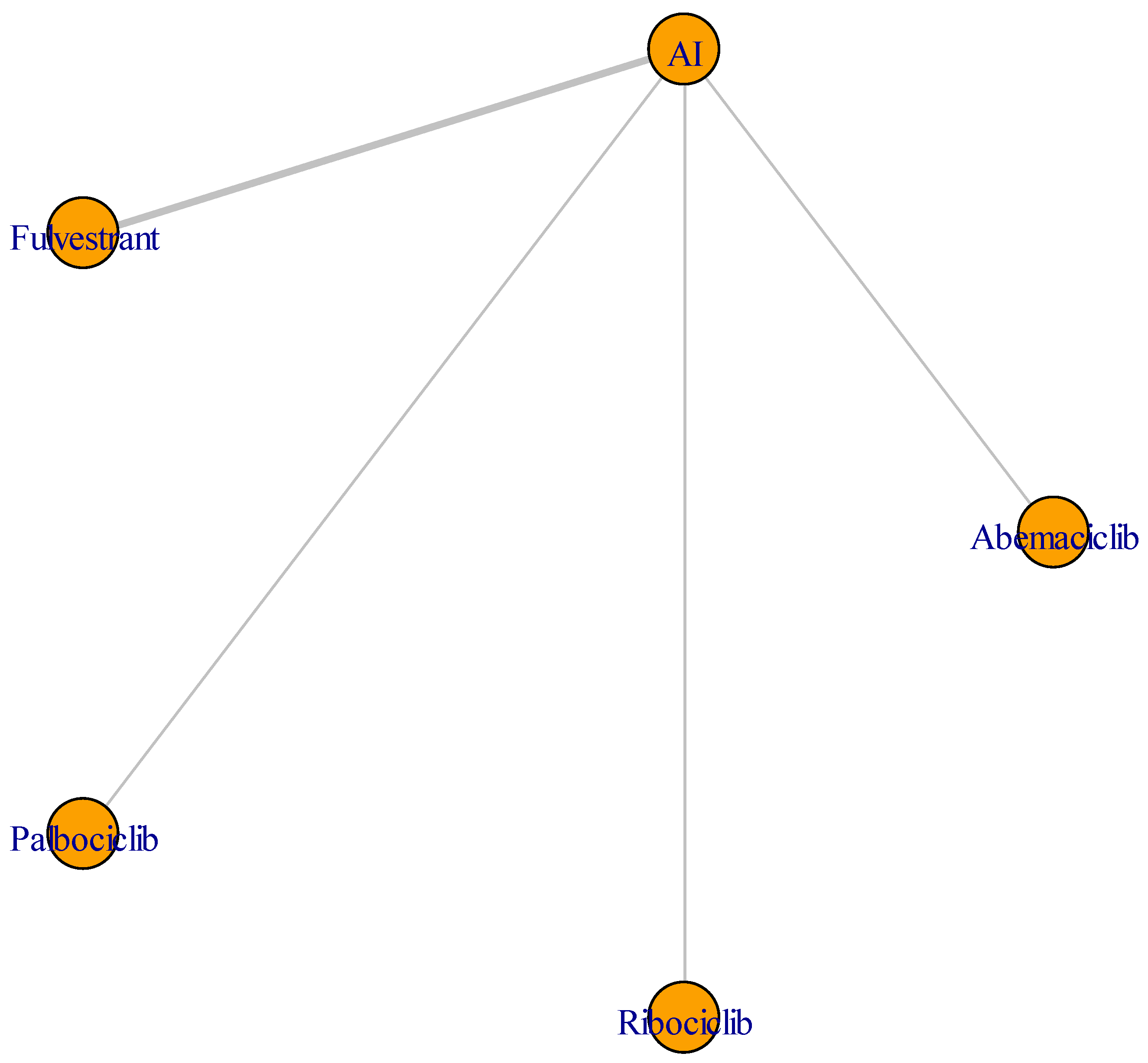
3.3. Outcomes
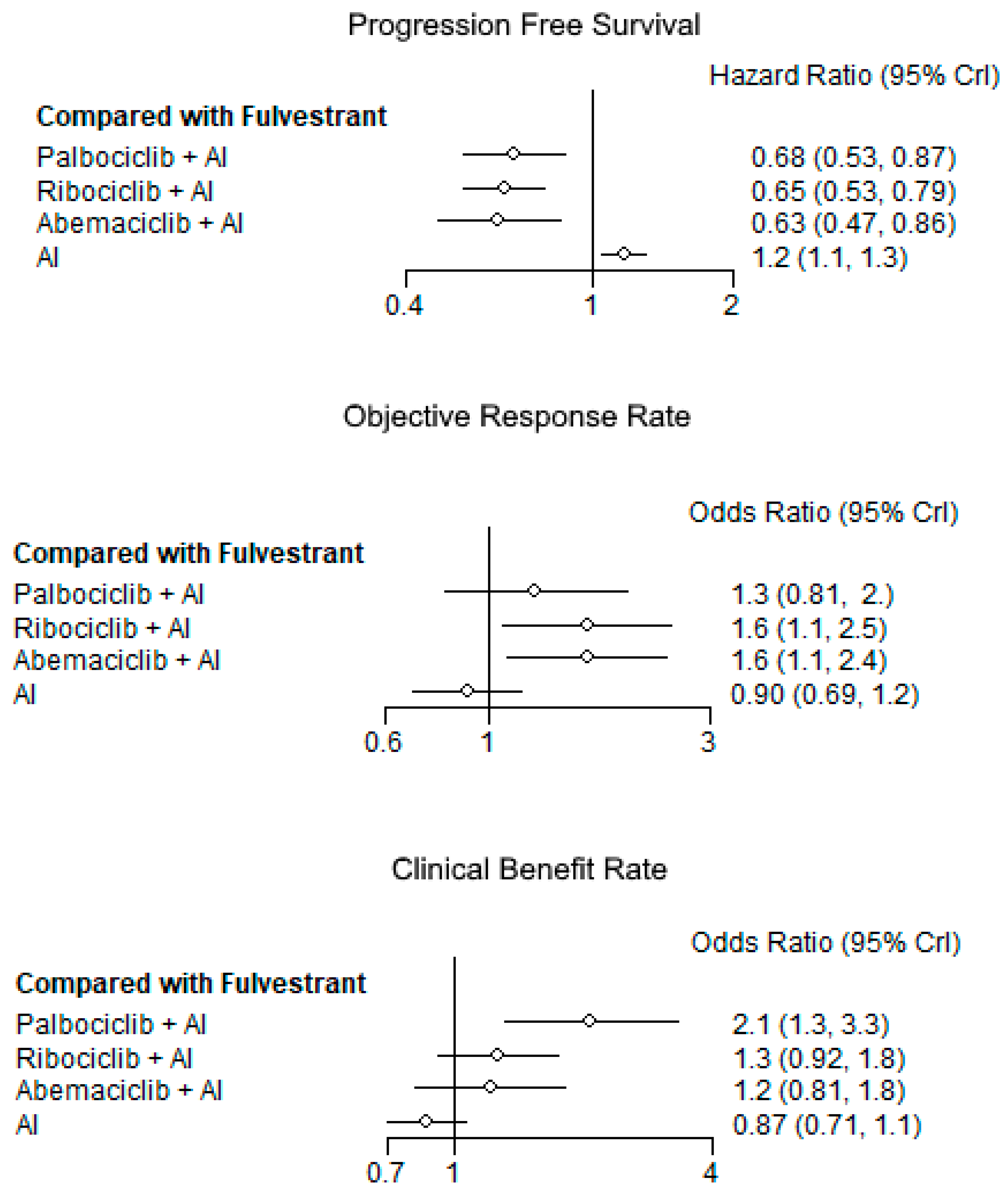
3.3.1. Progression-Free Survival
3.3.2. Objective Response
3.3.3. Clinical Benefit
3.3.4. Overall Survival
3.3.5. Safety Profile
3.4. Subgroup Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sorlie, T.; Perou, C.M.; Tibshirani, R.; Aas, T.; Geisler, S.; Johnsen, H.; Hastie, T.; Eisen, M.B.; van de Rijn, M.; Jeffrey, S.S.; et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc. Natl. Acad. Sci. USA 2001, 98, 10869–10874. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gonzalez-Angulo, A.M.; Morales-Vasquez, F.; Hortobagyi, G.N. Overview of resistance to systemic therapy in patients with breast cancer. Adv. Exp. Med. Biol. 2007, 608, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Rugo, H.S.; Rumble, R.B.; Macrae, E.; Barton, D.L.; Connolly, H.K.; Dickler, M.N.; Fallowfield, L.; Fowble, B.; Ingle, J.N.; Jahanzeb, M.; et al. Endocrine therapy for hormone receptor-positive metastatic breast cancer: American Society of Clinical Oncology Guideline. J. Clin. Oncol. 2016, 34, 3069–3103. [Google Scholar] [CrossRef] [PubMed]
- Kiang, D.T.; Kennedy, B.J. Tamoxifen (antiestrogen) therapy in advanced breast cancer. Ann. Intern. Med. 1977, 87, 687–690. [Google Scholar] [CrossRef]
- Robertson, J.F.R.; Bondarenko, I.M.; Trishkina, E.; Dvorkin, M.; Panasci, L.; Manikhas, A.; Shparyk, Y.; Cardona-Huerta, S.; Cheung, K.L.; Philco-Salas, M.J.; et al. Fulvestrant 500 mg versus anastrozole 1 mg for hormone receptor-positive advanced breast cancer (FALCON): An international, randomised, double-blind, phase 3 trial. Lancet 2016, 388, 2997–3005. [Google Scholar] [CrossRef]
- Di, L.A.; Jerusalem, G.; Petruzelka, L.; Torres, R.; Bondarenko, I.N.; Khasanov, R.; Verhoeven, D.; Pedrini, J.L.; Smirnova, I.; Lichinitser, M.R.; et al. Results of the CONFIRM phase III trial comparing fulvestrant 250 mg with fulvestrant 500 mg in postmenopausal women with estrogen receptor-positive advanced breast cancer. J. Clin. Oncol. 2010, 28, 4594–4600. [Google Scholar] [CrossRef]
- Mouridsen, H.; Gershanovich, M.; Sun, Y.; Perez-Carrion, R.; Boni, C.; Monnier, A.; Apffelstaedt, J.; Smith, R.; Sleeboom, H.P.; Jaenicke, F.; et al. Phase III study of letrozole versus tamoxifen as first-line therapy of advanced breast cancer in postmenopausal women: Analysis of survival and update of efficacy from the International Letrozole Breast Cancer Group. J. Clin. Oncol. 2003, 21, 2101–2109. [Google Scholar] [CrossRef]
- Nabholtz, J.M. Advanced breast cancer updates on anastrozole versus tamoxifen. J. Steroid Biochem. Mol. Biol. 2003, 86, 321–325. [Google Scholar] [CrossRef]
- Paridaens, R.J.; Dirix, L.Y.; Beex, L.V.; Nooij, M.; Cameron, D.A.; Cufer, T.; Piccart, M.J.; Bogaerts, J.; Therasse, P. Phase III study comparing exemestane with tamoxifen as first-line hormonal treatment of metastatic breast cancer in postmenopausal women: The European Organisation for Research and Treatment of Cancer Breast Cancer Cooperative Group. J. Clin. Oncol. 2008, 26, 4883–4890. [Google Scholar] [CrossRef]
- Mauri, D.; Pavlidis, N.; Polyzos, N.P.; Ioannidis, J.P. Survival with aromatase inhibitors and inactivators versus standard hormonal therapy in advanced breast cancer: Meta-analysis. J. Natl. Cancer Inst. 2006, 98, 1285–1291. [Google Scholar] [CrossRef]
- Schettini, F.; De Santo, I.; Rea, C.G.; De Placido, P.; Formisano, L.; Giuliano, M.; Arpino, G.; De Laurentiis, M.; Puglisi, F.; De Placido, S.; et al. CDK 4/6 inhibitors as single agent in advanced solid tumors. Front. Oncol. 2018, 8, 608. [Google Scholar] [CrossRef] [PubMed]
- Finn, R.S.; Martin, M.; Rugo, H.S.; Jones, S.; Im, S.A.; Gelmon, K.; Harbeck, N.; Lipatov, O.N.; Walshe, J.M.; Moulder, S.; et al. Palbociclib and letrozole in advanced breast cancer. N. Engl. J. Med. 2016, 375, 1925–1936. [Google Scholar] [CrossRef]
- Hortobagyi, G.N.; Stemmer, S.M.; Burris, H.A.; Yap, Y.S.; Sonke, G.S.; Paluch-Shimon, S.; Campone, M.; Petrakova, K.; Blackwell, K.L.; Winer, E.P.; et al. Updated results from MONALEESA-2, a phase III trial of first-line ribociclib plus letrozole versus placebo plus letrozole in hormone receptor-positive, HER2-negative advanced breast cancer. Ann. Oncol. 2018, 29, 1541–1547. [Google Scholar] [CrossRef] [PubMed]
- Goetz, M.P.; Toi, M.; Campone, M.; Sohn, J.; Paluch-Shimon, S.; Huober, J.; Park, I.H.; Trédan, O.; Chen, S.C.; Manso, L.; et al. MONARCH 3: Abemaciclibas initial therapy for advanced breast cancer. J. Clin. Oncol. 2017, 35, 3638–3646. [Google Scholar] [CrossRef] [PubMed]
- Tripathy, D.; Im, S.A.; Colleoni, M.; Franke, F.; Bardia, A.; Harbeck, N.; Hurvitz, S.A.; Chow, L.; Sohn, J.; Lee, K.S.; et al. Ribociclib plus endocrine therapy for premenopausal women with hormone-receptor-positive, advanced breast cancer (MONALEESA-7): A randomised phase 3 trial. Lancet Oncol. 2018, 19, 904–915. [Google Scholar] [CrossRef]
- Cristofanilli, M.; Turner, N.C.; Bondarenko, I.; Ro, J.; Im, S.A.; Masuda, N.; Colleoni, M.; DeMichele, A.; Loi, S.; Verma, S.; et al. Fulvestrant plus palbociclib versus fulvestrant plus placebo for treatment of hormone-receptor-positive, HER2-negative metastatic breast cancer that progressed on previous endocrine therapy (PALOMA-3): Final analysis of the multicentre, double-blind, phase 3 randomised controlled trial. Lancet Oncol. 2016, 17, 425–439. [Google Scholar] [CrossRef]
- Spring, L.M.; Zangardi, M.L.; Moy, B.; Bardia, A. Clinical management of potential toxicities and drug interactions related to cyclin-dependent kinase 4/6 inhibitors in breast cancer: Practical considerations and recommendations. Oncologist 2017, 22, 1039–1048. [Google Scholar] [CrossRef]
- Turner, N.C.; Slamon, D.J.; Ro, J.; Bondarenko, I.; Im, S.A.; Masuda, N.; Colleoni, M.; DeMichele, A.; Loi, S.; Verma, S.; et al. Overall survival with palbociclib and fulvestrant in advanced breast cancer. N. Engl. J. Med. 2018, 379, 1926–1936. [Google Scholar] [CrossRef]
- Hurvitz, S.A.; Im, S.A.; Lu, Y.S.; Colleoni, M.; Franke, F.A.; Bardia, A.; Harbeck, N.; Chow, L.W.C.; Sohn, J. Phase III MONALEESA-7 trial of premenopausal patients with HR+/HER2− advanced breast cancer (ABC) treated with endocrine therapy ± ribociclib: Overall survival (OS) results. J. Clin. Oncol. 2019. [CrossRef]
- Im, S.A.; Lu, Y.S.; Bardia, A.; Harbeck, N.; Colleoni, M.; Franke, F.; Chow, L.; Sohn, J.; Lee, K.S.; Campos-Gomez, S.; et al. Overall survival with ribociclib plus endocrine therapy in breast cancer. N. Engl. J. Med. 2019, 381, 307–316. [Google Scholar] [CrossRef]
- van Ommen-Nijhof, A.; Konings, I.R.; van Zeijl, C.J.J.; Uyl-de Groot, C.A.; van der Noort, V.; Jager, A.; Sonke, G.S.; SONIA Study Steering Committee. Selecting the optimal position of CDK 4/6 inhibitors in hormone receptor-positive advanced breast cancer – the SONIA study: Study protocol for a randomized controlled trial. BMC Cancer 2018, 18, 1146. [Google Scholar] [CrossRef] [PubMed]
- Cristofanilli, M.; DeMichele, A.; Giorgetti, C.; Turner, N.C.; Slamon, D.J.; Im, S.A.; Masuda, N.; Verma, S.; Loi, S.; Colleoni, M.; et al. Predictors of prolonged benefit from palbociclib plus fulvestrant in women with endocrine-resistant hormone receptor-positive/human epidermal growth factor receptor 2-negative metastatic breast cancer in PALOMA-3. Eur. J. Cancer 2018, 104, 21–31. [Google Scholar] [CrossRef] [PubMed]
- O’Leary, B.; Cutts, R.J.; Liu, Y.; Hrebien, S.; Huang, X.; Fenwick, K.; André, F.; Loibl, S.; Loi, S.; Garcia-Murillas, I.; et al. The genetic landscape and clonal evolution of breast cancer resistance to palbociclib plus fulvestrant in the PALOMA-3 trial. Cancer Discov. 2018, 8, 1390–1403. [Google Scholar] [CrossRef] [PubMed]
- O’Leary, B.; Hrebien, S.; Morden, J.P.; Beaney, M.; Fribbens, C.; Huang, X.; Liu, Y.; Bartlett, C.H.; Koehler, M.; Cristofanilli, M.; et al. Early circulating tumor DNA dynamics and clonal selection with palbociclib and fulvestrant for breast cancer. Nat. Commun. 2018, 9, 896. [Google Scholar] [CrossRef] [PubMed]
- Mehta, R.S.; Barlow, W.E.; Albain, K.S.; Vandenberg, T.A.; Dakhil, S.R.; Tirumali, N.R.; Lew, D.L.; Hayes, D.F.; Gralow, J.R.; Livingston, R.B.; et al. Combination anastrozole and fulvestrant in metastatic breast cancer. N. Engl. J. Med. 2012, 367, 435–444. [Google Scholar] [CrossRef]
- Mehta, R.S.; Barlow, W.E.; Albain, K.S.; Vandenberg, T.A.; Dakhil, S.R.; Tirumali, N.R.; Lew, D.L.; Hayes, D.F.; Gralow, J.R.; Linden, H.H.; et al. Overall survival with fulvestrant plus anastrozole in metastatic breast cancer. N. Engl. J. Med. 2019, 380, 1226–1234. [Google Scholar] [CrossRef]
- Bergh, J.; Jonsson, P.E.; Lidbrink, E.K.; Trudeau, M.; Eiermann, W.; Brattström, D.; Lindemann, J.P.; Wiklund, F.; Henriksson, R. FACT: An open-label randomized phase III study of fulvestrant and anastrozole in combination compared with anastrozole alone as first-line therapy for patients with receptor-positive postmenopausal breast cancer. J. Clin. Oncol. 2012, 30, 1919–1925. [Google Scholar] [CrossRef]
- Van Valkenhoef, G.; Lu, G.; de Brock, B.; Hillege, H.; Ades, A.E.; Welton, N.J. Automating network meta-analysis. Res. Synth. Methods 2012, 3, 285–299. [Google Scholar] [CrossRef]
- Van Valkenhoef, G. GEMTC: Network Meta-Analysis Using Bayesian Methods. R package version 0.8-2. Available online: https://rdrr.io/cran/gemtc/ (accessed on 25 October 2019).
- Vidula, N.; Rugo, H.S. Emerging data on improving response to hormone therapy: The role of novel targeted agents. Expert Rev. Anticancer Ther. 2018, 18, 3–18. [Google Scholar] [CrossRef]
- Başaran, G.A.; Twelves, C.; Dieras, V.; Cortés, J.; Awada, A. Ongoing unmet needs in treating estrogen receptor-positive/HER2-negative metastatic breast cancer. Cancer Treat. Rev. 2018, 63, 144–155. [Google Scholar] [CrossRef]
- Ding, W.; Li, Z.; Wang, C.; Ruan, G.; Chen, L.; Tu, C. The CDK 4/6 inhibitor in HR-positive advanced breast cancer: A systematic review and meta-analysis. Medicine (Baltimore) 2018, 97, e10746. [Google Scholar] [CrossRef] [PubMed]
- Corona, S.P.; Generali, D. Abemaciclib: A CDK 4/6 inhibitor for the treatment of HR+/. Drug Des. Devel. Ther. 2018, 12, 321–330. [Google Scholar] [CrossRef] [PubMed]
- Mills, C.E. Omics profiling of CDK 4/6 inhibitors reveals functionally important secondary targets of abemaciclib. In Proceedings of the Visualizing and Quantifying Drug Distribution in Tissue II, San Francisco, CA, USA, 14 March 2018; SPIE: Bellingham, WA, USA, 2018. [Google Scholar]
- André, F.; Ciruelos, E.; Rubovszky, G.; Campone, M.; Loibl, S.; Rugo, H.S.; Iwata, H.; Conte, P.; Mayer, I.A.; Kaufman, B.; et al. Alpelisib for PIK3CA-mutated, hormone receptor–positive advanced breast cancer. N. Engl. J. Med. 2019, 380, 1929–1940. [Google Scholar] [CrossRef] [PubMed]
- Ayyagari, R.; Tang, D.; Patterson-Lomba, O.; Zhou, Z.; Xie, J.; Chandiwana, D.; Dalal, A.A.; Niravath, P.A. Progression-free survival with endocrine-based therapies following progression on non-steroidal aromatase inhibitor among postmenopausal women with hormone receptor positive, human epidermal growth factor receptor-2 negative metastatic breast cancer: A network meta-analysis. Curr. Med. Res. Opin. 2018, 34, 1645–1652. [Google Scholar] [CrossRef] [PubMed]
- El, R.E.; Bakouny, Z.; Assi, T.; Kattan, J. Different inhibitors for the same target in metastatic luminal breast cancer: Is there any difference? Future Oncol. 2018, 14, 891–895. [Google Scholar] [CrossRef]

| Study and First Author | Publication Year | Phase | Setting | Post-Menopausal | RR (%) | CB (%) | Median PFS (months) | Median OS (months) |
|---|---|---|---|---|---|---|---|---|
| PALOMA-2 Finn [12] | 2016 | III | First-line therapy for MBC in patients not treated before for their metastatic disease. | Yes | 42.1 vs. 34.7 | 84.9 vs. 70.3 | 24.8 vs. 14.5 | NR |
| MONALEESA-2 Hotobagyi [13] | 2016 2018 | III | First-line therapy for locally advanced and MBC. Patients who had not received previous systemic therapy for advanced disease were eligible. Previous neoadjuvant or adjuvant therapy with a nonsteroidal AI was not allowed unless the disease-free interval was more than 12 months | Yes | 40.7 vs. 27.5 42.5 vs. 28.7 | 79.6 vs. 72.8 79.9 vs. 73.1 | NR vs. 14.7 25.3 vs. 16 | NR NR vs. 33 |
| MONARCH-3 Goetz [14] | 2017 | III | First-line therapy for locally advanced and MBC (endocrine therapy in the neoadjuvant or adjuvant setting was permitted if the patient had a disease-free interval 12 months from the completion of endocrine therapy) | Yes | 48.2 vs. 34.5 | 78 vs. 71.5 | NR vs. 14.7 | NR |
| MONALEESA-7 Tripathy [15] | 2018 2019 | III | First-line therapy for locally advanced or MBC (endocrine therapy and chemotherapy in the adjuvant or neoadjuvant setting was permitted, as was up to one line of chemotherapy for advanced disease). | Not (only pre and perimenopausal women were included and treated with goserelin) | 41 vs. 30 | 79 vs. 70 | 23.8 vs. 13.0 | NR NR vs. 40.9 |
| FALCON Robertson [5] | 2016 | III | First-line therapy for locally advanced or MBC (no previous adjuvant therapy was admitted, only a first-line CHT for metastatic disease was accepted) | Yes | 46 vs. 45 | 78 vs. 74 | 16.6 vs. 13.8 | NR |
| SWOG Mehta [26] | 2012 2019 | III | First line for de novo MBC or recurrent MBC after 12 months by the end of adjuvant CHT or HT | Yes | 27 vs. 22 | 73 vs. 70 | 15 vs. 13.5 15 vs. 13.5 | 47.7 vs. 41.3 49.8 vs. 42 |
| FACT Bergh [27] | 2012 | III | First-line therapy in recurrent MBC after or during primary treatment (with or without HT, CHT, RT). Patients treated with an adjuvant AI had to be relapse-free for more than one year after completion of this type of endocrine therapy (30.2% of the patients in the experimental arm were endocrine naive) | Yes (only 3% of patients wer in the premenopausal status and were treated with GnRH agonists) | 31.8 vs. 33.6 | 55 vs. 55.1 | 10.8 vs. 10.2 | 37.8 vs. 38.2 |
| Study | Treatment | AEs | G3 (%) | G4 (%) |
|---|---|---|---|---|
| PALOMA-2 | Palbociclib–letrozole | Any AEs | 62.2% | 13.5% |
| Neutropenia | 56.1% | 10.4% | ||
| Leukopenia | 24.1% | 0.7% | ||
| Placebo–letrozole | Any AEs | 22.1% | 2.3% | |
| Neutropenia | 0.9% | 0.5% | ||
| MONALEESA-2 | Ribociclib–letrozole | Neutropenia | 52.4% | 9.6% |
| Abnormal LFT | 8.4% | 1.8% | ||
| Leukopenia | 20.1% | 1.2% | ||
| Placebo–letrozole | Abnormal LFT | 2.4% | – | |
| Neutropenia, anemia, arthralgia | 1.2% | – | ||
| MONARCH-3 | Abemaciclib–nonsteroidal AI | Any AEs | 51.7% | 6.7% |
| Neutropenia | 22.0% | 1.8% | ||
| Leukopenia | 8.3% | 0.3% | ||
| ALT increase | 6.1% | 0.3% | ||
| Placebo–nonsteroidal AI | Any AEs | 22.4% | 2.5% | |
| Neutropenia | 0.6% | 0.6% | ||
| MONALEESA-7 | Ribociclib group | Any AEs | 63% | 14% |
| Neutropenia | 51% | 10% | ||
| Leukopenia | 13% | 1% | ||
| Placebo group | Any AEs | 26% | 4% | |
| Neutropenia | 3% | 1% | ||
| FALCON Robertson | Fulvestrant | Arthralgia (17%) | ||
| Hot flush, fatigue, nausea (11%) | ||||
| Back pain (9%) | ||||
| Anastrozole | Arthralgia, hot flush, nausea (10%) | |||
| SWOG Mehta | Anastrozole | Musculoskeletal pain, fatigue, hot flashes, mood alterations, GI symptoms | 15% (each 1–4%) | |
| Anastrozole–fulvestrant | Musculoskeletal pain, fatigue, hot flashes, mood alterations, GI symptoms | 13% (each 1–4%) | ||
| FACT Bergh | Anastrozole | GI symptoms (25.2%) | ||
| Joint disorders (27.6%) | ||||
| Hot flashes (13.8%) | ||||
| Anastrozole–fulvestrant | GI symptoms (28.9%) | |||
| Joint disorders (26.6%) | ||||
| Hot flashes (24.6%) | ||||
| Characteristics | PALOMA-2, n (%) | MONALEESA-2, n (%) | MONARCH-3, n (%) | MONALEESA-7, n (%) | FALCON a, n (%) | SWOG b, n (%) | FACT4, n (%) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| L. + Palb. | L. | L. + Rib. | L. | L or A. + Abem. | L. or A. | Rib. + T or nAIs | T or nAIs | F. | A. | F.+ A. | A. | F. + A. | A. | |
| No. of patients | 444 | 222 | 334 | 334 | 328 | 165 | 335 | 337 | 230 | 232 | 345 | 349 | 258 | 256 |
| Age: | ||||||||||||||
| • Median (range), years | 62(3089) | 61 (28–88) | 62 (23–91) | 63 (29–88) | 63 (38–87) | 63 (32–88) | 43 (25–58) | 45 (29–58) | 64 (38–87) | 62 (36–90) | 65 (36–91) | 65 (27–92) | 65 (33–86) | 63 (36–90) |
| • <65 years | 263 (59.2) | 141 (63.5) | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | 124 (48.1) | 145 (56.6) |
| • ≥65 years | 181 (40.8) | 81 (36.5) | NR | NR | NR | NR | NR | NR | 108 (47) | 91 (39) | NR | NR | 134 (51.9) | 111 (43.3) |
| Ethnicity: | ||||||||||||||
| • White | 344 (77.5) | 172 (77.5) | 269 (80.5) | 280 (83.8) | 186 (56.7) | 102 (61.8) | 187 (56) | 201 (60) | 175 (76) | 174 (75) | NR | NR | 242 (93.8) | 237 (92.6) |
| • Asian | 65 (14.6) | 30 (13.5) | 28 (8.4) | 23 (6.9) | 103 (31.4) | 45 (27.3) | 99 (30) | 99 (29) | 36 (16) | 34 (15) | NR | NR | 4 (1.6) | 2 (0.8) |
| • Black | 8 (1.8) | 3 (1.4) | 10 (3.0) | 7 (2.1) | NR | NR | 10 (3) | 9 (3) | NR | NR | NR | NR | 1 (0.4) | 2 (0.8) |
| • Other | 27 (6.1) | 17 (7.7) | 27 (8.1) | 24 (7.2) | 11 (3.4) | 7 (4.2) | 39 (12) | 28 (8) | 19 (8) | 24 (10) | NR | NR | 11 (4.3) | 15 (5.9) |
| Hormone receptor status: | ||||||||||||||
| • ER+, PgR+ | NR | NR | NR | NR | 255 (77.7) | 127 (77.0) | 290 (87) | 288 (85) | 175 (76) | 179 (77) | NR | NR | 193 (74.8) | 195 (76.2) |
| • ER+, PgR− | NR | NR | NR | NR | 70 (21.3) | 36 (21.8) | NR | NR | 44 (19) | 43 (19) | NR | NR | 60 (23.3) | 51 (19.9) |
| • Unknown | NR | NR | NR | NR | NR | NR | NR | NR | 10(4) | 7 (3) | NR | NR | 4 (1.6) | 6 (2.3) |
| • ER−PgR+ | NR | NR | NR | NR | NR | NR | NR | NR | 1 (<1) | 3 (1) | NR | NR | 1 (0.4) | 4 (1.6) |
| Performance status: | ||||||||||||||
| • ECOG 0 | 257 (57.9) | 102 (45.9) | 205 (61.4) | 202 (60.5) | 192 (58.5) | 104 (63.0) | 245 (73) | 255 (76) | 117 (51) | 115 (50) | NR | NR | NR | NR |
| • ECOG 1 | 178 (40.1) | 117 (52.7) | 129 (38.6) | 132 (39.5) | 136 (41.5) | 61 (37.0) | 87 (26) | 78 (23) | 106 (46) | 105 (45) | NR | NR | NR | NR |
| • ECOG 2 | 9 (2.0) | 3 (1.4) | NR | NR | NR | NR | 0 | 1 (<1) | 7 (3) | 12 (5) | NR | NR | NR | NR |
| • ECOG > 2 | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| • Unavailable | NR | NR | NR | NR | NR | NR | 3 (1) | 3 (1) | NR | NR | NR | NR | NR | NR |
| Disease stage: | ||||||||||||||
| • I | 51 (11.5) | 30 (13.5) | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| • II | 137 (30.9) | 68 (30.6) | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| • III | 72 (16.2) | 39 (17.6) | 1 (0.3) | 3 (0.9) | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| • IV | 138 (31.1) | 72 (32.4) | 333 (99.7) | 331 (99.1) | NR | NR | NR | NR | 202 (88) | 200 (86) | NR | NR | 245 (95) | 242 (94.5) |
| • Unknown | 36 (8.1) | 1 (0.5) | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| Site of metastasis: | ||||||||||||||
| • Bone only | 103 (23.2) | 48 (21.6) | 69 (20.1) | 78 (23.4) | 70 (21.3) | 39 (23.6) | 81 (24) | 78 (23) | 24 (10) | 24 (10) | 76 (22.0) | 75 (21.5) | 63 (24.4) | 71 (27.7) |
| • Visceral | 214 (48.2) | 110 (49.5) | 197 (59.0) | 196 (58.7) | 172 (52.4) | 89 (53.9) | 193 (58) | 188 (56) | 135 (59) | 119 (51) | 167 (48.4) | 181 (51.9) | 134 (51.9) | 124 (48.4) |
| • Non- visceral | 230 (51.8) | 112 (50.5) | NR | NR | NR | NR | NR | NR | 60 (26) | 81 (35) | 102 (29.6) | 93 (26.6) | 195 (28.1) | NR |
| • Lymph nodes | NR | NR | 133 (39.8) | 123 (36.8) | NR | NR | 142 (42) | 158 (47) | NR | NR | NR | NR | NR | NR |
| • Other | NR | NR | 45 (12.9) | 33 (9.9) | 86 (26.2) | 37 (22.4) | 8 (2) | 8(2) | 11 (4) | 8 (4) | NR | NR | 1 (0.4) | 1 (0.4) |
| Measurable disease: | ||||||||||||||
| • Yes | NR | NR | NR | NR | 267 (81.4) | 130 (78.8) | NR | NR | 193 (84) | 196 (84) | 188 (54.5) | 188 (53.9) | 129 (50.0) | 113 (44.1) |
| • No | NR | NR | NR | NR | 61 (18.6) | 35 (21.2) | NR | NR | NR | NR | 157 (45.5) | 161 (46.1) | 129 (50.0) | 143 (55.9) |
| Disease-free interval: | ||||||||||||||
| • De novo | 167 (37.6) | 81 (36.5) | 114 (34.1) | 113 (33.8) | NR | NR | 136 (41) | 134 (40) | NR | NR | NR | NR | NR | NR |
| • ≤12 months | 99 (22.3) | 48 (21.6) | 4 (1.2) | 10 (3.0) | NR | NR | 23 (7) | 13 (4) | NR | NR | NR | NR | 14 (5.4) | 18 (7.0) |
| • >12 months | 178 (40.1) | 93 (41.9) | 216 (64.7) | 210 (62.9) | NR | NR | 176 (53) | 190 (56) | NR | NR | NR | NR | 85 (32.9) | 78 (30.5) |
| • Unknown | NR | NR | NR | 1 (0.3) | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| No. of disease sites | ||||||||||||||
| • 0 | NR | NR | 2 (0.6) | 1 (0.3) | NR | NR | 1 (<1) | NR | NR | NR | NR | NR | NR | NR |
| • 1 | 138 (31.1) | 66 (29.7) | 100 (29.9) | 117 (35.0) | 96 (29.3) | 47 (28.5) | 112 (33) | 117 (35) | NR | NR | NR | NR | NR | NR |
| • 2 | 117 (26.4) | 52 (23.4) | 118 (35.3) | 103 (30.8) | 76 (23.2) | 42 (25.5) | 106 (32) | 99 (29) | NR | NR | NR | NR | NR | NR |
| • 3 | 112 (25.2) | 61 (27.5) | 114 (34.1) | 113 (33.8) | 154 (47)2 | 75 (45.5)2 | 116 (35) | 121 (36) | NR | NR | NR | NR | NR | NR |
| • ≥4 | 77 (17.3) | 43 (19.4) | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| Prior chemotherapy: | ||||||||||||||
| • Adjuvant | 180 (40.5) | 89 (40.1) | 146 (43.7) | 145 (43.4) | 125 (38.1) | 66 (40.0) | 138 (41) | 138 (41) | 35 (15) | 27 (12) | 103 (29.9) | 129 (37.0) | 108 (41.9) | 127 (49.6) |
| • Neoadjuvant | 54 (12.2) | 32 (14.4) | * | * | * | * | * | * | 11 (5) | 16 (7) | NR | NR | NR | NR |
| • Palliative | NR | NR | NR | NR | NR | NR | 47 (14) | 47 (14) | 36 (16) | 43 (19) | NR | NR | NR | NR |
| • None | NR | NR | NR | NR | 203 (61.9) | 99 (60.0) | 150 (45) | 152 (45) | NR | NR | 242 (70.1) | 220 (63.0) | NR | NR |
| Prior hormonal therapy: | ||||||||||||||
| • Adjuvant | 249 (56.1) | 126 (56.8) | 175 (52.4) | 171 (51.2) | 150 (45.7) | 80 (48.5) | 127 (38) | 141 (42) | 2 (1) | 1 (<1) | 139 (40.3) | 141 (40.4) | 180 (69.8) | 168 (65.6) |
| • Neoadjuvant | NR | * | * | |||||||||||
| • None | NR | NR | NR | NR | 178 (54.3) | 85 (51.5) | 208 (62) | 196 (58) | NR | NR | 206 (59.7) | 208 (59.6) | NR | NR |
| Type of adjuvant ET: | ||||||||||||||
| • Tamoxifen | 209 (47.1) | 98 (44.1) | 140 (41.9) | 145 (43.4) | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| • Anastrozole | 56 (12.6) | 29 (13.1) | 47 (14.1) | 42 (12.6) | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| • Letrozole | 36 (8.1) | 16 (7.2) | 34 (10.2) | 25 (7.5) | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| • Exemestane | 30 (6.8) | 13 (5.9) | 19 (5.7) | 25 (7.5) | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| • Other | NR | NR | 8 (2.4) | 7 (2.1) | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rossi, V.; Berchialla, P.; Giannarelli, D.; Nisticò, C.; Ferretti, G.; Gasparro, S.; Russillo, M.; Catania, G.; Vigna, L.; Mancusi, R.L.; et al. Should All Patients With HR-Positive HER2-Negative Metastatic Breast Cancer Receive CDK 4/6 Inhibitor As First-Line Based Therapy? A Network Meta-Analysis of Data from the PALOMA 2, MONALEESA 2, MONALEESA 7, MONARCH 3, FALCON, SWOG and FACT Trials. Cancers 2019, 11, 1661. https://doi.org/10.3390/cancers11111661
Rossi V, Berchialla P, Giannarelli D, Nisticò C, Ferretti G, Gasparro S, Russillo M, Catania G, Vigna L, Mancusi RL, et al. Should All Patients With HR-Positive HER2-Negative Metastatic Breast Cancer Receive CDK 4/6 Inhibitor As First-Line Based Therapy? A Network Meta-Analysis of Data from the PALOMA 2, MONALEESA 2, MONALEESA 7, MONARCH 3, FALCON, SWOG and FACT Trials. Cancers. 2019; 11(11):1661. https://doi.org/10.3390/cancers11111661
Chicago/Turabian StyleRossi, Valentina, Paola Berchialla, Diana Giannarelli, Cecilia Nisticò, Gianluigi Ferretti, Simona Gasparro, Michelangelo Russillo, Giovanna Catania, Leonardo Vigna, Rossella Letizia Mancusi, and et al. 2019. "Should All Patients With HR-Positive HER2-Negative Metastatic Breast Cancer Receive CDK 4/6 Inhibitor As First-Line Based Therapy? A Network Meta-Analysis of Data from the PALOMA 2, MONALEESA 2, MONALEESA 7, MONARCH 3, FALCON, SWOG and FACT Trials" Cancers 11, no. 11: 1661. https://doi.org/10.3390/cancers11111661






