Risk Factors for Severe Diarrhea with an Afatinib Treatment of Non-Small Cell Lung Cancer: A Pooled Analysis of Clinical Trials
Abstract
:1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Study Design and Patients
4.2. Predictor and Outcome Data
4.3. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Greenhalgh, J.; Dwan, K.; Boland, A.; Bates, V.; Vecchio, F.; Dundar, Y.; Jain, P.; Green, J.A. First-line treatment of advanced epidermal growth factor receptor (EGFR) mutation positive non-squamous non-small cell lung cancer. Cochrane Database Syst. Rev. 2016. [Google Scholar] [CrossRef] [PubMed]
- Park, K.; Tan, E.H.; O’Byrne, K.; Zhang, L.; Boyer, M.; Mok, T.; Hirsh, V.; Yang, J.C.H.; Lee, K.H.; Lu, S.; et al. Afatinib versus gefitinib as first-line treatment of patients with EGFR mutation-positive non-small-cell lung cancer (LUX-Lung 7): A phase 2B, open-label, randomised controlled trial. Lancet Oncol. 2016, 17, 577–589. [Google Scholar] [CrossRef]
- Takeda, M.; Okamoto, I.; Nakagawa, K. Pooled safety analysis of EGFR-TKI treatment for EGFR mutation-positive non-small cell lung cancer. Lung Cancer 2015, 88, 74–79. [Google Scholar] [CrossRef] [PubMed]
- Barron, F.; De la Torre-Vallejo, M.; Luna-Palencia, R.L.; Cardona, A.F.; Arrieta, O. The safety of afatinib for the treatment of non-small cell lung cancer. Expert Opin. Drug Saf. 2016, 15, 1563–1572. [Google Scholar] [CrossRef] [PubMed]
- Wada, Y.; Koyama, S.; Kuraishi, H.; Miyahara, T.; Yoshiike, F.; Agatsuma, T.; Yamamoto, R.; Ono, Y.; Suzuki, T.; Hachiya, T.; et al. Clinical analysis of patients treated with afatinib for advanced non-small cell lung cancer: A Nagano Lung Cancer Research Group observational study. Respir. Investig. 2016, 54, 462–467. [Google Scholar] [CrossRef] [PubMed]
- Arrieta, O.; De la Torre-Vallejo, M.; Lopez-Macias, D.; Orta, D.; Turcott, J.; Macedo-Perez, E.O.; Sanchez-Lara, K.; Ramirez-Tirado, L.A.; Baracos, V.E. Nutritional Status, Body Surface, and Low Lean Body Mass/Body Mass Index Are Related to Dose Reduction and Severe Gastrointestinal Toxicity Induced by Afatinib in Patients With Non-Small Cell Lung Cancer. Oncologist 2015, 20, 967–974. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ueda, Y.; Suzuki, H.; Kondo, Y.; Okamoto, N.; Kumode, A.; Usui, N.; Nakano, H.; Kimura, T.; Sando, M.; Shimura, K.; et al. Risk Factors of Afatinib Induced Diarrhea: A Retrospective Study. Iryo Yakugaku (Jpn. J. Pharm. Health Care Sci.) 2016, 42, 670–677. [Google Scholar] [CrossRef]
- Giotrif: European Public Assessment Report—Product Information. 2013. Available online: http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/002280/human_med_001698.jsp (accessed on 20 April 2018).
- Freiwald, M.; Schmid, U.; Fleury, A.; Wind, S.; Stopfer, P.; Staab, A. Population pharmacokinetics of afatinib, an irreversible ErbB family blocker, in patients with various solid tumors. Cancer Chemother. Pharmacol. 2014, 73, 759–770. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.C.; Sequist, L.V.; Zhou, C.; Schuler, M.; Geater, S.L.; Mok, T.; Hu, C.P.; Yamamoto, N.; Feng, J.; O‘Byrne, K.; et al. Effect of dose adjustment on the safety and efficacy of afatinib for EGFR mutation-positive lung adenocarcinoma: Post hoc analyses of the randomized LUX-Lung 3 and 6 trials. Ann. Oncol. 2016, 27, 2103–2110. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.-L.; Sequist, L.V.; Tan, E.-H.; Geater, S.L.; Orlov, S.; Zhang, L.; Lee, K.H.; Tsai, C.-M.; Kato, T.; Barrios, C.H.; et al. Afatinib as First-line Treatment of Older Patients With EGFR Mutation-Positive Non-Small-Cell Lung Cancer: Subgroup Analyses of the LUX-Lung 3, LUX-Lung 6, and LUX-Lung 7 Trials. Clin. Lung Cancer 2018, 19, e465–e479. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.-J.; Tsai, M.-J.; Hung, J.-Y.; Lee, M.-H.; Tsai, Y.-M.; Tsai, Y.-C.; Hsu, J.-F.; Liu, T.-C.; Huang, M.-S.; Chong, I.-W. The clinical efficacy of Afatinib 30 mg daily as starting dose may not be inferior to Afatinib 40 mg daily in patients with stage IV lung Adenocarcinoma harboring exon 19 or exon 21 mutations. BMC Pharmacol. Toxicol. 2017, 18, 82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soria, J.-C.; Ohe, Y.; Vansteenkiste, J.; Reungwetwattana, T.; Chewaskulyong, B.; Lee, K.H.; Dechaphunkul, A.; Imamura, F.; Nogami, N.; Kurata, T.; et al. Osimertinib in Untreated EGFR-Mutated Advanced Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2018, 378, 113–125. [Google Scholar] [CrossRef] [PubMed]
- Hirsh, V. Turning EGFR mutation-positive non-small-cell lung cancer into a chronic disease: Optimal sequential therapy with EGFR tyrosine kinase inhibitors. Ther. Adv. Med. Oncol. 2018, 10. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.C.; Shih, J.Y.; Su, W.C.; Hsia, T.C.; Tsai, C.M.; Ou, S.H.; Yu, C.J.; Chang, G.C.; Ho, C.L.; Sequist, L.V.; et al. Afatinib for patients with lung adenocarcinoma and epidermal growth factor receptor mutations (LUX-Lung 2): A phase 2 trial. Lancet Oncol. 2012, 13, 539–548. [Google Scholar] [CrossRef]
- Miller, V.A.; Hirsh, V.; Cadranel, J.; Chen, Y.M.; Park, K.; Kim, S.W.; Zhou, C.; Su, W.C.; Wang, M.; Sun, Y.; et al. Afatinib versus placebo for patients with advanced, metastatic non-small-cell lung cancer after failure of erlotinib, gefitinib, or both, and one or two lines of chemotherapy (LUX-Lung 1): A phase 2b/3 randomised trial. Lancet Oncol. 2012, 13, 528–538. [Google Scholar] [CrossRef]
- Sequist, L.V.; Yang, J.C.; Yamamoto, N.; O’Byrne, K.; Hirsh, V.; Mok, T.; Geater, S.L.; Orlov, S.; Tsai, C.M.; Boyer, M.; et al. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J. Clin. Oncol. 2013, 31, 3327–3334. [Google Scholar] [CrossRef] [PubMed]
- Katakami, N.; Atagi, S.; Goto, K.; Hida, T.; Horai, T.; Inoue, A.; Ichinose, Y.; Koboyashi, K.; Takeda, K.; Kiura, K.; et al. LUX-Lung 4: A phase II trial of afatinib in patients with advanced non-small-cell lung cancer who progressed during prior treatment with erlotinib, gefitinib, or both. J. Clin. Oncol. 2013, 31, 3335–3341. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.L.; Zhou, C.; Hu, C.P.; Feng, J.; Lu, S.; Huang, Y.; Li, W.; Hou, M.; Shi, J.H.; Lee, K.Y.; et al. Afatinib versus cisplatin plus gemcitabine for first-line treatment of Asian patients with advanced non-small-cell lung cancer harbouring EGFR mutations (LUX-Lung 6): An open-label, randomised phase 3 trial. Lancet Oncol. 2014, 15, 213–222. [Google Scholar] [CrossRef]
- Cappuzzo, F.; Finocchiaro, G.; Grossi, F.; Bidoli, P.; Favaretto, A.; Marchetti, A.; Valente, M.L.; Cseh, A.; Clementi, L.; Massey, D.; et al. Phase II study of afatinib, an irreversible ErbB family blocker, in EGFR FISH-positive non-small-cell lung cancer. J. Thorac. Oncol. 2015, 10, 665–672. [Google Scholar] [CrossRef] [PubMed]
- De Greve, J.; Moran, T.; Graas, M.P.; Galdermans, D.; Vuylsteke, P.; Canon, J.L.; Schallier, D.; Decoster, L.; Teugels, E.; Massey, D.; et al. Phase II study of afatinib, an irreversible ErbB family blocker, in demographically and genotypically defined lung adenocarcinoma. Lung Cancer 2015, 88, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Levey, A.S.; Bosch, J.P.; Lewis, J.B.; Greene, T.; Rogers, N.; Roth, D. A more accurate method to estimate glomerular filtration rate from serum creatinine: A new prediction equation. Modification of Diet in Renal Disease Study Group. Ann. Intern. Med. 1999, 130, 461–470. [Google Scholar] [CrossRef] [PubMed]
- Cleveland, W.S.; Devlin, S.J. Locally Weighted Regression: An Approach to Regression Analysis by Local Fitting. J. Am. Stat. Assoc. 1988, 83, 596–610. [Google Scholar] [CrossRef]
- Steyerberg, E.W.; Vergouwe, Y. Towards better clinical prediction models: Seven steps for development and an ABCD for validation. Eur. Heart J. 2014, 35, 1925–1931. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sauerbrei, W.; Meier-Hirmer, C.; Benner, A.; Royston, P. Multivariable regression model building by using fractional polynomials: Description of SAS, STATA and R programs. Comput. Stat. Data Anal. 2006, 50, 3464–3485. [Google Scholar] [CrossRef]
- Van Calster, B.; Nieboer, D.; Vergouwe, Y.; De Cock, B.; Pencina, M.J.; Steyerberg, E.W. A calibration hierarchy for risk models was defined: From utopia to empirical data. J. Clin. Epidemiol. 2016, 74, 167–176. [Google Scholar] [CrossRef] [PubMed]
| Characteristic | Total No. 1151 |
|---|---|
| Age (years) | 60 (52–68) |
| Sex | |
| Male | 443 (38%) |
| Female | 708 (62%) |
| Race | |
| Asian | 839 (73%) |
| White | 299 (26%) |
| Other | 13 (1%) |
| ECOG PS | |
| 0 | 385 (33%) |
| 1 | 726 (63%) |
| 2 | 40 (3%) |
| Prior EGFR inhibitor | 479 (42%) |
| Prior chemotherapy | 595 (52%) |
| Afatinib starting dose | |
| 40 mg | 498 (43%) |
| 50 mg | 653 (57%) |
| Weight (kg) | |
| Median (IQR) | 61 (53–70) |
| Missing | 3 (<1%) |
| Body mass index (kg/m2) | |
| Median (IQR) | 23 (21–26) |
| Missing | 10 (1%) |
| Body surface area (m2) | |
| Median (IQR) | 1.7 (1.5–1.8) |
| Missing | 10 (1%) |
| eGFR (mL/min/1.73 m2) | |
| Median (IQR) | 91 (76–109) |
| Missing | 5 (<1%) |
| Hemoglobin (g/L) | |
| Median (IQR) | 128 (117–138) |
| Missing | 5 (<1%) |
| Baseline Characteristics | Univariate Analysis * | Multivariable Analysis * | |||||
|---|---|---|---|---|---|---|---|
| Events/N (%) | OR | 95% CI | p | OR | 95% CI | p | |
| Sex | <0.001 | <0.001 | |||||
| Male | 44/443 (10%) | 1.00 | 1.00 | ||||
| Female | 140/708 (20%) | 2.22 | 1.53–3.21 | 2.04 | 1.36–3.07 | ||
| Age (years) | <0.001 | 0.008 | |||||
| 27–49 | 21/237 (9%) | 1.00 | 1.00 | ||||
| 50–59 | 40/319 (13%) | 1.44 | 0.82–2.53 | 1.38 | 0.78–2.47 | ||
| 60–69 | 78/373 (21%) | 2.57 | 1.52–4.33 | 2.32 | 1.35–4.00 | ||
| 70–86 | 45/222 (20%) | 2.24 | 1.27–3.96 | 1.97 | 1.08–3.61 | ||
| Race † | 0.080 | 0.079 | |||||
| Asian | 121/839 (14%) | 1.00 | 1.00 | ||||
| Non-Asian | 63/312 (20%) | 1.45 | 0.96–2.20 | 1.49 | 0.96–2.32 | ||
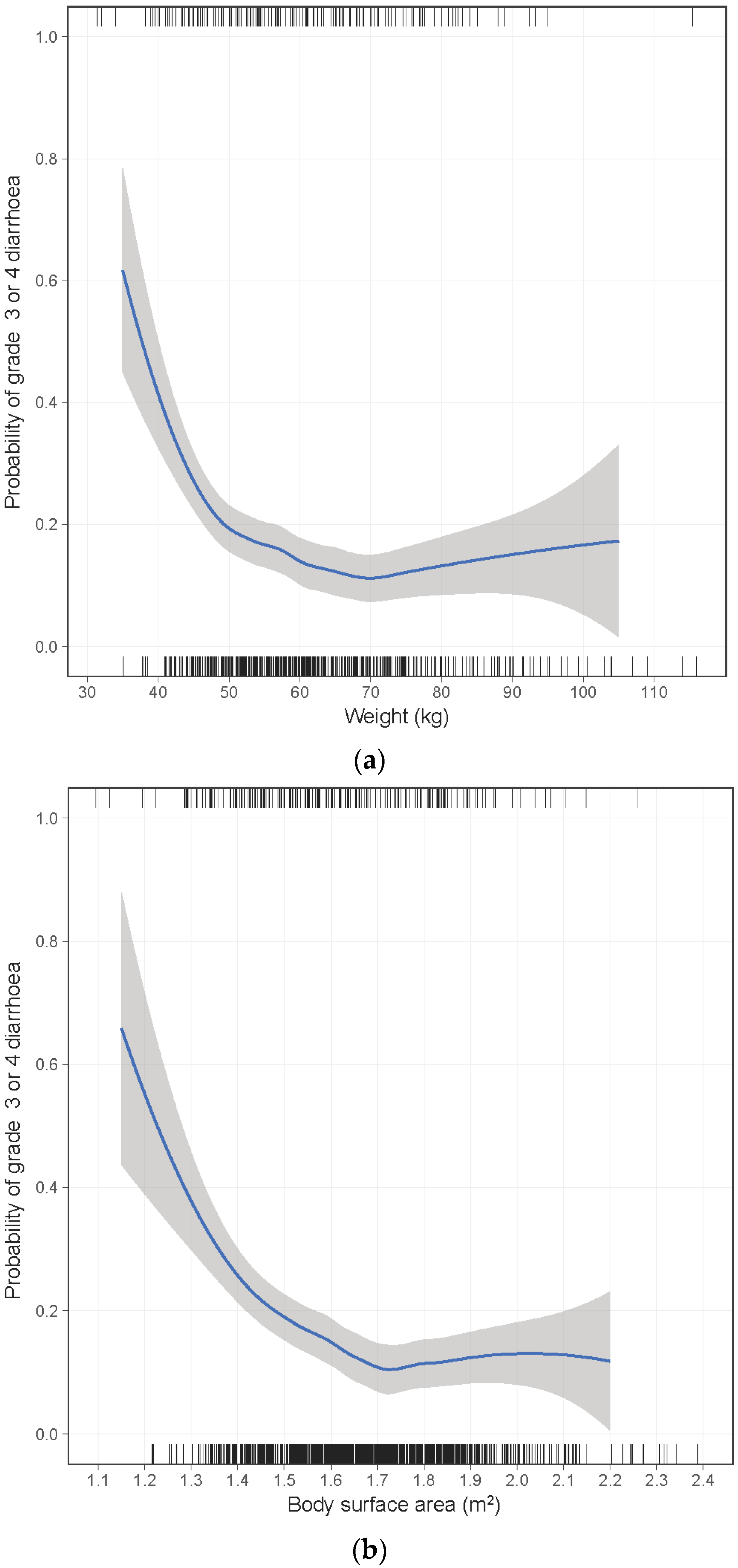
| Weight (kg) | <0.001 | 0.003 | |||||
| ≥50 | 138/977 (14%) | 1.00 | 1.00 | ||||
| 45–49 | 22/102 (22%) | 1.60 | 0.94–2.71 | 1.51 | 0.85–2.65 | ||
| 40–44 | 16/56 (29%) | 2.11 | 1.12–3.99 | 1.98 | 1.00–3.93 | ||
| <40 | 8/13 (62%) | 8.81 | 2.72–28.5 | 7.93 | 2.32–27.1 | ||
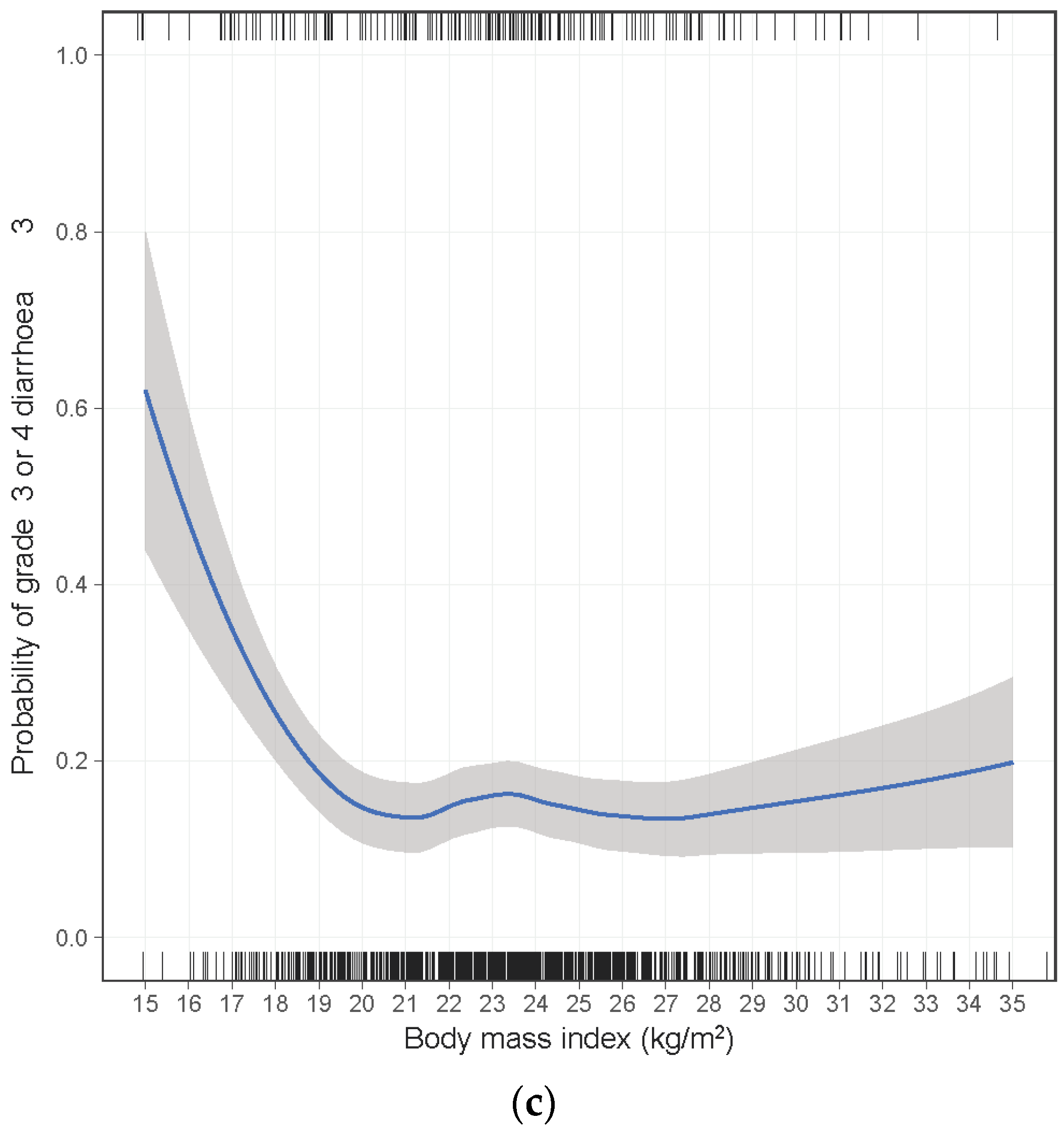
| BMI (kg/m2) | 0.002 | ||||||
| ≥18.5 | 157/1063 (15%) | 1.00 | |||||
| 17.0–18.4 | 13/58 (22%) | 1.50 | 0.77–2.93 | ||||
| 16.0–16.9 | 7/14 (50%) | 5.11 | 1.68–15.5 | ||||
| <16.0 | 4/6 (67%) | 10.2 | 1.66–62.5 | ||||
| BSA (m2) | <0.001 | ||||||
| ≥1.50 | 121/898 (13%) | 1.00 | |||||
| 1.40–1.49 | 32/155 (21%) | 1.65 | 1.05–2.60 | ||||
| 1.30–1.39 | 18/70 (26%) | 2.13 | 1.17–3.86 | ||||
| <1.30 | 9/17 (53%) | 5.46 | 1.95–15.3 | ||||
| eGFR (mL/min/1.73 m2) | 0.066 | 0.248 | |||||
| ≥90 | 74/591 (13%) | 1.00 | 1.00 | ||||
| 60–89 | 92/478 (19%) | 1.54 | 1.09–2.19 | 1.46 | 1.00–2.12 | ||
| 45–59 | 14/64 (22%) | 1.60 | 0.82–3.12 | 1.27 | 0.63–2.57 | ||
| <45 | 4/13 (31%) | 2.17 | 0.63–7.46 | 1.72 | 0.48–6.16 | ||
| ECOG PS | 0.460 | 0.306 | |||||
| 0 | 71/385 (18%) | 1.00 | 1.00 | ||||
| 1–2 | 113/766 (15%) | 0.88 | 0.62–1.24 | 0.82 | 0.57–1.19 | ||
| Hemoglobin (g/L) | 0.061 | 0.600 | |||||
| 75–117 | 56/294 (19%) | 1.00 | 1.00 | ||||
| 118–128 | 55/288 (19%) | 1.07 | 0.70–1.64 | 1.03 | 0.66–1.61 | ||
| 129–138 | 38/281 (14%) | 0.69 | 0.43–1.09 | 0.76 | 0.47–1.23 | ||
| 139–185 | 34/283 (12%) | 0.63 | 0.39–1.02 | 0.91 | 0.54–1.53 | ||
| Risk Factors 1 | Events/Patients (%) | |
|---|---|---|
| 40 mg Afatinib | 50 mg Afatinib | |
| 0 | 5/90 (6%) | 9/119 (8%) |
| 1 | 17/238 (7%) | 51/312 (16%) |
| 2 | 23/152 (15%) | 64/198 (32%) |
| 3 | 6/18 (33%) | 9/21 (43%) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hopkins, A.M.; Nguyen, A.-M.; Karapetis, C.S.; Rowland, A.; Sorich, M.J. Risk Factors for Severe Diarrhea with an Afatinib Treatment of Non-Small Cell Lung Cancer: A Pooled Analysis of Clinical Trials. Cancers 2018, 10, 384. https://doi.org/10.3390/cancers10100384
Hopkins AM, Nguyen A-M, Karapetis CS, Rowland A, Sorich MJ. Risk Factors for Severe Diarrhea with an Afatinib Treatment of Non-Small Cell Lung Cancer: A Pooled Analysis of Clinical Trials. Cancers. 2018; 10(10):384. https://doi.org/10.3390/cancers10100384
Chicago/Turabian StyleHopkins, Ashley M., Anh-Minh Nguyen, Christos S. Karapetis, Andrew Rowland, and Michael J. Sorich. 2018. "Risk Factors for Severe Diarrhea with an Afatinib Treatment of Non-Small Cell Lung Cancer: A Pooled Analysis of Clinical Trials" Cancers 10, no. 10: 384. https://doi.org/10.3390/cancers10100384





