Hydrophilic Surface Modification of PDMS Microchannel for O/W and W/O/W Emulsions
Abstract
:1. Introduction
2. Experimental Methodology
2.1. Coating Setup
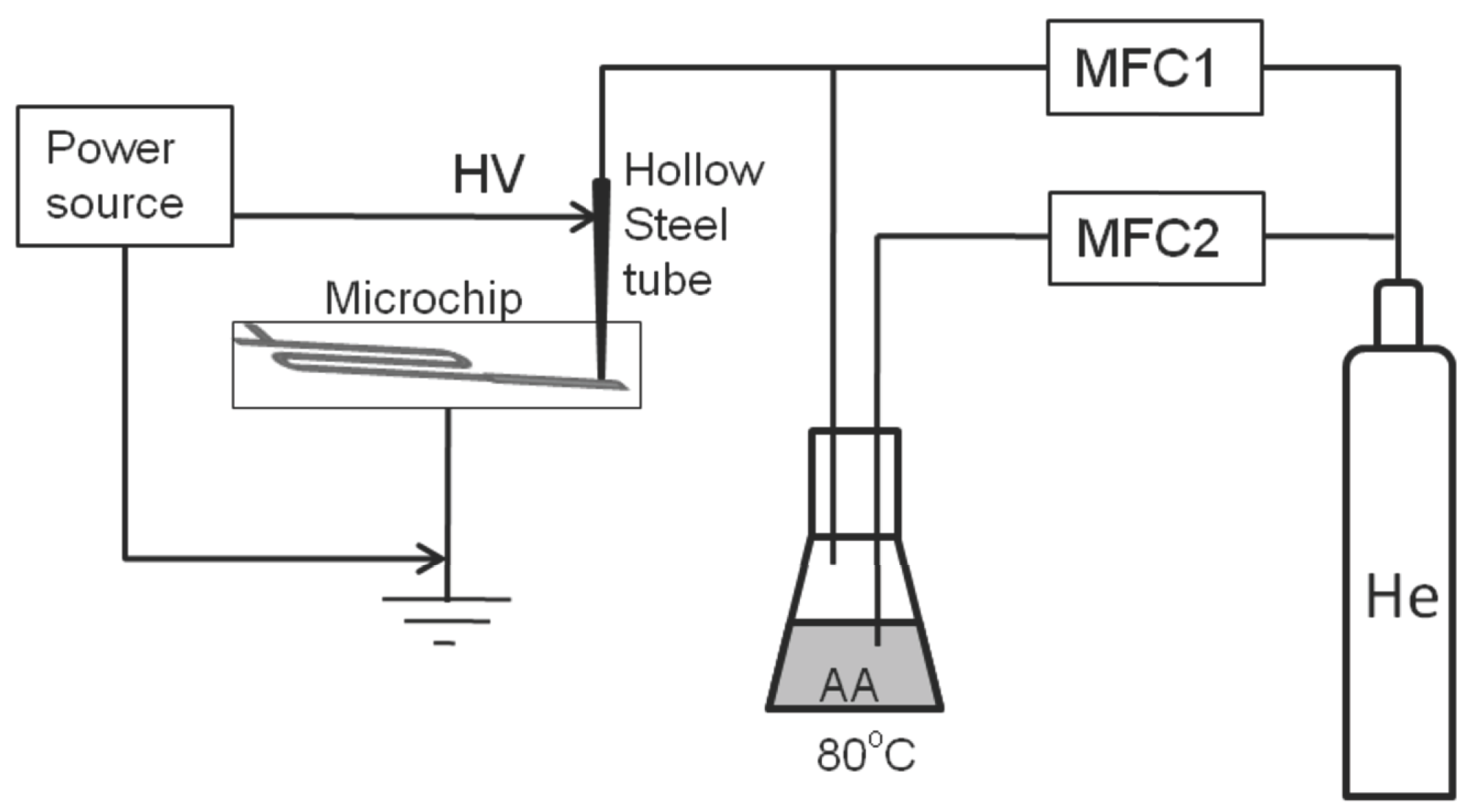
2.2. Emulsification Setup
2.3. Plasma Deposition Process
3. Results and Discussions
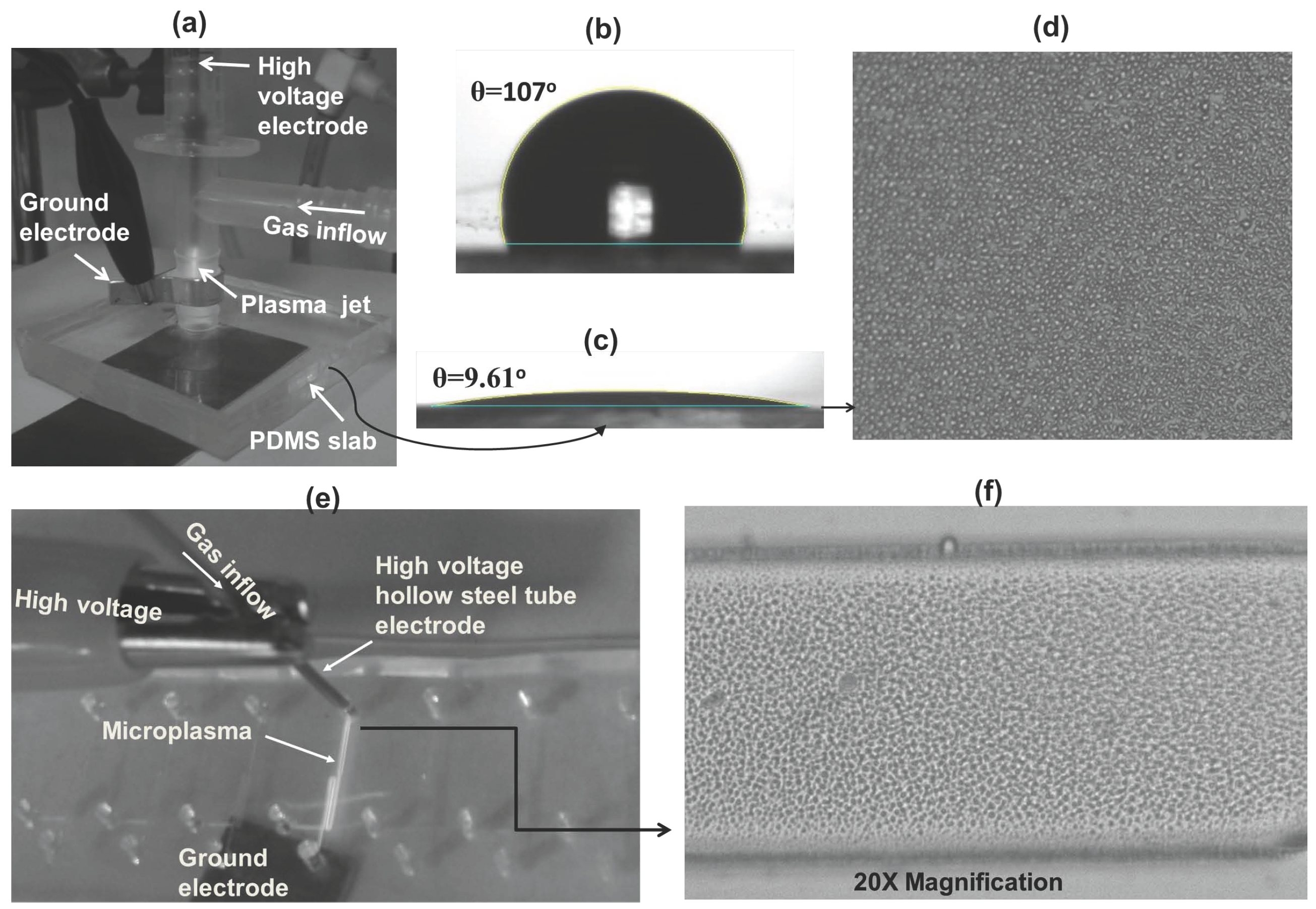
3.1. Contact Angle Measurement
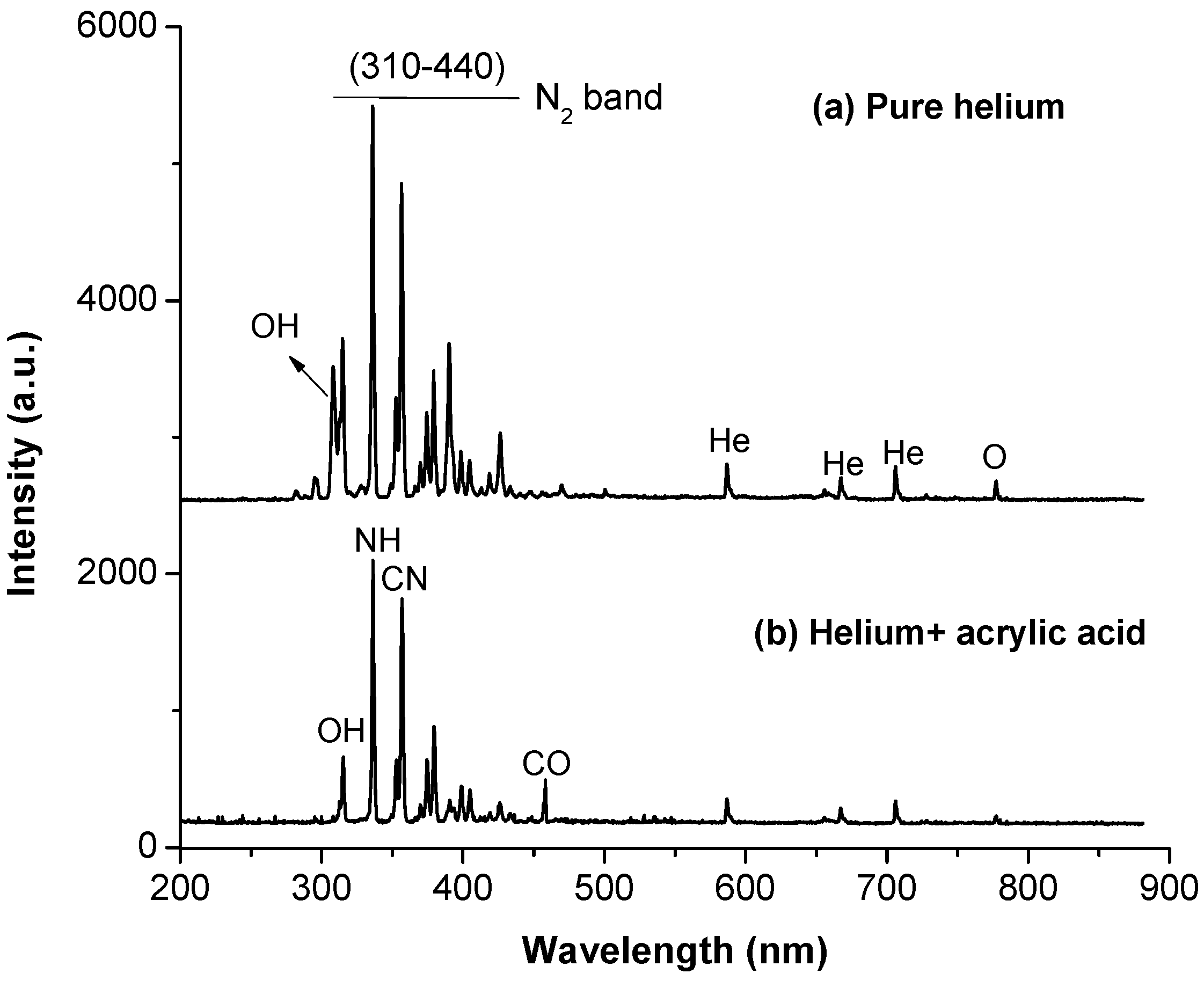
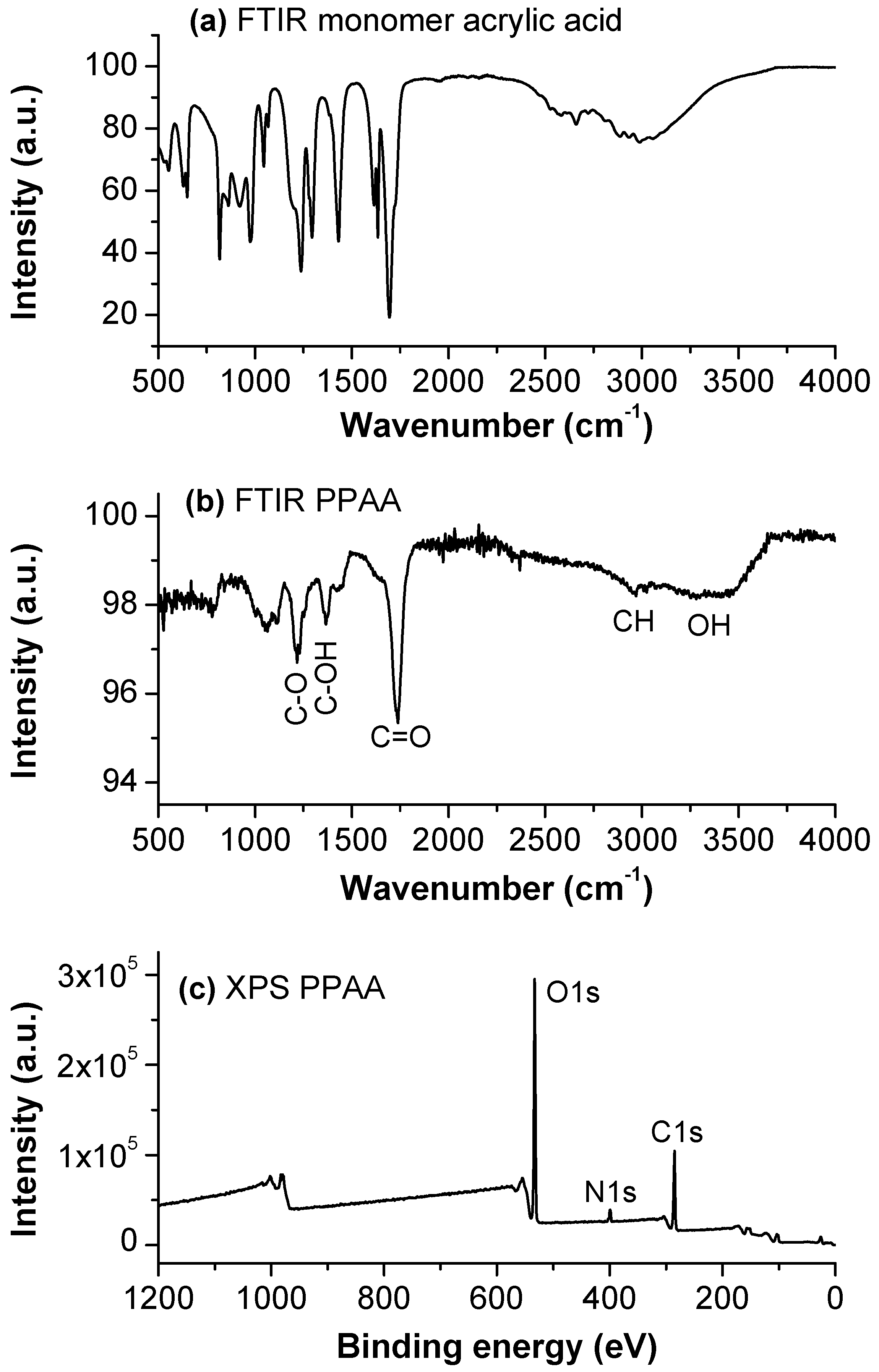
3.2. Plasma and Film Characterization in Microchip
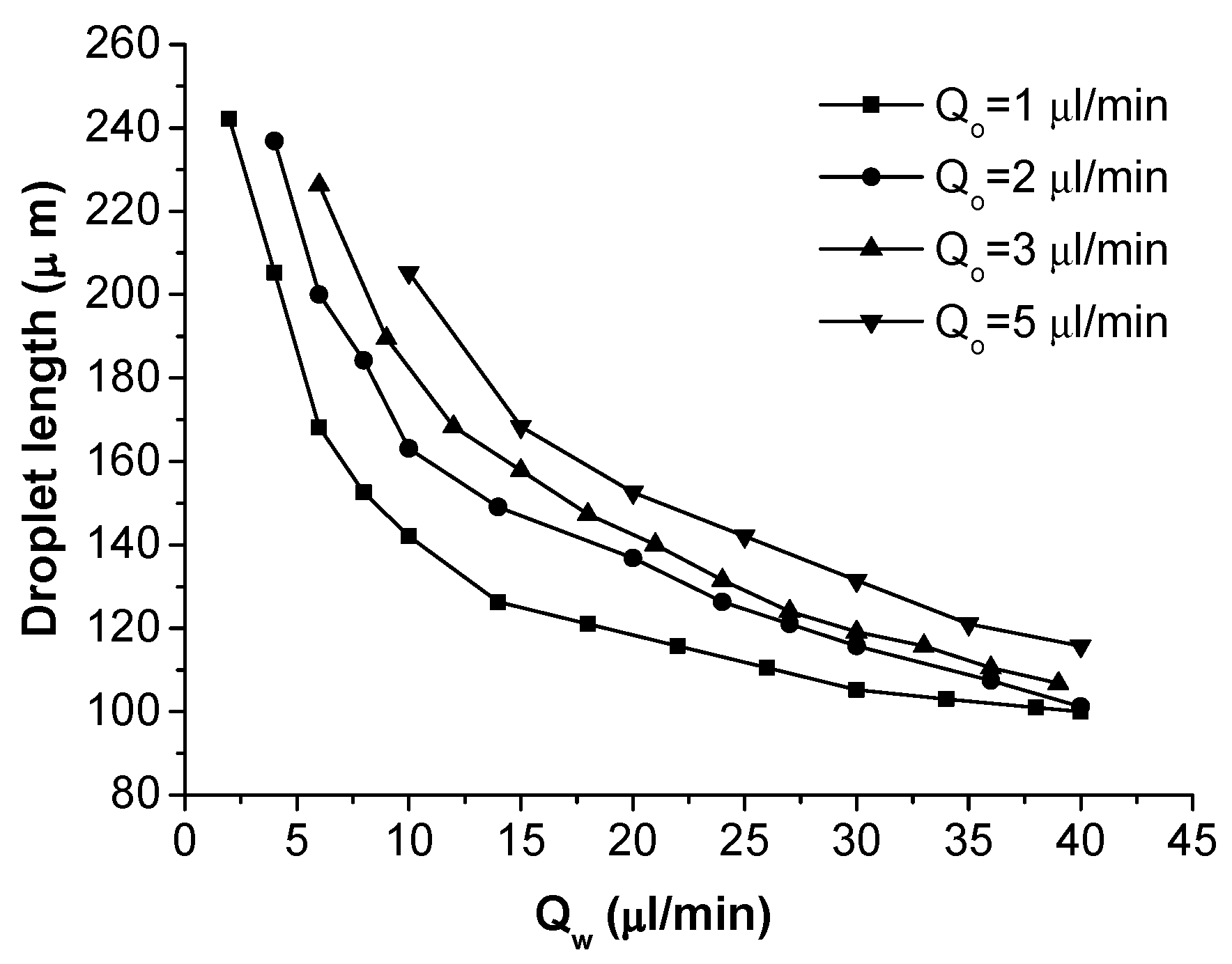
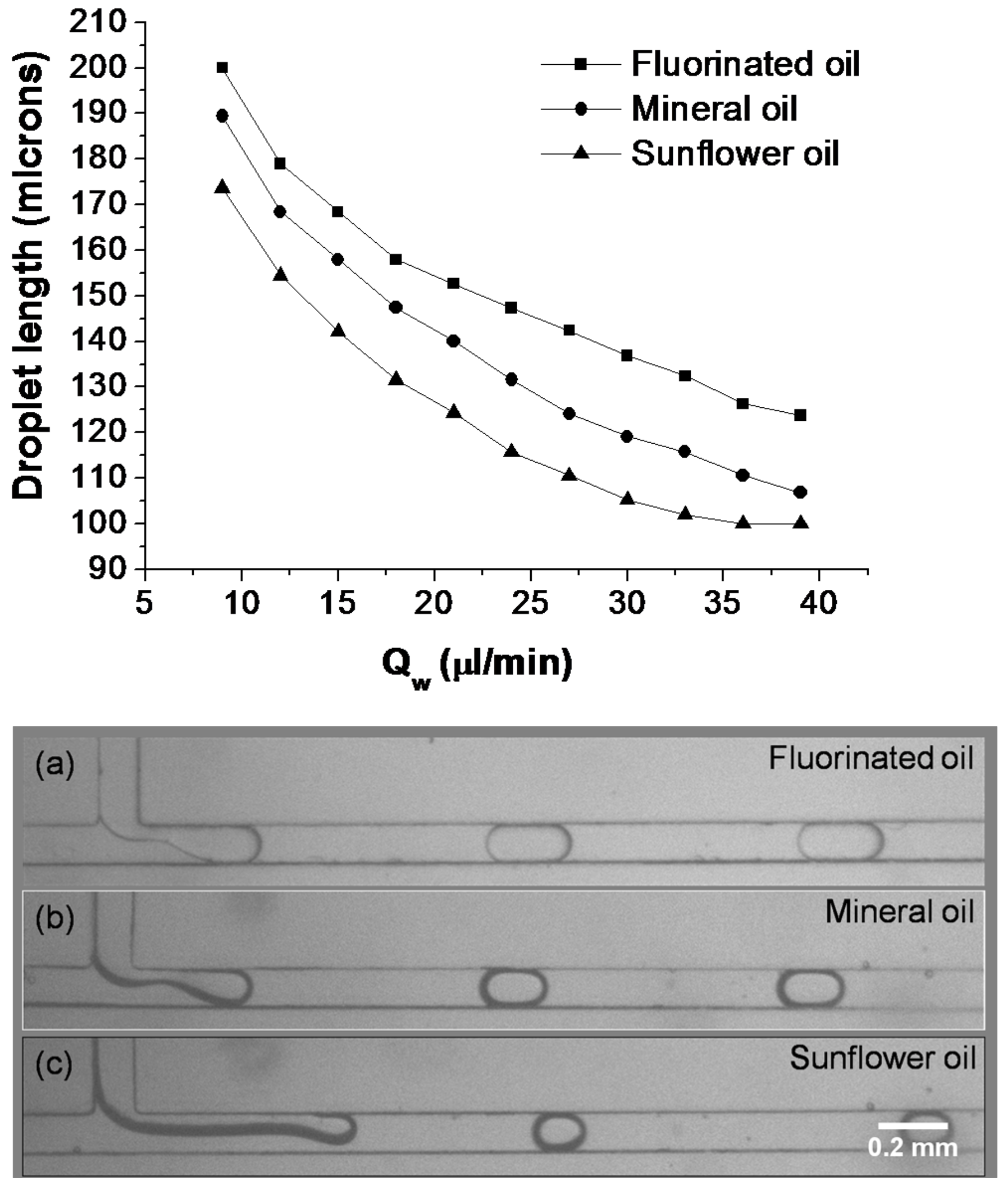
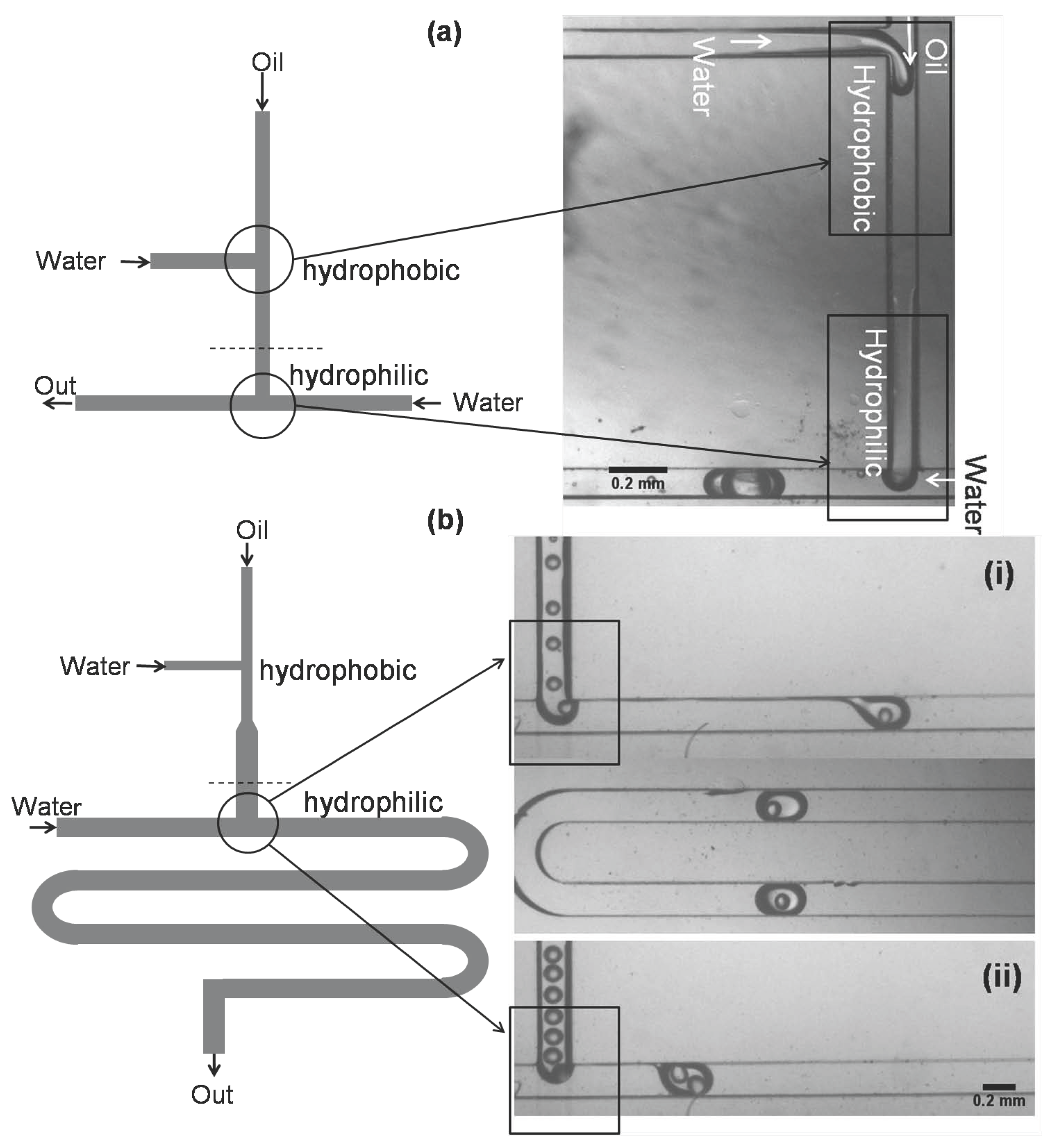
3.3. Formation of Oil-in-Water Microdroplets

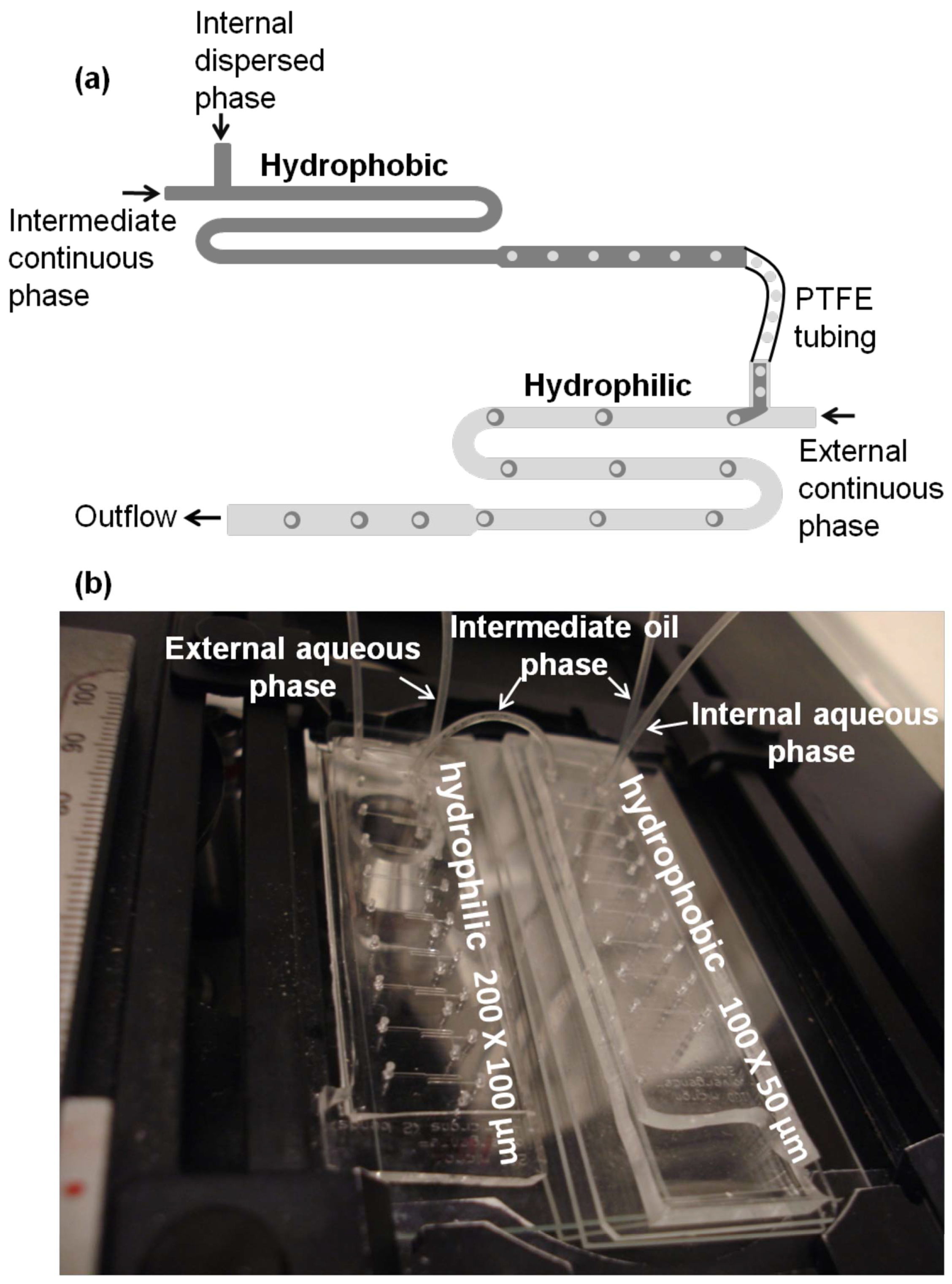
3.4. Microfluidic System for Double Emulsions
3.4.1. Two Microchip Module
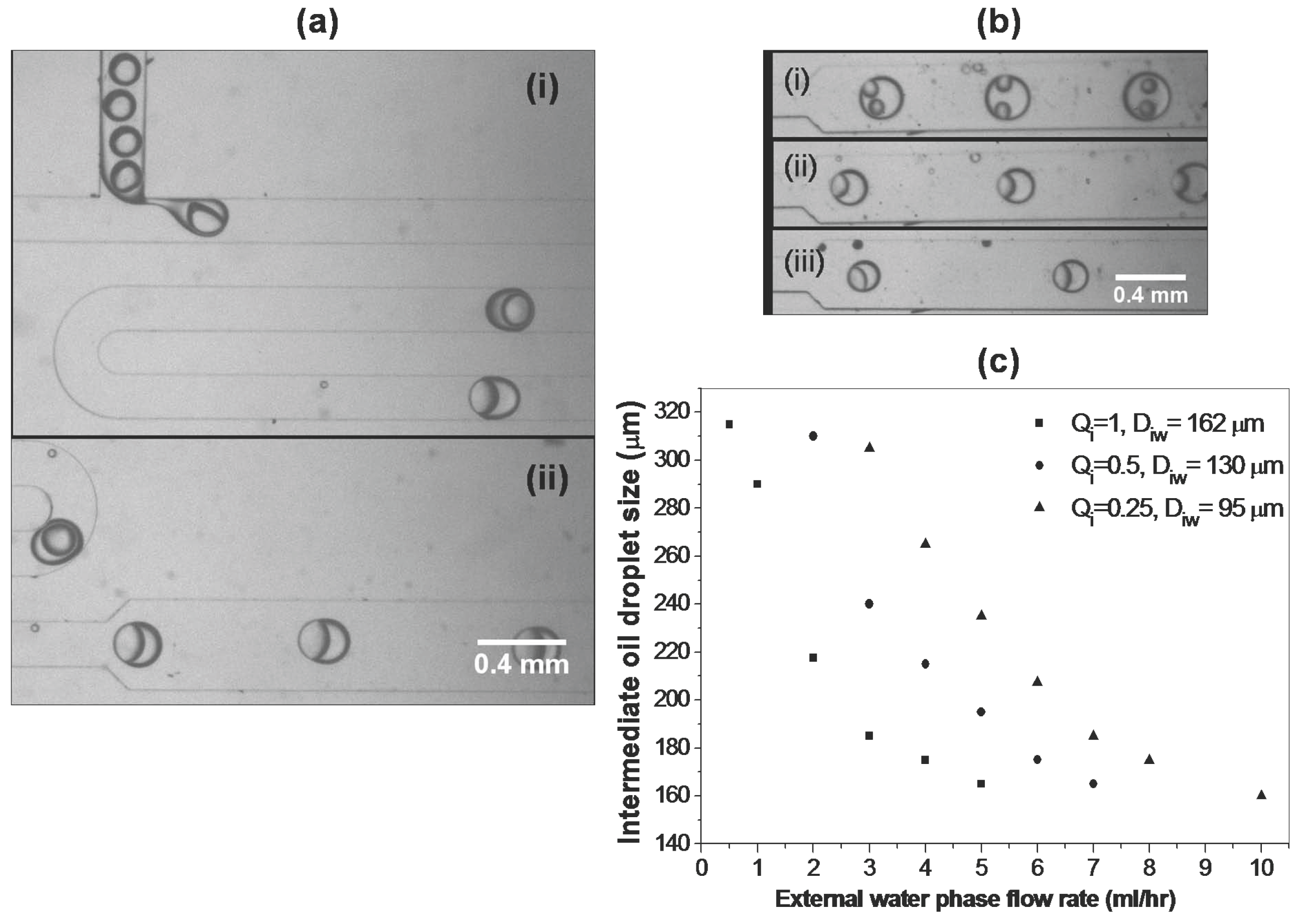
3.4.2. Single microchip module
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Gunther, A.; Jensenb, K.F. Multiphase microfluidics: from flow characteristics to chemical and materials synthesis. Lab Chip 2006, 6, 1487–1503. [Google Scholar] [CrossRef]
- Casadevall i Solvas, X.; de Mello, A. Droplet microfluidics: Recent developments and future applications. Chem. Commun. 2011, 47, 1936–1942. [Google Scholar] [CrossRef] [PubMed]
- McClements, J.D. Food Emulsions: Principle, Practices and Techniques; CRC Press: Boca Raton, FL, USA, 1999. [Google Scholar]
- Urban, K.; Wagner, G.; Schaffner, D.; Roglin, D.; Ulrich, J. Rotor-stator and disc systems for emulsification processes. Chem. Eng. Technol. 2006, 29, 24–31. [Google Scholar]
- Seemann, R.; Brinkmann, M.; Pfohl, T.; Herminghaus, S. Droplet based microfluidics. Rep. Prog. Phys. 2012, 75, 016601. [Google Scholar] [CrossRef]
- Song, H.; Chen, D.L.; Ismagilov, R.F. Reactions in droplets in microfluidic channels. Angew. Chem. Int. Ed. 2006, 45, 7336–7356. [Google Scholar] [CrossRef] [PubMed]
- Belder, D. Microfluidics with droplets. Angew. Chem. Int. Ed. 2005, 44, 3521–3522. [Google Scholar] [CrossRef] [PubMed]
- Casadevall i Solvas, X.; Niu, X.; Leeper, K.; Cho, S.; Chang, S.I.k.; Edel, J.B.; de Mello, A.J. Fluorescence detection methods for microfluidic droplet platforms. J. Vis. Exp. 2011, 58, 1–7. [Google Scholar]
- Utada, A.S.; Lorenceau, E.; Link, D.R.; Kaplan, P.D.; Stone, H.A.; Weitz, D.A. Monodisperse double emulsions generated from a microcapillary. Science 2005, 308, 537–541. [Google Scholar] [CrossRef] [PubMed]
- Shah, R.K.; Shum, H.C.; Rowat, A.C.; Lee, D.; Agresti, J.J.; Utada, A.S.; Chu, L.Y.; Kim, J.W.; Nieves, A.F.; Martinez, C.J.; et al. Designer emulsions using microfluidics. Mater. Today 2008, 11, 18–27. [Google Scholar] [CrossRef]
- Matsumoto, S.; Kita, Y.; Yonezawa, D. An attempt at preparing water-in-oil-in-water multiple phase emulsions. J. Colloid. Interface. Sci. 1976, 57, 353–361. [Google Scholar] [CrossRef]
- De Luca, M.; Rocha-Filho, P.; Grossiord, J.L.; Rabaron, A.; Vaution, C.; Seiller, M. Les émulsions multiples. Int. J. Cosmetic. Sci. 1991, 13, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Shum, H.C.; Kim, J.W.; Weitz, D.A. Microfluidic fabrication of monodisperse biocompatible and biodegradable polymersomes with controlled permeability. J. Am. Chem. Soc. 2008, 130, 9543–9549. [Google Scholar] [CrossRef]
- Shum, H.C.; Lee, D.; Yoon, I.; Kodger, T.; Weitz, D.A. Double emulsion templated monodisperse phospholipid vesicles. Langmuir 2008, 24, 7651–7653. [Google Scholar] [CrossRef] [PubMed]
- Bashir, S.; Rees, J.M.; Zimmerman, W.B. Simulations of microfluidic droplet formation using the two-phase level set method. Chem. Eng. Sci. 2011, 66, 4733–4741. [Google Scholar] [CrossRef]
- Bashir, S.; Casadevall i Solvas, X.; Bashir, M.; Rees, J.M.; Zimmerman, W.B. Dynamic wetting in microfluidic droplet formation. BioChip J. 2014, 8, 122–128. [Google Scholar] [CrossRef]
- Okushima, S.; Nisisako, T.; Tori, T.; Higuchi, T. Controlled production of monodisperse double emulsions by two-step droplet breakup in microfluidic devices. Langmuir 2004, 20, 9905–9908. [Google Scholar] [CrossRef] [PubMed]
- Honest Makamba, H.; Kim, J.H.; Park, N.; Hahn, J.H. Surface modification of poly(dimethylsiloxane) microchannels. Electrophoresis 2003, 24, 3607–3619. [Google Scholar] [CrossRef] [PubMed]
- Fritz, J.L.; Owen, M.J.; Adhesion, J. Hydrophobic recovery of plasma-treated polydimethylsiloxane. J. Adhesion 1995, 54, 33–45. [Google Scholar] [CrossRef]
- Roman, G.T.; Hlaus, T.; Bass, K.J.; Seelhammer, T.G.; Culbertson, C.T. Sol-gel modified poly(dimethylsiloxane) microfluidic devices with high electroosmotic mobilities and hydrophilic channel wall characteristics. Anal. Chem. 2005, 77, 1414–1422. [Google Scholar] [CrossRef] [PubMed]
- Bauer, W.A.C.; Fischlechner, M.; Abell, C.; Huck, W.T.S. Hydrophilic PDMS microchannels for high-throughput formation of oil-in-water microdroplets and water-in-oil-in-water double emulsions. Lab Chip 2010, 10, 1814–1819. [Google Scholar] [CrossRef] [PubMed]
- Ji, J.; Zhao, Y.; Guo, L.; Liu, B.; Jib, C.; Yang, P. Interfacial organic synthesis in a simple droplet-based microfluidic system. Lab Chip 2012, 12, 1373–1377. [Google Scholar] [CrossRef] [PubMed]
- Delamarche, E.; Donzel, C.; Kamounah, F.S.; Wolf, H.; Geissler, M.; Stutz, R.; Winkel, P.S.; Michel, B.; Mathieu, H.J.; Schaumburg, K. Microcontact printing using poly(dimethylsiloxane) stamps hydrophilized by poly(ethylene oxide) silanes. Langmuir 2003, 19, 8749–8758. [Google Scholar] [CrossRef]
- Yao, M.; Fang, J. Hydrophilic PEO-PDMS for microfluidic applications. J. Micromech. Microeng. 2012, 22, 025012. [Google Scholar] [CrossRef]
- Seo, M.; Paquet, C.; Nie, Z.; Xua, S.; Kumacheva, E. Microfluidic consecutive flow-focusing droplet generators. Soft. Matter. 2007, 3, 986–992. [Google Scholar] [CrossRef]
- Rohr, T.; Ogletree, D.F.; Svec, F.; Frechet, J.M.J. Surface functionalization of thermoplastic ploymers for the fabrication of microfluidics devices by photoinitiated grafting. Adv. Funct. Mater. 2003, 13, 264–270. [Google Scholar] [CrossRef]
- Hu, S.; Ren, S.; Bachman, M.; Sims, C.E.; Li, G.P.; Allbritton, N. Surface modification of poly(dimethylsiloxane) microfluidic devices by ultraviolet polymer grafting. Anal. Chem. 2002, 74, 4117–4123. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.; Hang, M.; Wang, W.; Tian, Y.; Wang, L.; McCarthy, T.; Chen, W. Simple and improved approaches to long-lasting, hydrophilic silicones derived from commercially available precursors. Appl. Mater. Interfaces 2014, 6, 22876–22883. [Google Scholar] [CrossRef] [PubMed]
- Kovach, K.; Capadona, J.; Sen Gupta, A.; Potkay, J. The effects of PEG-based surface modification of PDMS microchannels on long-term hemocompatibility. J. Biomed. Mater. Res. 2014, 102, 4195–4205. [Google Scholar] [CrossRef] [PubMed]
- Barbier, V.; Tatoulian, M.; Tori, T.; Arefi-Khonsari, F.; Ajdari, A.; Tabeling, P. Stable modification of PDMS surface properties by plasma polymerization: Application to the formation of double Emulsions in microfluidic systems. Langmuir 2006, 22, 5230–5232. [Google Scholar] [CrossRef] [PubMed]
- Wong, T.; Ho, C.M. Surface molecular property modifications for poly(dimethylsiloxane) (PDMS) based microfluidic devices. Microfluid. Nanofluid. 2009, 7, 291–306. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Khodakov, D.A.; Ellis, A.V.; Voelcker, N.H. Surface modification for PDMS-based microfluidic devices. Electrophoresis 2012, 33, 89–104. [Google Scholar] [CrossRef]
- Bashir, M.; Bashir, S. Hydrophobic–hydrophilic character of hexamethyldisiloxane films polymerized by atmospheric pressure plasma jet. Plasma Chem. Plasma Process. 2015, 35, 739–755. [Google Scholar] [CrossRef]
- Tendero, C.; Tixier, C.; Tristant, P.; Desmaison, J.; Leprince, P. Atmospheric pressure plasmas: A review. Spectrochim. Acta Part B 2006, 61, 2–30. [Google Scholar] [CrossRef]
- Bashir, M.; Rees, J.M.; Zimmerman, W.B. Plasma polymerization in a microcapillary using an atmospheric pressue dielectric barrier discharge. Surf. Coat. Tech. 2013, 234, 82–91. [Google Scholar] [CrossRef]
- Evju, J.K.; Howell, P.B.; Locascio, L.E.; Tarlova, M.J.; Hickmanb, J.J. Atmospheric pressure microplasmas for modifying sealed microfluidic devices. Appl. Phys. Lett. 2004, 84, 1668–1670. [Google Scholar] [CrossRef]
- Priest, C.; Gruner, P.J.; Szili, E.J.; Al-Bataineh, S.A.; Bradley, J.W.; Ralston, J.; Steeleb, D.A.; Shortb, R.D. Microplasma patterning of bonded microchannels using high-precision injected electrodes. Lab Chip 2010, 11, 541–544. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, X.; Cheng, C.; Wang, L.; Zhao, E.; Wang, X.; Wen, W. Selective modification for polydimethylsiloxane chip by micro-plasma. J. Mater. Sci. 2013, 48, 1310–1314. [Google Scholar] [CrossRef]
- Jidenko, N.; Petit, M.; Borra, J.P. Electrical characterization of microdischarges produced by dielectric barrier discharge in dry air at atmospheric pressure. J. Phys. D Appl. Phys. 2006, 39, 281–293. [Google Scholar] [CrossRef]
- Koo, I.G.; Cho, J.H.; Choi, M.Y.; Lee, W.M. Room-temperature slot microplasma in atmospheric pressure air between cylindrical electrodes with a nanoporous alumina dielectric. Appl. Phys. Lett. 2007, 91, 041502. [Google Scholar] [CrossRef]
- Bashir, M.; Rees, J.M.; Bashir, S.; Zimmerman, W.B. Characterization of atmospheric pressure microplasma produced from argon and a mixture of argon-ethylenediamine. Phys. Lett. A. 2014, 378, 2395–2405. [Google Scholar] [CrossRef]
- Chen, G.; Chen, S.; Chen, W.; Yang, S. Biofilm deposited by the atmospheric plasma liquid sputtering. Surf. Coat. Technol. 2008, 202, 4741–4745. [Google Scholar] [CrossRef]
- Tran, D.T.; Mori, S.; Tsuboi, D.; Suzuki, M. Formation of plasma-polymerized top layers on composite membranes: Influence on separation efficiency. Plasma Process. Polym. 2009, 6, 110–118. [Google Scholar] [CrossRef]
- Massines, F.; Gouda, G. A comparison of polypropylene-surface treatment by filamentary, homogeneous and glow discharges in helium at atmospheric pressure. J. Phys. D Appl. Phys. 1998, 31, 3411–3420. [Google Scholar] [CrossRef]
- Wan, J.; Wang, S.; Song, M.; Jia, X.; Yang, J. Plasma-induced direct-grafting on Polytetrafluoroethylene films by Quasi-glow discharge at atmospheric pressure. Plasma Process. Polym. 2009, 6, 825–830. [Google Scholar] [CrossRef]
- Morent, R.; De Geyter, N.; Van Vlierberghe, S.; Vanderleyden, E.; Dubruel, P.; Leys, C.; Schacht, E. Deposition of polyacrylic acid films by means of an atmospheric pressure dielectric barrier discharge. Plasma Chem. Plasma Process. 2009, 29, 103–117. [Google Scholar] [CrossRef]
- Bashir, M.; Rees, J.M.; Bashir, S.; Zimmerman, W.B. Microplasma copolymerization of amine and Si containing precursors. Thin Solid Film. 2014, 564, 186–194. [Google Scholar] [CrossRef]
- Thorsen, T.; Roberts, R.W.; Arnold, F.H.; Quake, S.R. Dynamic pattern formation in a vesicle-generating microfluidic device. Phys. Rev. Lett. 2001, 86, 4163–4166. [Google Scholar] [CrossRef] [PubMed]
- Nisisako, T.; Tori, T.; Higuchi, T. Droplet formation in a microchannel network. Lab Chip 2002, 2, 24–26. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.H.; Li, S.W.; Tan, J.; Wang, Y.J.; Luo, G.S. Controllable preparation of monodisperse O/W and W/O emulsions in the same microfluidic device. Langmuir 2006, 22, 7943–7946. [Google Scholar] [CrossRef] [PubMed]
- Nisisako, T. Microstructured devices for preparing controlled multiple emulsions. Chem. Eng. Technol. 2008, 31, 1091–1098. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bashir, S.; Bashir, M.; Solvas, X.C.i.; Rees, J.M.; Zimmerman, W.B. Hydrophilic Surface Modification of PDMS Microchannel for O/W and W/O/W Emulsions. Micromachines 2015, 6, 1445-1458. https://doi.org/10.3390/mi6101429
Bashir S, Bashir M, Solvas XCi, Rees JM, Zimmerman WB. Hydrophilic Surface Modification of PDMS Microchannel for O/W and W/O/W Emulsions. Micromachines. 2015; 6(10):1445-1458. https://doi.org/10.3390/mi6101429
Chicago/Turabian StyleBashir, Shazia, Muhammad Bashir, Xavier Casadevall i Solvas, Julia M. Rees, and William B. Zimmerman. 2015. "Hydrophilic Surface Modification of PDMS Microchannel for O/W and W/O/W Emulsions" Micromachines 6, no. 10: 1445-1458. https://doi.org/10.3390/mi6101429




