Advances in NIR-II Fluorescent Nanoprobes: Design Principles, Optical Engineering, and Emerging Translational Directions
Abstract
1. Introduction
Scope and Objectives of This Review
- •
- To summarize recent advances in fluorescent nanoprobes for biomedical imaging and disease diagnosis.
- •
- To focus on four main classes of nanoprobes: quantum dots, carbon dots, upconversion nanoparticles, and dye-doped silica nanoparticles.
- •
- To highlight functionalization strategies, including targeted, activatable, and ratiometric probes, especially those operating in the NIR and NIR-II regions.
- •
- To discuss biomedical applications such as early disease detection, real-time monitoring, and image-guided surgery.
- •
- To evaluate translational challenges including toxicity, reproducibility of synthesis, and regulatory approval.
- •
- To provide a comparative overview of different fluorescent nanoprobe systems in terms of their optical and biological properties.
- •
- To explore the design strategies that improve specificity, minimize background signals, and increase imaging depth.
- •
- To review applications that demonstrate clinical relevance, with a focus on in vivo imaging and biosensing.
- •
- To identify the barriers that hinder the translation of nanoprobes from laboratory to bedside.
- •
- To propose future directions such as the integration of artificial intelligence, multimodal imaging, and precision medicine approaches.
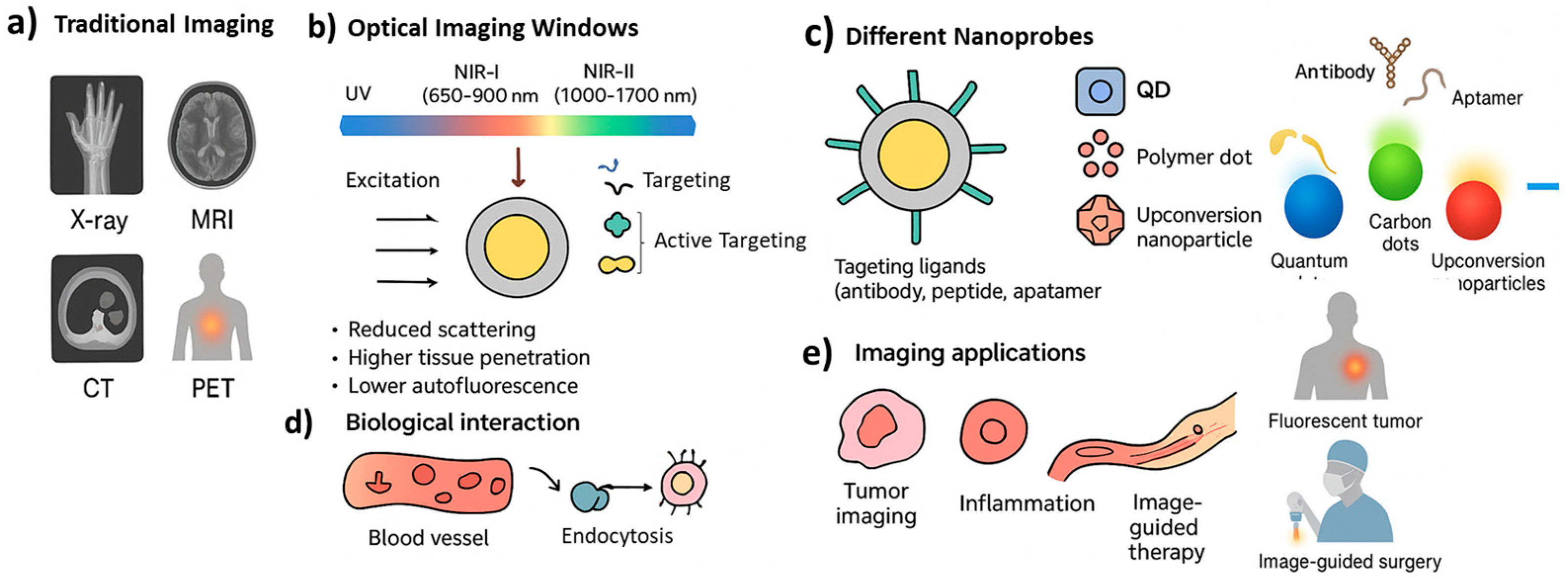
2. Fundamentals of Fluorescent Nanoprobes
2.1. Basic Principles of Fluorescence and Imaging
2.2. Key Optical and Physicochemical Properties
2.3. Advantages over Conventional Probes
3. Classes of NIR-II Fluorescent Nanoprobes
3.1. Quantum Dots
3.2. Carbon Dots
3.3. Upconversion Nanoparticles
3.3.1. Mechanism of Upconversion Luminescence
- •
- Excited-state absorption (ESA): A single ion absorbs two or more photons in succession, moving stepwise to higher excited states before emitting a photon of shorter wavelength.
- •
- Energy transfer upconversion (ETU): An energy donor ion (commonly Yb3+) absorbs NIR light and transfers the excitation energy to a nearby acceptor ion (such as Er3+ or Tm3+), which then emits higher-energy photons.
- •
- Photon avalanche (PA): A positive feedback process where initial excitation triggers a chain of absorption and emission events, leading to rapid signal amplification.
3.3.2. Advantages in Deep-Tissue Imaging
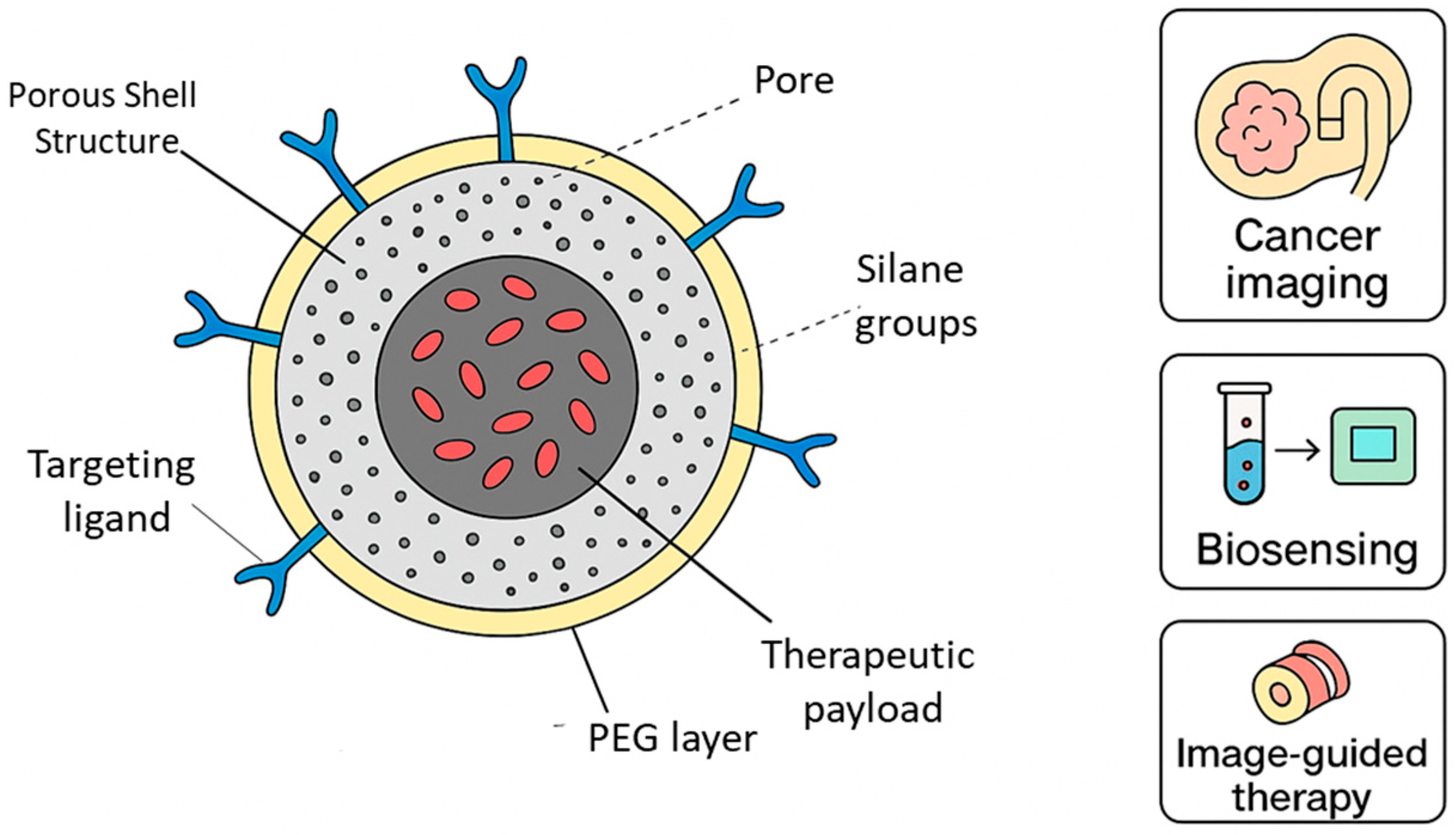
3.4. Dye-Doped Silica Nanoparticles
4. Functionalization and Targeting Strategies
4.1. Surface Modification and Bioconjugation
4.2. Tumor-Targeted and Organ-Specific Probes
4.3. Activatable and Ratiometric Probes
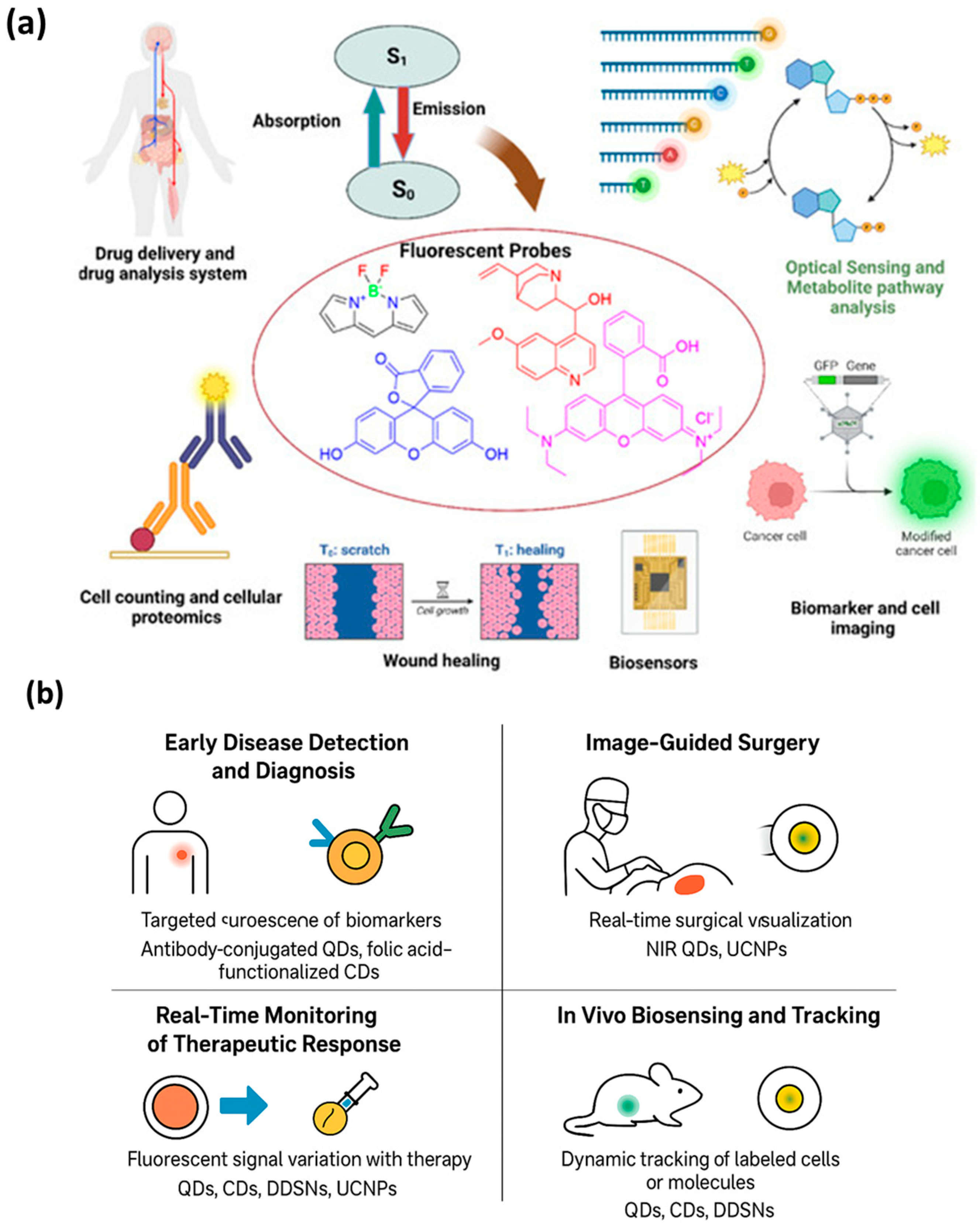
5. Biomedical Applications of Fluorescent Nanoprobes
5.1. Early Disease Detection and Diagnosis
5.2. Image-Guided Surgery
5.3. Real-Time Monitoring of Therapeutic Response
5.4. In Vivo Biosensing and Tracking
6. Translation from Bench to Bedside
7. Challenges and Limitations
7.1. Biocompatibility and Long-Term Safety
7.2. Stability and Reproducibility of Synthesis
7.3. Imaging Depth and Real-Time Monitoring Constraints
8. Future Perspectives
8.1. Integration with Artificial Intelligence
8.2. Multimodal Imaging and Theranostics
8.3. Emerging Trends in Precision Medicine
9. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bruno, F.; Arrigoni, F.; Mariani, S.; Splendiani, A.; Di Cesare, E.; Masciocchi, C.; Barile, A. Advanced Magnetic Resonance Imaging (MRI) of Soft Tissue Tumors: Techniques and Applications. La Radiol. Med. 2019, 124, 243–252. [Google Scholar] [CrossRef]
- Kim, H.; Park, S.Y.; Jung, J.; Kim, J.-H.; Hahn, S.Y.; Shin, J.H.; Oh, Y.L.; Chung, M.K.; Kim, H.I.; Kim, S.W.; et al. Improved Survival after Early Detection of Asymptomatic Distant Metastasis in Patients with Thyroid Cancer. Sci. Rep. 2019, 9, 18745. [Google Scholar] [CrossRef] [PubMed]
- Mieog, J.S.D.; Achterberg, F.B.; Zlitni, A.; Hutteman, M.; Burggraaf, J.; Swijnenburg, R.-J.; Gioux, S.; Vahrmeijer, A.L. Fundamentals and Developments in Fluorescence-Guided Cancer Surgery. Nat. Rev. Clin. Oncol. 2022, 19, 9–22. [Google Scholar] [CrossRef]
- Man, F.; Lammers, T.; de Rosales, R.T.M. Imaging Nanomedicine-Based Drug Delivery: A Review of Clinical Studies. Mol. Imaging Biol. 2018, 20, 683–695. [Google Scholar] [CrossRef]
- Zhao, Z.; Ukidve, A.; Kim, J.; Mitragotri, S. Targeting Strategies for Tissue-Specific Drug Delivery. Cell 2020, 181, 151–167. [Google Scholar] [CrossRef]
- Yang, Y.; Xie, Y.; Zhang, F. Second Near-Infrared Window Fluorescence Nanoprobes for Deep-Tissue in Vivo Multiplexed Bioimaging. Adv. Drug Deliv. Rev. 2023, 193, 114697. [Google Scholar] [CrossRef]
- Liu, S.; Dong, W.; Gao, H.; Song, Z.; Cheng, Z. Near-Infrared-II Fluorescent Probes for Analytical Applications: From In Vitro Detection to In Vivo Imaging Monitoring. Acc. Chem. Res. 2025, 58, 543–554. [Google Scholar] [CrossRef]
- Irving, P.M.; Gecse, K.B. Optimizing Therapies Using Therapeutic Drug Monitoring: Current Strategies and Future Perspectives. Gastroenterology 2022, 162, 1512–1524. [Google Scholar] [CrossRef]
- He, J.; Li, C.; Ding, L.; Huang, Y.; Yin, X.; Zhang, J.; Zhang, J.; Yao, C.; Liang, M.; Pirraco, R.P.; et al. Tumor Targeting Strategies of Smart Fluorescent Nanoparticles and Their Applications in Cancer Diagnosis and Treatment. Adv. Mater. 2019, 31, 1902409. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhang, W.; Deng, Y. Advances and Challenges for Hydrovoltaic Intelligence. ACS Nano 2023, 17, 14229–14252. [Google Scholar] [CrossRef] [PubMed]
- Parvin, N.; Kumar, V.; Mandal, T.K.; Joo, S.W. Advancements in Nanoporous Materials for Biomedical Imaging and Diagnostics. J. Funct. Biomater. 2024, 15, 226. [Google Scholar] [CrossRef]
- Liu, H.; Ju, Z.; Hui, X.; Li, W.; Lv, R. Upconversion and NIR-II Luminescent Rare Earth Nanoparticles Combined with Machine Learning for Cancer Theranostics. Nanoscale 2024, 16, 16697–16705. [Google Scholar] [CrossRef]
- Kuperkar, K.; Atanase, L.; Bahadur, A.; Crivei, I.; Bahadur, P. Degradable Polymeric Bio(Nano)Materials and Their Biomedical Applications: A Comprehensive Overview and Recent Updates. Polymers 2024, 16, 206. [Google Scholar] [CrossRef]
- Yang, T.; Qin, J.; Zhang, J.; Guo, L.; Yang, M.; Wu, X.; You, M.; Peng, H. Recent Progresses in NIR-II Luminescent Bio/Chemo Sensors Based on Lanthanide Nanocrystals. Chemosensors 2022, 10, 206. [Google Scholar] [CrossRef]
- Dogantzis, N.P.; Hodgson, G.K.; Impellizzeri, S. Optical Writing and Single Molecule Reading of Photoactivatable and Silver Nanoparticle-Enhanced Fluorescence. Nanoscale Adv. 2020, 2, 1956–1966. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, W.; Xu, X.; Li, M.; Xi, P. High-Spatiotemporal-Resolution Structured Illumination Microscopy: Principles, Instrumentation, and Applications. Photonics Insights 2025, 4, R01. [Google Scholar] [CrossRef]
- Lu, Y.; Jabbari, P.; Mukhamedshin, A.; Zvyagin, A.V. Fluorescence Lifetime Imaging in Drug Delivery Research. Adv. Drug Deliv. Rev. 2025, 218, 115521. [Google Scholar] [CrossRef]
- del Rosal, B.; Benayas, A. Strategies to Overcome Autofluorescence in Nanoprobe-Driven In Vivo Fluorescence Imaging. Small Methods 2018, 2, 1800075. [Google Scholar] [CrossRef]
- Parvin, N.; Mandal, T.K.; Roy, P. Polyelectrolyte Carbon Quantum-Dots: New Player as a Noninvasive Imaging Probe in Drosophila. J. Nanosci. Nanotechnol. 2013, 13, 6499–6505. [Google Scholar] [CrossRef]
- Liang, W.; He, S.; Wu, S. Fluorescence Imaging in Second Near-infrared Window: Developments, Challenges, and Opportunities. Adv. NanoBiomed Res. 2022, 2, 202200087. [Google Scholar] [CrossRef]
- Ma, T.; Xia, T. Nanoparticle-Based Activatable Probes for Bioimaging. Adv. Biol. 2021, 5, 202000193. [Google Scholar] [CrossRef]
- Cai, H.; Dai, X.; Wang, X.; Tan, P.; Gu, L.; Luo, Q.; Zheng, X.; Li, Z.; Zhu, H.; Zhang, H.; et al. A Nanostrategy for Efficient Imaging-Guided Antitumor Therapy through a Stimuli-Responsive Branched Polymeric Prodrug. Adv. Sci. 2020, 7, 1903243. [Google Scholar] [CrossRef] [PubMed]
- Mimona, M.A.; Rimon, M.I.H.; Zohura, F.T.; Sony, J.M.; Rim, S.I.; Arup, M.M.R.; Mobarak, M.H. Quantum Dot Nanomaterials: Empowering Advances in Optoelectronic Devices. Chem. Eng. J. Adv. 2025, 21, 100704. [Google Scholar] [CrossRef]
- Kim, D.; Kim, J.; Park, Y.I.; Lee, N.; Hyeon, T. Recent Development of Inorganic Nanoparticles for Biomedical Imaging. ACS Cent. Sci. 2018, 4, 324–336. [Google Scholar] [CrossRef]
- Rokesh, K.; Sakar, M.; Do, T.-O. Emerging Hybrid Nanocomposite Photocatalysts for the Degradation of Antibiotics: Insights into Their Designs and Mechanisms. Nanomaterials 2021, 11, 572. [Google Scholar] [CrossRef] [PubMed]
- Kong, S.; Liu, H.; Zhang, Y.; Fan, J.; Huang, W. Clinical Applications of Nanoprobes of High-Resolution in Vivo Imaging. iScience 2025, 28, 111459. [Google Scholar] [CrossRef]
- Parvin, N.; Kumar, V.; Park, S.; Mandal, T.K.; Joo, S.W. Innovative Wearable Electronics: Next-Generation Nitrogen-Doped Lutetium-Carbon Microspheres Composites for Robust Energy Harvesting. Small 2025, 21, 2407386. [Google Scholar] [CrossRef]
- Jena, S.; Parker, L.L. Fluorescence Lifetime Imaging Probes for Cell-Based Measurements of Enzyme Activity; Spring: Berlin/Heidelberg, Germany, 2022; pp. 133–162. [Google Scholar] [CrossRef]
- Kumar, M.; Kulkarni, P.; Liu, S.; Chemuturi, N.; Shah, D.K. Nanoparticle Biodistribution Coefficients: A Quantitative Approach for Understanding the Tissue Distribution of Nanoparticles. Adv. Drug Deliv. Rev. 2023, 194, 114708. [Google Scholar] [CrossRef]
- Qu, K.; Yuan, Z.; Wang, Y.; Song, Z.; Gong, X.; Zhao, Y.; Mu, Q.; Zhan, Q.; Xu, W.; Wang, L. Structures, Properties, and Applications of Zwitterionic Polymers. ChemPhysMater 2022, 1, 294–309. [Google Scholar] [CrossRef]
- Wahyudi, S.; Bahtiar, A.; Panatarani, C.; Anas; Risdiana. Recent Advanced Carbon Dots Derived Natural Products and Aptasensor-Based Carbon Dots for Detection of Pesticides. Sens. Bio-Sens. Res. 2023, 41, 100576. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Z.; Du, Z.; Pan, J.; Huang, Y. Multifunctional Upconversion Nanoparticles Transforming Photoacoustic Imaging: A Review. Nanomaterials 2025, 15, 1074. [Google Scholar] [CrossRef]
- Khalid, A.; Tomljenovic-Hanic, S. Emerging Fluorescent Nanoparticles for Non-Invasive Bioimaging. Molecules 2024, 29, 5594. [Google Scholar] [CrossRef]
- Olmedo-Gaya, M.-V.; Romero-Olid, M.-N.; Ocaña-Peinado, F.M.; Vallecillo-Rivas, M.; Vallecillo, C.; Reyes-Botella, C. Influence of Different Surgical Techniques on Primary Implant Stability in the Posterior Maxilla: A Randomized Controlled Clinical Trial. Clin. Oral Investig. 2023, 27, 3499–3508. [Google Scholar] [CrossRef]
- Jiang, Y.; Luo, X.; Chen, L.; Lin, H.; Gao, J. Multicolor 19F Magnetic Resonance Imaging: A Promising Medical Technique for in Vivo Visualization of Multiple Biological Targets. Fundam. Res. 2023, 3, 529–533. [Google Scholar] [CrossRef]
- Wang, X.; Guo, Y.; Chen, D.; Hou, Y.; He, Q.; Gao, X.; Ye, Z.; Zhang, Q.; Lu, J. Carbonaceous Sulfur Host for Cathodes in Room-Temperature Metal–Sulfur Batteries. ACS Energy Lett. 2024, 9, 3093–3135. [Google Scholar] [CrossRef]
- Al-Thani, A.N.; Jan, A.G.; Abbas, M.; Geetha, M.; Sadasivuni, K.K. Nanoparticles in Cancer Theragnostic and Drug Delivery: A Comprehensive Review. Life Sci. 2024, 352, 122899. [Google Scholar] [CrossRef] [PubMed]
- Isuri, R.K.; Williams, J.; Rioux, D.; Dorval, P.; Chung, W.; Dancer, P.-A.; Delikatny, E.J. Clinical Integration of NIR-II Fluorescence Imaging for Cancer Surgery: A Translational Evaluation of Preclinical and Intraoperative Systems. Cancers 2025, 17, 2676. [Google Scholar] [CrossRef] [PubMed]
- Yoshioka, N.; Okubo, T.; Suzuki, Y.; Koizumi, Y.; Mizukami, W. Hunting for Quantum-Classical Crossover in Condensed Matter Problems. npj Quantum Inf. 2024, 10, 45. [Google Scholar] [CrossRef]
- Mitrofanov, A.; Oleneva, P.; Shugurov, S.; Prikhodko, I.; Dmitrenko, M.; Penkova, A.; Selyutin, A. Rapid Green Synthesis of CdSe Quantum Dots with Controlled Size and Tunable Properties. Phys. B Condens. Matter 2026, 722, 418054. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, S.; Zhang, Y.; Al-Hartomy, O.A.; Wageh, S.; Al-Sehemi, A.G.; Hao, Y.; Gao, L.; Wang, H.; Zhang, H. Colloidal Quantum Dots: Synthesis, Composition, Structure, and Emerging Optoelectronic Applications. Laser Photon. Rev. 2023, 17, 2200551. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, M.; Pan, Z.; Biesold, G.M.; Liang, S.; Rao, H.; Lin, Z.; Zhong, X. Colloidal Inorganic Ligand-Capped Nanocrystals: Fundamentals, Status, and Insights into Advanced Functional Nanodevices. Chem. Rev. 2022, 122, 4091–4162. [Google Scholar] [CrossRef] [PubMed]
- Parvin, N.; Joo, S.W.; Mandal, T.K. Nanomaterial-Based Strategies to Combat Antibiotic Resistance: Mechanisms and Applications. Antibiotics 2025, 14, 207. [Google Scholar] [CrossRef]
- Wieszczycka, K.; Staszak, K.; Woźniak-Budych, M.J.; Litowczenko, J.; Maciejewska, B.M.; Jurga, S. Surface Functionalization—The Way for Advanced Applications of Smart Materials. Coord. Chem. Rev. 2021, 436, 213846. [Google Scholar] [CrossRef]
- Kuo, C.-W.; Smith, A.M. Digital and Absolute Assays for Low Abundance Molecular Biomarkers. Acc. Chem. Res. 2023, 56, 1031–1042. [Google Scholar] [CrossRef] [PubMed]
- Kunachowicz, D.; Kłosowska, K.; Sobczak, N.; Kepinska, M. Applicability of Quantum Dots in Breast Cancer Diagnostic and Therapeutic Modalities—A State-of-the-Art Review. Nanomaterials 2024, 14, 1424. [Google Scholar] [CrossRef]
- Jiang, Z.; Jiang, N.; Sun, M.; Wang, Z.; Guo, Y.; Gui, B.; Chen, Y.; Tian, Z.; Hu, B. Quantum Dot Nanoprobes for Microvascular Thromboembolism Therapy via Ultrasound and Near-Infrared Dual-Mode Approach. Adv. Healthc. Mater. 2025, 14, 2500315. [Google Scholar] [CrossRef]
- Yan, J.; Cheng, L.; Li, Y.; Wang, R.; Wang, J. Advancements in Single-Molecule Fluorescence Detection Techniques and Their Expansive Applications in Drug Discovery and Neuroscience. Biosensors 2025, 15, 283. [Google Scholar] [CrossRef]
- Yang, Y.; Jiang, Q.; Zhang, F. Nanocrystals for Deep-Tissue In Vivo Luminescence Imaging in the Near-Infrared Region. Chem. Rev. 2024, 124, 554–628. [Google Scholar] [CrossRef]
- Lv, Y.; Fan, J.; Zhao, M.; Wu, R.; Li, L.S. Recent Advances in Quantum Dot-Based Fluorescence-Linked Immunosorbent Assays. Nanoscale 2023, 15, 5560–5578. [Google Scholar] [CrossRef]
- Basavaraj, N.; Sekar, A.; Yadav, R. Review on Green Carbon Dot-Based Materials for the Photocatalytic Degradation of Dyes: Fundamentals and Future Perspective. Mater. Adv. 2021, 2, 7559–7582. [Google Scholar] [CrossRef]
- Šafranko, S.; Šubarić, D.; Jerković, I.; Jokić, S. Citrus By-Products as a Valuable Source of Biologically Active Compounds with Promising Pharmaceutical, Biological and Biomedical Potential. Pharmaceuticals 2023, 16, 1081. [Google Scholar] [CrossRef]
- Song, J.; Kang, M.; Ji, S.; Ye, S.; Guo, J. Research on Red/Near-Infrared Fluorescent Carbon Dots Based on Different Carbon Sources and Solvents: Fluorescence Mechanism and Biological Applications. Nanomaterials 2025, 15, 81. [Google Scholar] [CrossRef] [PubMed]
- Zha, S.; Liu, H.; Li, H.; Li, H.; Wong, K.-L.; All, A.H. Functionalized Nanomaterials Capable of Crossing the Blood–Brain Barrier. ACS Nano 2024, 18, 1820–1845. [Google Scholar] [CrossRef]
- Li, L.; Dong, T. Photoluminescence Tuning in Carbon Dots: Surface Passivation or/and Functionalization, Heteroatom Doping. J. Mater. Chem. C 2018, 6, 7944–7970. [Google Scholar] [CrossRef]
- Mandal, T.K.; Parvin, N.; Mishra, K.; Mohandoss, S.; Lee, Y.R. Sensitive and Selective Fluorometric Determination of DNA by Using Layered Hexagonal Nanosheets of a Covalent Organic Framework Prepared from P-Phenylenediamine and Benzene-1,3,5-Tricarboxaldehyde. Microchim. Acta 2019, 186, 833. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.; Yang, T.; Zhao, Z.; Zhu, T.; Zhang, Q.; Hou, W.; Yuan, W.Z. Nonconventional Luminophores: Characteristics, Advancements and Perspectives. Chem. Soc. Rev. 2021, 50, 12616–12655. [Google Scholar] [CrossRef] [PubMed]
- Mondal, J.; Lamba, R.; Yukta, Y.; Yadav, R.; Kumar, R.; Pani, B.; Singh, B. Advancements in Semiconductor Quantum Dots: Expanding Frontiers in Optoelectronics, Analytical Sensing, Biomedicine, and Catalysis. J. Mater. Chem. C 2024, 12, 10330–10389. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, X.; Xian, M.; Dong, C.; Shuang, S. Folic Acid-Conjugated Green Luminescent Carbon Dots as a Nanoprobe for Identifying Folate Receptor-Positive Cancer Cells. Talanta 2018, 183, 39–47. [Google Scholar] [CrossRef]
- Yang, J.; Li, Z.; Jia, Q. Design of Dual-Emission Fluorescence Sensor Based on Cu Nanoclusters with Solvent-Dependent Effects: Visual Detection of Water via a Smartphone. Sens. Actuators B Chem. 2019, 297, 126807. [Google Scholar] [CrossRef]
- Huang, L.; Su, Y.; Zhang, D.; Zeng, Z.; Hu, X.; Hong, S.; Lin, X. Recent Theranostic Applications of Hydrogen Peroxide-Responsive Nanomaterials for Multiple Diseases. RSC Adv. 2023, 13, 27333–27358. [Google Scholar] [CrossRef]
- Jiang, P.; Gao, N.; Chang, G.; Wu, Y. Biosensors for Early Detection of Parkinson’s Disease: Principles, Applications, and Future Prospects. Biosensors 2025, 15, 280. [Google Scholar] [CrossRef] [PubMed]
- Parvin, N.; Mandal, T.K.; Nagajyothi, P.C.; Reddy, P.M.; Reddy, N.R.; Joo, S.W. Highly Fluorescent Doped Fe3O4@C Nanoparticles Cross the Blood–Brain Barrier: Help in Brain Imaging and Blocking the Life Cycle of Mosquitoes. J. Clust. Sci. 2021, 32, 1761–1767. [Google Scholar] [CrossRef]
- Zhang, Y.; Wei, C.; Lv, F.; Liu, T. Real-Time Imaging Tracking of a Dual-Fluorescent Drug Delivery System Based on Doxorubicin-Loaded Globin- Polyethylenimine Nanoparticles for Visible Tumor Therapy. Colloids Surf. B Biointerfaces 2018, 170, 163–171. [Google Scholar] [CrossRef]
- Mandal, T.K.; Parvin, N.; Joo, S.W. PH-Responsive Biocompatible Fluorescent Core-Shell Nanogel for Intracellular Imaging and Control Drug Release. Part. Part. Syst. Charact. 2021, 38, 2100110. [Google Scholar] [CrossRef]
- Dong, C.; Wang, Y.; Chen, T.; Ren, W.; Gao, C.; Ma, X.; Gao, X.; Wu, A. Carbon Dots in the Pathological Microenvironment: ROS Producers or Scavengers? Adv. Healthc. Mater. 2024, 13, 2402108. [Google Scholar] [CrossRef]
- Farshidfar, N.; Fooladi, S.; Nematollahi, M.H.; Iravani, S. Carbon Dots with Tissue Engineering and Regenerative Medicine Applications. RSC Adv. 2023, 13, 14517–14529. [Google Scholar] [CrossRef]
- Lee, Y.B.; Kyun, M.-L.; Lee, Y.J.; Shim, H.-E.; Huh, K.M.; Kang, S.-W. Cyclodextrins as Multifunctional Tools for Advanced Biomaterials in Tissue Repair and Regeneration. Bioact. Mater. 2025, 49, 627–651. [Google Scholar] [CrossRef]
- Karimov, D.N.; Demina, P.A.; Koshelev, A.V.; Rocheva, V.V.; Sokovikov, A.V.; Generalova, A.N.; Zubov, V.P.; Khaydukov, E.V.; Koval’chuk, M.V.; Panchenko, V.Y. Upconversion Nanoparticles: Synthesis, Photoluminescence Properties, and Applications. Nanotechnol. Russ. 2020, 15, 655–678. [Google Scholar] [CrossRef]
- Parvin, N.; Mandal, T.; Joo, S. Application of Bimetallic Heterojunction Nanoparticle-Based Multishelled Porous Hollow Microspheres as a Two-in-One Inorganic UV Filter. ACS Sustain. Chem. Eng. 2023, 11, 16133–16143. [Google Scholar] [CrossRef]
- Chang, Y. Mechanism in Rare-Earth-Doped Luminescence Nanomaterials. In Photofunctional Nanomaterials for Biomedical Applications; Wiley: Hoboken, NJ, USA, 2025; pp. 77–115. [Google Scholar] [CrossRef]
- Chen, L.-C.; Wang, E.; Tai, C.-S.; Chiu, Y.-C.; Li, C.-W.; Lin, Y.-R.; Lee, T.-H.; Huang, C.-W.; Chen, J.-C.; Chen, W.L. Improving the Reproducibility, Accuracy, and Stability of an Electrochemical Biosensor Platform for Point-of-Care Use. Biosens. Bioelectron. 2020, 155, 112111. [Google Scholar] [CrossRef]
- Ubah, P.; Esan, O.; Okoronkwo, E. Advances in Upconversion Nanoparticle Synthesis Methods for Photovoltaic Solar Cell. ChemRxiv 2025. [Google Scholar] [CrossRef]
- Nannuri, S.H.; Rao, P.; Singh, S.; Misra, S.K.; George, S.D. Upconversion Phenomenon and Its Implications in Core–Shell Architecture; Spring: Berlin/Heidelberg, Germany, 2023; pp. 97–126. [Google Scholar] [CrossRef]
- Xu, Y.; Yang, W.; Zhang, B. ROS-Responsive Probes for Low-Background Optical Imaging: A Review. Biomed. Mater. 2021, 16, 022002. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Sun, R.; Xia, K.; Xia, Q.; Liu, Y.; Zhang, X. Design and Application of Fluorescent Probes to Detect Cellular Physical Microenvironments. Chem. Rev. 2024, 124, 1738–1861. [Google Scholar] [CrossRef]
- Li, X.; Jian, M.; Sun, Y.; Zhu, Q.; Wang, Z. The Peptide Functionalized Inorganic Nanoparticles for Cancer-Related Bioanalytical and Biomedical Applications. Molecules 2021, 26, 3228. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Li, D.; Li, C.; Zhuang, Z.; Chu, C.; (Ken) Ostrikov, K.; Thompson, E.W.; Liu, G.; Wang, P. A Review on Reactive Oxygen Species (ROS)-Inducing Nanoparticles Activated by Uni- or Multi-Modal Dynamic Treatment for Oncotherapy. Nanoscale 2023, 15, 11813–11833. [Google Scholar] [CrossRef]
- Lebaka, V.R.; Ravi, P.; Reddy, M.C.; Thummala, C.; Mandal, T.K. Zinc Oxide Nanoparticles in Modern Science and Technology: Multifunctional Roles in Healthcare, Environmental Remediation, and Industry. Nanomaterials 2025, 15, 754. [Google Scholar] [CrossRef] [PubMed]
- Mandal, T.K. Nanomaterial-Enhanced Hybrid Disinfection: A Solution to Combat Multidrug-Resistant Bacteria and Antibiotic Resistance Genes in Wastewater. Nanomaterials 2024, 14, 1847. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Wang, Y.; Xu, X.; Liu, Y.; Lin, B.; Zhang, M.; Zhang, J.; Wan, S.; Yang, C.; Tan, W. Aptamer-Based Detection of Circulating Targets for Precision Medicine. Chem. Rev. 2021, 121, 12035–12105. [Google Scholar] [CrossRef]
- Sokolov, I. Ultrabright Fluorescent Particles via Physical Encapsulation of Fluorescent Dyes in Mesoporous Silica: A Mini-Review. Nanoscale 2024, 16, 10994–11004. [Google Scholar] [CrossRef]
- Klippel, N.; Jung, G.; Kickelbick, G. Hybrid Inorganic-Organic Fluorescent Silica Nanoparticles—Influence of Dye Binding Modes on Dye Leaching. J. Sol-Gel Sci. Technol. 2023, 107, 2–19. [Google Scholar] [CrossRef]
- Kumeria, T. Advances on Porous Nanomaterials for Biomedical Application (Drug Delivery, Sensing, and Tissue Engineering). ACS Biomater. Sci. Eng. 2022, 8, 4025–4027. [Google Scholar] [CrossRef] [PubMed]
- Díez-Pascual, A.M. Surface Engineering of Nanomaterials with Polymers, Biomolecules, and Small Ligands for Nanomedicine. Materials 2022, 15, 3251. [Google Scholar] [CrossRef] [PubMed]
- Mahammad, A.; Akbar, I.; Mukhtar, S. Theranostic Application of Smart Nanomaterials in Target Drug Delivery; Spring: Berlin/Heidelberg, Germany, 2025; pp. 25–54. [Google Scholar] [CrossRef]
- Schueder, F.; Lara-Gutiérrez, J.; Beliveau, B.J.; Saka, S.K.; Sasaki, H.M.; Woehrstein, J.B.; Strauss, M.T.; Grabmayr, H.; Yin, P.; Jungmann, R. Multiplexed 3D Super-Resolution Imaging of Whole Cells Using Spinning Disk Confocal Microscopy and DNA-PAINT. Nat. Commun. 2017, 8, 2090. [Google Scholar] [CrossRef]
- Xu, C.; Du, D.; Han, Z.; Si, H.; Li, W.; Li, L.; Tang, B. Separation and Analysis of Rare Tumor Cells in Various Body Fluids Based on Microfluidic Technology for Clinical Applications. Anal. Chem. 2025, 97, 7567–7588. [Google Scholar] [CrossRef]
- Shu, T.; Hunter, H.; Zhou, Z.; Sun, Y.; Cheng, X.; Ma, J.; Su, L.; Zhang, X.; Serpe, M.J. Portable Point-of-Care Diagnostic Devices: An Updated Review. Anal. Methods 2021, 13, 5418–5435. [Google Scholar] [CrossRef]
- Li, X.; Peng, X.; Zoulikha, M.; Boafo, G.F.; Magar, K.T.; Ju, Y.; He, W. Multifunctional Nanoparticle-Mediated Combining Therapy for Human Diseases. Signal Transduct. Target. Ther. 2024, 9, 1. [Google Scholar] [CrossRef]
- Parvin, N.; Mandal, T.K.; Joo, S.-W. The Impact of COVID-19 on RNA Therapeutics: A Surge in Lipid Nanoparticles and Alternative Delivery Systems. Pharmaceutics 2024, 16, 1366. [Google Scholar] [CrossRef]
- Song, G.; Li, C.; Wang, T.; Lim, K.H.; Hu, F.; Cheng, S.; Hondo, E.; Liu, S.; Kawi, S. Hierarchical Hollow Carbon Particles with Encapsulation of Carbon Nanotubes for High Performance Supercapacitors. Small 2024, 20, 2305517. [Google Scholar] [CrossRef]
- Wang, X.; Ding, Q.; Groleau, R.R.; Wu, L.; Mao, Y.; Che, F.; Kotova, O.; Scanlan, E.M.; Lewis, S.E.; Li, P.; et al. Fluorescent Probes for Disease Diagnosis. Chem. Rev. 2024, 124, 7106–7164. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, K.; Prasad, B.L.V. Surface Functionalization of Inorganic Nanoparticles with Ligands: A Necessary Step for Their Utility. Chem. Soc. Rev. 2023, 52, 2573–2595. [Google Scholar] [CrossRef] [PubMed]
- Hussain, Z.; Hussain Al-Shadidi, J.R.M.; Jagal, J.; Rawas-Qalaji, M. Overcoming Stealthing Effect of PEGylation on Chitosan Nanoparticles: Optimization, Characterization, and Assessment of Cytotoxicity, Apoptosis, Cellular Internalization, and Antimetastatic Efficacy. Int. J. Biol. Macromol. 2025, 321, 146514. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Z.; Chen, S.; Chen, Y. Zwitterionic Polymer: A New Paradigm for Protein Conjugation beyond PEG. ChemMedChem 2023, 18, e202300245. [Google Scholar] [CrossRef]
- Ghosal, K.; Bhattacharyya, S.K.; Mishra, V.; Zuilhof, H. Click Chemistry for Biofunctional Polymers: From Observing to Steering Cell Behavior. Chem. Rev. 2024, 124, 13216–13300. [Google Scholar] [CrossRef]
- Khan, I.A.; Yu, T.; Yang, M.; Liu, J.; Chen, Z. A Systematic Review of Toxicity, Biodistribution, and Biosafety in Upconversion Nanomaterials: Critical Insights into Toxicity Mitigation Strategies and Future Directions for Safe Applications. BME Front. 2025, 6, 0120. [Google Scholar] [CrossRef]
- Sharifi, M.; Cho, W.C.; Ansariesfahani, A.; Tarharoudi, R.; Malekisarvar, H.; Sari, S.; Bloukh, S.H.; Edis, Z.; Amin, M.; Gleghorn, J.P.; et al. An Updated Review on EPR-Based Solid Tumor Targeting Nanocarriers for Cancer Treatment. Cancers 2022, 14, 2868. [Google Scholar] [CrossRef]
- Akpa, P.A.; Peter, I.E.; Onwuka, A.M.; Obi, B.C.; Akunne, M.O.; Nworu, C.S.; Ejikeme, P.M.; Akunne, T.C.; Attama, A.A.; Akah, P.A. Nanotheranostics: Platforms, Current Applications, and Mechanisms of Targeting in Breast and Prostate Cancers. J. Nanotheranostics 2023, 4, 346–383. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, J.; He, W.; Li, C.; Wang, Y. Nanomaterials for Targeting Liver Disease: Research Progress and Future Perspectives. Nano Biomed. Eng. 2023, 15, 199–224. [Google Scholar] [CrossRef]
- Bertolini, M.; Wong, M.S.; Mendive-Tapia, L.; Vendrell, M. Smart Probes for Optical Imaging of T Cells and Screening of Anti-Cancer Immunotherapies. Chem. Soc. Rev. 2023, 52, 5352–5372. [Google Scholar] [CrossRef]
- Varadharajan, S.; Vasanthan, K.S.; Mathur, V.; Hariperumal, N.; Mazumder, N. Green Synthesis and Multifaceted Applications: Challenges and Innovations in Carbon Dot Nanocomposites. Discov. Nano 2024, 19, 205. [Google Scholar] [CrossRef]
- Ilieş, B.D.; Yildiz, I.; Abbas, M. Peptide-conjugated Nanoparticle Platforms for Targeted Delivery, Imaging, and Biosensing Applications. ChemBioChem 2024, 25, e202300867. [Google Scholar] [CrossRef]
- Ji, X.; Wang, N.; Wang, J.; Wang, T.; Huang, X.; Hao, H. Non-Destructive Real-Time Monitoring and Investigation of the Self-Assembly Process Using Fluorescent Probes. Chem. Sci. 2024, 15, 3800–3830. [Google Scholar] [CrossRef]
- Fu, Q.; Yang, X.; Wang, M.; Zhu, K.; Wang, Y.; Song, J. Activatable Probes for Ratiometric Imaging of Endogenous Biomarkers In Vivo. ACS Nano 2024, 18, 3916–3968. [Google Scholar] [CrossRef] [PubMed]
- Tauser, R.-G.; Lupascu, F.-G.; Profire, B.-S.; Iacob, A.-T.; Vasincu, I.-M.; Apotrosoaei, M.; Chirliu, O.-M.; Lupascu, D.; Profire, L. Aptamer-Nanoconjugates as Potential Theranostics in Major Neuro-Oncological and Neurodegenerative Disorders. Pharmaceutics 2025, 17, 1106. [Google Scholar] [CrossRef]
- Hang, Y.; Boryczka, J.; Wu, N. Visible-Light and near-Infrared Fluorescence and Surface-Enhanced Raman Scattering Point-of-Care Sensing and Bio-Imaging: A Review. Chem. Soc. Rev. 2022, 51, 329–375. [Google Scholar] [CrossRef]
- Zhao, X.; Zhang, J.; Shi, L.; Xian, M.; Dong, C.; Shuang, S. Folic Acid-Conjugated Carbon Dots as Green Fluorescent Probes Based on Cellular Targeting Imaging for Recognizing Cancer Cells. RSC Adv. 2017, 7, 42159–42167. [Google Scholar] [CrossRef]
- Usha, S.P.; Manoharan, H.; Deshmukh, R.; Álvarez-Diduk, R.; Calucho, E.; Sai, V.V.R.; Merkoçi, A. Attomolar Analyte Sensing Techniques (AttoSens): A Review on a Decade of Progress on Chemical and Biosensing Nanoplatforms. Chem. Soc. Rev. 2021, 50, 13012–13089. [Google Scholar] [CrossRef]
- Rainu, S.K.; Ramachandran, R.G.; Parameswaran, S.; Krishnakumar, S.; Singh, N. Advancements in Intraoperative Near-Infrared Fluorescence Imaging for Accurate Tumor Resection: A Promising Technique for Improved Surgical Outcomes and Patient Survival. ACS Biomater. Sci. Eng. 2023, 9, 5504–5526. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, Z.; Zhang, Z.; Yang, H.; Wang, H.; Yang, Z.; Xu, Y.; Li, S.; Yang, D. Recent Advances in Silica-Based Nanomaterials for Enhanced Tumor Imaging and Therapy. ACS Appl. Bio Mater. 2024, 7, 7133–7169. [Google Scholar] [CrossRef] [PubMed]
- Joshi, S.; Moody, A.; Budthapa, P.; Gurung, A.; Gautam, R.; Sanjel, P.; Gupta, A.; Aryal, S.P.; Parajuli, N.; Bhattarai, N. Advances in Natural-Product-Based Fluorescent Agents and Synthetic Analogues for Analytical and Biomedical Applications. Bioengineering 2024, 11, 1292. [Google Scholar] [CrossRef] [PubMed]
- Núñez-Salinas, A.; Parra-Garretón, C.; Acuña, D.; Peñaloza, S.; Günther, G.; Bollo, S.; Arriagada, F.; Morales, J. Nanoradiopharmaceuticals: Design Principles, Radiolabeling Strategies, and Biomedicine Applications. Pharmaceutics 2025, 17, 912. [Google Scholar] [CrossRef] [PubMed]
- Maharjan, P.S.; Bhattarai, H.K. Singlet Oxygen, Photodynamic Therapy, and Mechanisms of Cancer Cell Death. J. Oncol. 2022, 2022, 7211485. [Google Scholar] [CrossRef]
- Yu, H.; Tang, K.; Cai, Z.; Lin, X.; Huang, Y.; Yu, T.; Zhang, Q.; Wang, Q.; Wu, L.; Yang, L.; et al. Carbon Dots-Based Nanozyme for Drug-Resistant Lung Cancer Therapy by Encapsulated Doxorubicin/SiRNA Cocktail. Int. J. Nanomed. 2023, 18, 933–948. [Google Scholar] [CrossRef]
- Cai, H.; Abbas, K.; Yang, Y.; Li, Z.; Bi, H. The Application of Carbon Dots in Tumor Immunotherapy: Researches and Prospects. Appl. Res. 2023, 2, e202300001. [Google Scholar] [CrossRef]
- Grasso, G.; Colella, F.; Forciniti, S.; Onesto, V.; Iuele, H.; Siciliano, A.C.; Carnevali, F.; Chandra, A.; Gigli, G.; del Mercato, L.L. Fluorescent Nano- and Microparticles for Sensing Cellular Microenvironment: Past, Present and Future Applications. Nanoscale Adv. 2023, 5, 4311–4336. [Google Scholar] [CrossRef]
- Chaturvedi, S.; Maheshwari, D.; Chawathe, A.; Sharma, N. Current Analytical Approaches for Characterizing Nanoparticle Sizes in Pharmaceutical Research. J. Nanopart. Res. 2024, 26, 19. [Google Scholar] [CrossRef]
- Sajjadi, M.; Nasrollahzadeh, M.; Jaleh, B.; Soufi, G.J.; Iravani, S. Carbon-Based Nanomaterials for Targeted Cancer Nanotherapy: Recent Trends and Future Prospects. J. Drug Target. 2021, 29, 716–741. [Google Scholar] [CrossRef]
- Subhan, M.A.; Yalamarty, S.S.K.; Filipczak, N.; Parveen, F.; Torchilin, V.P. Recent Advances in Tumor Targeting via EPR Effect for Cancer Treatment. J. Pers. Med. 2021, 11, 571. [Google Scholar] [CrossRef]
- Liu, Q.; Wu, D.; Ma, Y.; Cao, Y.; Pang, Y.; Tang, M.; Pu, Y.; Zhang, T. Intracellular Reactive Oxygen Species Trigger Mitochondrial Dysfunction and Apoptosis in Cadmium Telluride Quantum Dots-Induced Liver Damage. NanoImpact 2022, 25, 100392. [Google Scholar] [CrossRef]
- Xu, Y.; Fourniols, T.; Labrak, Y.; Préat, V.; Beloqui, A.; des Rieux, A. Surface Modification of Lipid-Based Nanoparticles. ACS Nano 2022, 16, 7168–7196. [Google Scholar] [CrossRef] [PubMed]
- Samadzadeh, M.; Khosravi, A.; Zarepour, A.; Jamalipour Soufi, G.; Hekmatnia, A.; Zarrabi, A.; Iravani, S. Molecular Imaging Using (Nano)Probes: Cutting-Edge Developments and Clinical Challenges in Diagnostics. RSC Adv. 2025, 15, 24696–24725. [Google Scholar] [CrossRef]
- Desai, N.; Rana, D.; Patel, M.; Bajwa, N.; Prasad, R.; Vora, L.K. Nanoparticle Therapeutics in Clinical Perspective: Classification, Marketed Products, and Regulatory Landscape. Small 2025, 21, 2502315. [Google Scholar] [CrossRef]
- Huang, Y.; Pan, M.; Chen, B. A Systematic Review and Meta-Analysis of Sentinel Lymph Node Biopsy in Gastric Cancer, an Optimization of Imaging Protocol for Tracer Mapping. World J. Surg. 2021, 45, 1126–1134. [Google Scholar] [CrossRef]
- Zhao, Y.-Y.; Lu, L.; Jeong, H.; Kim, H.; Li, X.; Zhang, H.; Yoon, J. Enhancing Biosafety in Photodynamic Therapy: Progress and Perspectives. Chem. Soc. Rev. 2025, 54, 7749–7768. [Google Scholar] [CrossRef]
- Minasian, L.M.; Pinsky, P.; Katki, H.A.; Dickherber, T.; Han, P.K.J.; Harris, L.; Patriotis, C.; Srivastava, S.; Weil, C.J.; Prorok, P.C.; et al. Study Design Considerations for Trials to Evaluate Multicancer Early Detection Assays for Clinical Utility. JNCI J. Natl. Cancer Inst. 2023, 115, 250–257. [Google Scholar] [CrossRef] [PubMed]
- Ayoubi, M.; Naserzadeh, P.; Hashemi, M.T.; Reza Rostami, M.; Tamjid, E.; Tavakoli, M.M.; Simchi, A. Biochemical Mechanisms of Dose-Dependent Cytotoxicity and ROS-Mediated Apoptosis Induced by Lead Sulfide/Graphene Oxide Quantum Dots for Potential Bioimaging Applications. Sci. Rep. 2017, 7, 12896. [Google Scholar] [CrossRef] [PubMed]
- Siddique, S.; Chow, J.C.L. Application of Nanomaterials in Biomedical Imaging and Cancer Therapy. Nanomaterials 2020, 10, 1700. [Google Scholar] [CrossRef]
- Yang, G.; Phua, S.Z.F.; Bindra, A.K.; Zhao, Y. Degradability and Clearance of Inorganic Nanoparticles for Biomedical Applications. Adv. Mater. 2019, 31, 1805730. [Google Scholar] [CrossRef]
- Xu, C.; Ivanovski, S. Clinical Translation of Personalized Bioengineered Implant Scaffolds. Nat. Rev. Bioeng. 2025, 3, 390–407. [Google Scholar] [CrossRef]
- Soman, S.; Kulkarni, S.; Sherin, F.; Roy, A.A.; Mukharya, A.; Pokale, R.; Mutalik, S. Bioinspired Quantum Dots: Advancing Diagnostic and Therapeutic Strategies in Breast Cancer. RSC Adv. 2025, 15, 27738–27771. [Google Scholar] [CrossRef]
- Kashyap, A.; Rapsomaniki, M.A.; Barros, V.; Fomitcheva-Khartchenko, A.; Martinelli, A.L.; Rodriguez, A.F.; Gabrani, M.; Rosen-Zvi, M.; Kaigala, G. Quantification of Tumor Heterogeneity: From Data Acquisition to Metric Generation. Trends Biotechnol. 2022, 40, 647–676. [Google Scholar] [CrossRef]
- Shakeri, A.; Khan, S.; Didar, T.F. Conventional and Emerging Strategies for the Fabrication and Functionalization of PDMS-Based Microfluidic Devices. Lab Chip 2021, 21, 3053–3075. [Google Scholar] [CrossRef]
- Prud’homme, A.; Nabki, F. Cost-Effective Photoacoustic Imaging Using High-Power Light-Emitting Diodes Driven by an Avalanche Oscillator. Sensors 2025, 25, 1643. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wang, S.; Zhang, F. Near-Infrared Luminescence High-Contrast in Vivo Biomedical Imaging. Nat. Rev. Bioeng. 2023, 1, 60–78. [Google Scholar] [CrossRef]
- Agbeyangi, A.; Suleman, H. Advances and Challenges in Low-Resource-Environment Software Systems: A Survey. Informatics 2024, 11, 90. [Google Scholar] [CrossRef]
- Suo, J.; Zhang, W.; Gong, J.; Yuan, X.; Brady, D.J.; Dai, Q. Computational Imaging and Artificial Intelligence: The Next Revolution of Mobile Vision. Proc. IEEE 2023, 111, 1607–1639. [Google Scholar] [CrossRef]
- Mao, J.; He, H. Deep Learning in Fluorescence Imaging and Analysis. J. Intell. Med. 2024, 1, 42–62. [Google Scholar] [CrossRef]
- Cui, Y.; Zhang, Z.; Shi, Y.; Hu, Y. Chemical Imaging for Biological Systems: Techniques, AI-Driven Processing, and Applications. J. Mater. Chem. B 2025, 13, 6916–6948. [Google Scholar] [CrossRef] [PubMed]
- Eftekharifar, M.; Heidari, R.; Mohaghegh, N.; Najafabadi, A.H.; Heidari, H. Advances in Photoactivated Carbon-Based Nanostructured Materials for Targeted Cancer Therapy. Adv. Drug Deliv. Rev. 2025, 222, 115604. [Google Scholar] [CrossRef]
- Dey, S.; Hassan, S.; Pandey, R.K. Nanomedicine in Targeted Drug Delivery: Precision Therapeutics for Personalized Medicine; Springer: Cham, Switzerland, 2024; pp. 179–231. [Google Scholar] [CrossRef]
- Capobianco, E. High-Dimensional Role of AI and Machine Learning in Cancer Research. Br. J. Cancer 2022, 126, 523–532. [Google Scholar] [CrossRef]
- Parvin, N.; Aslam, M.; Joo, S.W.; Mandal, T.K. Nano-Phytomedicine: Harnessing Plant-Derived Phytochemicals in Nanocarriers for Targeted Human Health Applications. Molecules 2025, 30, 3177. [Google Scholar] [CrossRef] [PubMed]
- Parvin, N.; Joo, S.W.; Jung, J.H.; Mandal, T.K. Multimodal AI in Biomedicine: Pioneering the Future of Biomaterials, Diagnostics, and Personalized Healthcare. Nanomaterials 2025, 15, 895. [Google Scholar] [CrossRef]
- Hussain, D.; Abbas, N.; Khan, J. Recent Breakthroughs in PET-CT Multimodality Imaging: Innovations and Clinical Impact. Bioengineering 2024, 11, 1213. [Google Scholar] [CrossRef]
- Wu, Y.; Shang, J.; Zhang, X.; Li, N. Advances in Molecular Imaging and Targeted Therapeutics for Lymph Node Metastasis in Cancer: A Comprehensive Review. J. Nanobiotechnol. 2024, 22, 783. [Google Scholar] [CrossRef]
- Malhotra, K.; Hrovat, D.; Kumar, B.; Qu, G.; Van Houten, J.; Ahmed, R.; Piunno, P.A.E.; Gunning, P.T.; Krull, U.J. Lanthanide-Doped Upconversion Nanoparticles: Exploring A Treasure Trove of NIR-Mediated Emerging Applications. ACS Appl. Mater. Interfaces 2023, 15, 2499–2528. [Google Scholar] [CrossRef] [PubMed]
- Sola, F.; Montanari, M.; Fiorani, M.; Barattini, C.; Ciacci, C.; Burattini, S.; Lopez, D.; Ventola, A.; Zamai, L.; Ortolani, C.; et al. Fluorescent Silica Nanoparticles Targeting Mitochondria: Trafficking in Myeloid Cells and Application as Doxorubicin Delivery System in Breast Cancer Cells. Int. J. Mol. Sci. 2022, 23, 3069. [Google Scholar] [CrossRef]
- Ma, X.; Mao, M.; He, J.; Liang, C.; Xie, H.-Y. Nanoprobe-Based Molecular Imaging for Tumor Stratification. Chem. Soc. Rev. 2023, 52, 6447–6496. [Google Scholar] [CrossRef] [PubMed]
- Perluigi, M.; Di Domenico, F.; Butterfield, D.A. Oxidative Damage in Neurodegeneration: Roles in the Pathogenesis and Progression of Alzheimer Disease. Physiol. Rev. 2024, 104, 103–197. [Google Scholar] [CrossRef]
- Himawan, A.; Vora, L.K.; Permana, A.D.; Sudir, S.; Nurdin, A.R.; Nislawati, R.; Hasyim, R.; Scott, C.J.; Donnelly, R.F. Where Microneedle Meets Biomarkers: Futuristic Application for Diagnosing and Monitoring Localized External Organ Diseases. Adv. Healthc. Mater. 2023, 12, 2202066. [Google Scholar] [CrossRef]




| Aspect/Category | Core Concept and Mechanism | Biomedical Significance/Impact | Representative Examples/Key Features | References |
|---|---|---|---|---|
| Basic Principles of Fluorescence | Fluorescence arises from photon absorption, excitation to a higher energy state, and emission at a longer wavelength (Stokes shift). Core parameters include quantum yield and fluorescence lifetime. | Determines brightness, sensitivity, and contrast in biomedical imaging; essential for rational probe design. | Quantum dots and carbon dots exhibit high quantum yields; lifetime imaging improves precision compared with intensity-based detection. | [15,16,17,18] |
| Fluorescence Lifetime Imaging and Lifetime-Based Environmental Sensing (Unified Entry) | Measures decay time instead of intensity. Lifetime responds to microenvironmental factors such as pH, viscosity, oxygen level, and molecular interactions. | Provides concentration-independent contrast, reduces autofluorescence interference, and enables real-time sensing of metabolic or biochemical changes. | Used to map enzyme activity, metabolic states, drug-release dynamics, and intracellular environment variations in living tissues. | [17,18,24,25,26] |
| Optical Penetration and NIR Imaging | NIR and NIR-II wavelengths penetrate deeper into tissues due to lower scattering and absorption. | Enables high-resolution, deep-tissue visualization suitable for in vivo imaging. | NIR-II quantum dots, carbon dots, and upconversion nanoparticles visualize tumors and vasculature several millimeters below the surface. | [12,19] |
| Activatable and Responsive Fluorescent Nanoprobes | Probes switch from “off” to “on” in response to biochemical triggers (enzymes, pH, redox). | Enhances site-specific imaging and reduces background noise. | Enzyme-activated carbon dots; redox-responsive silica nanoprobes for tumor and inflammation detection. | [20] |
| Key Optical Parameters | Includes absorption/emission spectra, brightness, quantum yield, photostability, and lifetime. | Influences imaging sensitivity, durability under illumination, and quantitative accuracy. | Quantum dots and UCNPs offer high photostability; NIR-II probes reduce tissue autofluorescence. | [19,21,22,23] |
| Physicochemical Properties (Size, Surface, Stability) | Size, charge, and surface chemistry regulate colloidal stability, biodistribution, and clearance routes. | Ensures predictable in vivo behavior and contributes to safety. | Nanoparticles < 6 nm undergo renal clearance; PEGylated or zwitterionic coatings reduce protein adsorption. | [27,28] |
| Degradability and Biocompatibility | Biodegradable and carbon-based nanoprobes break down into non-toxic products. | Reduces long-term accumulation and improves clinical applicability. | Carbon dots and organic nanoprobes degrade naturally, minimizing organ retention. | [29,30,31] |
| Brightness and Sensitivity | Multiple fluorophores or quantum confinement effects amplify emission. | Enables imaging at low probe concentrations, reducing systemic exposure. | Quantum dots and dye-doped silica nanoparticles enable bright, sensitive detection at cellular resolution. | [32] |
| Photostability | Resistance to photobleaching maintains stable emission during prolonged imaging. | Essential for intraoperative visualization and long-term monitoring. | Quantum dots, UCNPs, and metal nanoclusters sustain emission under continuous excitation. | [33] |
| Spectral Tunability | Emission wavelength controlled by nanoparticle size, dopant type, or surface chemistry. | Supports multiplex imaging to track multiple biological targets simultaneously. | Size-tuned quantum dots and doped UCNPs covering visible to NIR spectra. | [34] |
| Activatable and Ratiometric Designs | Dual- or multi-emission systems enable internal calibration or environment-triggered signal changes. | Improves measurement accuracy for dynamic biological processes. | pH-sensitive and redox-responsive carbon dots for ratiometric biosensing. | [35] |
| Multifunctionality and Theranostics | Combines imaging and therapy on a single nanoprobe (drug loading, photothermal agents). | Enables real-time tracking of treatment response and image-guided therapy. | Quantum dots or silica nanoprobes integrated with drugs and targeting ligands. | [36] |
| Deep-Tissue and Intraoperative Imaging | NIR-II nanoprobes achieve enhanced contrast and spatial resolution beyond visible-range dyes. | Supports early detection and clear visualization of tumors and vasculature during surgery. | NIR-II emitting nanoprobes for cancer diagnosis and surgical navigation. | [37] |
| Class of Nanoprobe | Core Structure and Mechanism | Key Optical and Physicochemical Properties | Biomedical Applications and Functional Advantages | Representative References |
|---|---|---|---|---|
| Quantum Dots (QDs) | Semiconductor nanocrystals (2–10 nm) composed of a core (CdSe, CdTe, InP) and a passivating shell (ZnS, ZnSe) that enhances quantum yield and stability. Optical emission arises from quantum confinement, where energy levels are size-dependent. |
|
| [12,30,38,39,40,41,42,43,44,45,46,47,48,49] |
| Carbon Dots (CDs) | Nanoscale carbon particles (<10 nm) with graphitic or amorphous carbon cores and abundant surface functional groups (–COOH, –OH, –NH2). Synthesized via bottom-up (pyrolysis, hydrothermal, microwave) or top-down (laser ablation, oxidation) methods. |
|
| [11,14,31,43,48,50,51,52,53,54,55,56,57,,58,59,60,61,62,63,64,65,66,67] |
| Upconversion Nanoparticles (UCNPs) | Inorganic crystalline hosts (e.g., NaYF4) doped with lanthanide ions (Yb3+, Er3+, Tm3+). Emit higher-energy photons upon sequential absorption of multiple NIR photons via energy transfer upconversion (ETU), excited-state absorption (ESA), or photon avalanche (PA). |
|
| [18,56,68,69,70,71,72,73,74,75,76,77,78,79,80] |
| Dye-Doped Silica Nanoparticles (DDSNs) | Organic fluorescent dyes encapsulated or covalently bound within an amorphous silica matrix formed via sol–gel or microemulsion synthesis. The silica shell prevents dye leaching and aggregation-induced quenching. |
|
| [26,81,82,83,84,85,86,87,88,89,90,91] |
| Strategy/Probe Type | Key Mechanism or Functional Approach | Advantages and Biomedical Impact | Representative Examples/Applications | References |
|---|---|---|---|---|
| Surface Modification and Bioconjugation | Surface coatings (PEG, Zwitterionic polymers, Dextran, chitosan); Covalent (Carbodiimide, Click chemistry) and noncovalent (Electrostatic, hydrophobic, Biotin–streptavidin) conjugation | Enhances colloidal stability, Solubility, and Circulation time, and reduces nonspecific interactions; Enables specific ligand attachment for biosensing or targeting | PEGylated quantum dots for prolonged circulation; Silica-coated UCNPs for reduced toxicity; Amino-functionalized carbon dots for biocompatibility | [92,93,94,95,96] |
| Tumor-Targeted and Organ-Specific Probes | Passive targeting via EPR effect; active targeting using ligands such as antibodies, Peptides, aptamers, or Small molecules | Enables selective accumulation in tumor or organ-specific tissues; Improves imaging contrast and reduces systemic toxicity | Trastuzumab-modified QDs for HER2 imaging; Folic acid-conjugated CDs for ovarian cancer; angiopep-2 peptides for brain targeting; glycyrrhetinic acid-modified NPs for liver imaging | [97,98,99,100] |
| Activatable Probes | Designed to respond to physiological or pathological stimuli such as low pH, GSH concentration, Hypoxia, or enzyme activity | Provides “off–on” fluorescence activation, improving specificity and minimizing background signal | pH-responsive UCNPs for acidic tumor detection; Enzyme-cleavable peptide–carbon dots for protease activity imaging | [101,102] |
| Ratiometric Probes | Utilize two emission bands: one responsive to analyte changes and one constant as an internal reference | Allow quantitative imaging independent of probe concentration, photobleaching, or uneven illumination | Dual-emission CDs for intracellular pH sensing; dual-dye DDSNs for ion detection; UCNP-based ratiometric oxygen sensors for tumor hypoxia imaging | [103,104] |
| Application Area | Mechanism or Working Principle | Biomedical Advantages and Impact | Representative Examples/Probe Types | References |
|---|---|---|---|---|
| Early Disease Detection and Diagnosis | Targeted fluorescence of biomarkers via ligand–nanoprobe conjugation; detection of disease-associated biomolecules at low concentrations | Enables ultra-sensitive and early identification of cancer, infection, and metabolic disorders; supports preclinical and clinical diagnostics | Antibody-conjugated QDs for tumor antigen detection; folic acid–functionalized CDs for cancer imaging; UCNP-based immunoassays for viral antigen detection | [91,105,106,107,108] |
| Image-Guided Surgery | Real-time intraoperative fluorescence visualization of diseased tissue; NIR or visible light imaging for tumor margin delineation | Enhances surgical precision and reduces recurrence; provides high-contrast imaging and deep-tissue visualization | NIR QDs and UCNPs for deep-tissue imaging; targeted CDs and DDSNs for tumor margin detection; activatable probes for enzyme-triggered fluorescence | [48,109,110] |
| Real-Time Monitoring of Therapeutic Response | Fluorescent signal variation with therapeutic activity (e.g., drug release, apoptosis, ROS generation) | Enables real-time assessment of therapy efficacy and treatment adjustment; supports personalized medicine | QDs, CDs, and DDSNs as theranostic platforms for drug delivery and tracking; UCNPs for photodynamic therapy monitoring; activatable and ratiometric probes for oxidative stress imaging | [111,112,113,114] |
| In Vivo Biosensing and Tracking | Dynamic tracking of labeled cells, molecules, or ions within living organisms; fluorescence-based biosensing of physiological changes | Allows non-invasive monitoring of cell migration, immune responses, and metabolic processes in vivo | QDs or CDs for immune cell tracking; UCNPs for deep-tissue biosensing; DDSNs for pH, glucose, and ROS detection in live models | [48] |
| Stage/Aspect | Critical Focus/Barrier | Mitigation Strategies/Considerations | Illustrative Examples or Trends | References |
|---|---|---|---|---|
| Preclinical Characterization & Safety | Ensuring reproducible nanoprobe physicochemical properties (size, charge, fluorescence), assessing cytotoxicity, stability, and cellular uptake | Rigorous standardization, long-term in vitro assays, surface passivation and coatings to suppress ion leaching | Use of cadmium-free QDs, PEG or lipid coatings to improve biocompatibility | [117,118,119,120,121] |
| In Vivo Biodistribution & Pharmacokinetics | Achieving favorable tumor accumulation while minimizing off-target organ retention and long-term persistence | Optimization of size (<10 nm for renal clearance), active targeting ligands, stealth coatings, real-time imaging of distribution | Studies exploring UCNPs or silica-coated probes in rodent tumor models | [119]; also see clinical-translation reviews |
| Toxicity and Biosafety Evaluation | Avoiding dose-dependent toxicity, immunogenicity, complement activation, accumulation in reticuloendothelial organs | Employ inert matrix materials (silica, carbon dots), surface shielding (PEG, zwitterions), detailed long-term toxicity studies | Transition to silicon QDs, biocompatible nanoprobes such as dye-doped silica or carbon dots | [119,120] |
| Regulatory & Manufacturing Challenges | Meeting standards for reproducibility, batch-to-batch consistency, quality control, and regulatory classification (diagnostic agent, device, or combination product) | Early engagement with regulatory bodies, adherence to GMP-like practices, simplified nanoprobe designs | Pilot translation efforts in sentinel lymph node imaging using silica-encapsulated dyes or ICG-loaded nanoparticles | [122,123,124] |
| Clinical Feasibility and Trials | Demonstrating safety, biodistribution, diagnostic/therapeutic efficacy, and patient benefit in early-phase trials | Well-designed Phase I/II studies, careful dose escalation, imaging endpoints, comparison with standard-of-care diagnostics | Clinical trials with ICG-silica nanoparticles for sentinel lymph node mapping, and cadmium-free QDs in pilot imaging studies | [124,125] |
| Translational Trends & Future Directions | Integration with multimodal imaging, AI-assisted image analysis, simplified architectures to reduce complexity, regulatory harmonization | Combine fluorescence with MRI, PET, or photoacoustic modalities; use smart or activatable probes; standardize translational pathways | Review emphasis on NIR-II probes under evaluation, and challenges in scaling targeted fluorescent probes for intraoperative navigation |
| Challenge Area | Underlying Cause/Mechanism | Impact on Biomedical Application | Proposed Mitigation Strategies | References |
|---|---|---|---|---|
| Biocompatibility and Long-Term Safety | Interaction of nanoparticles with biological systems influenced by size, charge, and surface chemistry; release of toxic ions (e.g., Cd2+, Pb2+) from semiconductor QDs; accumulation of non-biodegradable materials in organs | Potential cytotoxicity, oxidative stress, inflammation, immune activation, and long-term organ retention hinder clinical use | Use of cadmium-free or silicon-based QDs; application of biocompatible materials (carbon dots, DDSNs, UCNPs); PEGylation, zwitterionic or lipid coatings to improve circulation and reduce immune response | [127,128,129,130] |
| Stability and Reproducibility of Synthesis | Variations in precursor purity, synthesis temperature, dopant ratios, and reaction time lead to inconsistent particle size, morphology, or optical properties | Batch-to-batch variability causes inconsistent fluorescence output, targeting accuracy, and quantitative imaging reliability | Development of standardized and scalable synthesis protocols; quality control during nanocrystal growth; surface passivation; rigorous control of dopant concentration and reaction kinetics | [131,132,133] |
| Imaging Depth and Real-Time Monitoring Constraints | Light scattering, Absorption, and Autofluorescence in tissues limit penetration depth; Dynamic physiological processes affect temporal signal stability | Restricts visualization of deep-seated or moving tissues; Reduces imaging resolution and accuracy in vivo | Employ near-infrared (NIR-I/NIR-II) or upconversion nanoprobes; integrate multimodal imaging (MRI, CT, photoacoustic); use AI-assisted image reconstruction and advanced optical detectors | [104,134,135,136,137] |
| Emerging Direction/Technology | Key Principles and Mechanisms | Advantages and Biomedical Implications | Representative Examples/Applications | References |
|---|---|---|---|---|
| Integration with Artificial Intelligence (AI) | Application of machine learning (ML) and deep learning (DL) algorithms for image analysis, signal processing, and pattern recognition of fluorescence imaging data | Enhances imaging accuracy, reduces background noise, and enables automated quantitative analysis; facilitates real-time diagnostic decision-making and predictive modeling | CNN-assisted identification of tumor margins using UCNP images; AI-based signal reconstruction for background autofluorescence correction; predictive algorithms for therapy response and disease progression | [138,139,140,141] |
| AI for Data-driven Biomarker Discovery and Drug Screening | High-throughput AI algorithms analyzing large fluorescence datasets from multiplexed probes for biomarker identification | Accelerates identification of molecular signatures and drug responses; enables multidimensional correlation analysis of optical, biochemical, and morphological data | AI processing of multiplexed carbon dot or QD fluorescence signals to identify novel biomarkers for early cancer and inflammatory disease detection | [142,143,144] |
| Multimodal Imaging Systems | Integration of fluorescence imaging with complementary modalities (MRI, CT, PET, photoacoustic imaging) within a single nanoparticle platform | Provides synergistic anatomical and molecular information; improves spatial resolution, imaging depth, and diagnostic accuracy | QDs or UCNPs conjugated with gadolinium or Fe3O4 for MRI-fluorescence dual imaging; PET-fluorescence hybrids for precise tumor localization | [145,146] |
| Theranostic Nanoparticles | Combination of therapeutic (drug delivery, PDT/PTT) and diagnostic (fluorescence imaging) functions in a single nanoprobe | Enables image-guided therapy, real-time tracking of treatment efficacy, and reduced off-target toxicity | UCNPs activating photosensitizers under NIR light for PDT; dye-doped silica nanoparticles co-loaded with chemotherapeutics for image-guided drug release | [147,148] |
| Smart and Activatable Nanoprobes | Fluorescent probes responsive to multiple physiological stimuli such as pH, enzyme activity, redox potential, and temperature | Provides spatiotemporal imaging of dynamic biological processes and tumor microenvironments; enhances diagnostic specificity | Dual-responsive probes that emit distinct signals under acidic and enzymatic conditions in cancer tissues; ROS-sensitive carbon dots for inflammation tracking | [149] |
| Multiplexed and Multicolor Imaging | Encoding multiple fluorophores within one nanoprobe or using spectral separation to monitor several biomarkers simultaneously | Enables simultaneous tracking of multiple cellular pathways or disease markers; enhances diagnostic depth in complex diseases | Multicolor QDs or CDs enabling simultaneous imaging of cancer biomarkers, neurotransmitters, and inflammatory mediators | [150] |
| Integration with Wearable/Implantable Devices | Embedding nanoprobes in microneedles, bioresponsive hydrogels, or flexible implants for continuous optical biosensing | Allows real-time, minimally invasive monitoring of metabolites and biomarkers in interstitial fluids or tissues | Fluorescent nanoprobe-integrated microneedles for glucose or lactate sensing; hydrogel-based sensors for pH and ion fluctuations in chronic diseases | [151] |
| Convergence toward Precision Medicine | Synergistic integration of AI, multimodal imaging, and smart nanoprobes for individualized diagnosis and treatment | Enables non-invasive, longitudinal patient monitoring and adaptive therapy optimization; supports precision oncology and chronic disease management | AI-guided multimodal theranostic platforms using UCNPs or dye-doped silica nanoprobes for personalized image-guided interventions | [138] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parvin, N.; Aslam, M.; Alam, M.N.; Mandal, T.K. Advances in NIR-II Fluorescent Nanoprobes: Design Principles, Optical Engineering, and Emerging Translational Directions. Micromachines 2025, 16, 1371. https://doi.org/10.3390/mi16121371
Parvin N, Aslam M, Alam MN, Mandal TK. Advances in NIR-II Fluorescent Nanoprobes: Design Principles, Optical Engineering, and Emerging Translational Directions. Micromachines. 2025; 16(12):1371. https://doi.org/10.3390/mi16121371
Chicago/Turabian StyleParvin, Nargish, Mohammad Aslam, Md Najib Alam, and Tapas K. Mandal. 2025. "Advances in NIR-II Fluorescent Nanoprobes: Design Principles, Optical Engineering, and Emerging Translational Directions" Micromachines 16, no. 12: 1371. https://doi.org/10.3390/mi16121371
APA StyleParvin, N., Aslam, M., Alam, M. N., & Mandal, T. K. (2025). Advances in NIR-II Fluorescent Nanoprobes: Design Principles, Optical Engineering, and Emerging Translational Directions. Micromachines, 16(12), 1371. https://doi.org/10.3390/mi16121371







