Convenient Heme Nanorod Modified Electrode for Quercetin Sensing by Two Common Electrochemical Methods
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Instrumentation
2.3. Fabrication of Sensor and Qu Sensing
2.4. Preparation of Actual Sample
3. Results and Discussion
3.1. Electrochemical Properties of Different Sensors
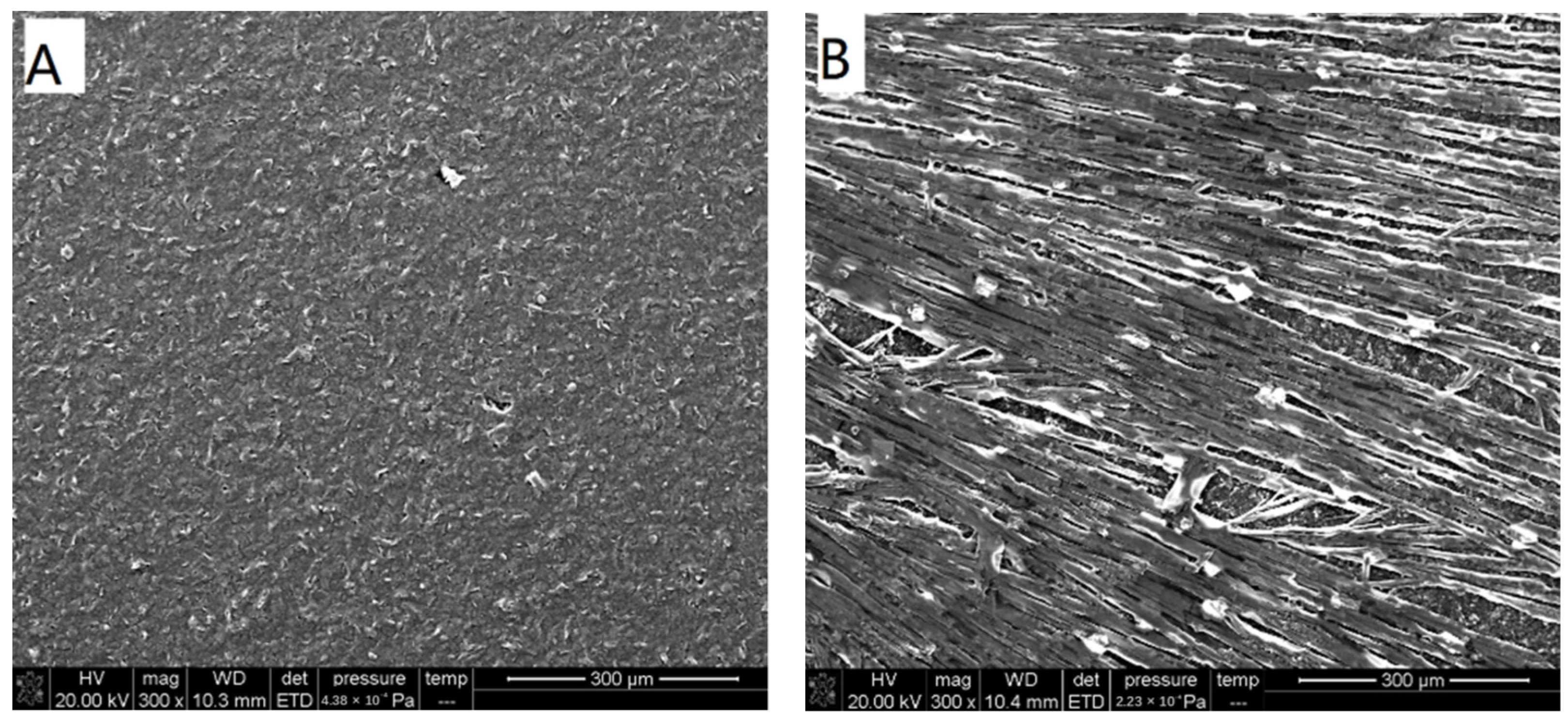
3.2. SEM Characterization of Heme/GCE
3.3. Optimization of Experimental Conditions
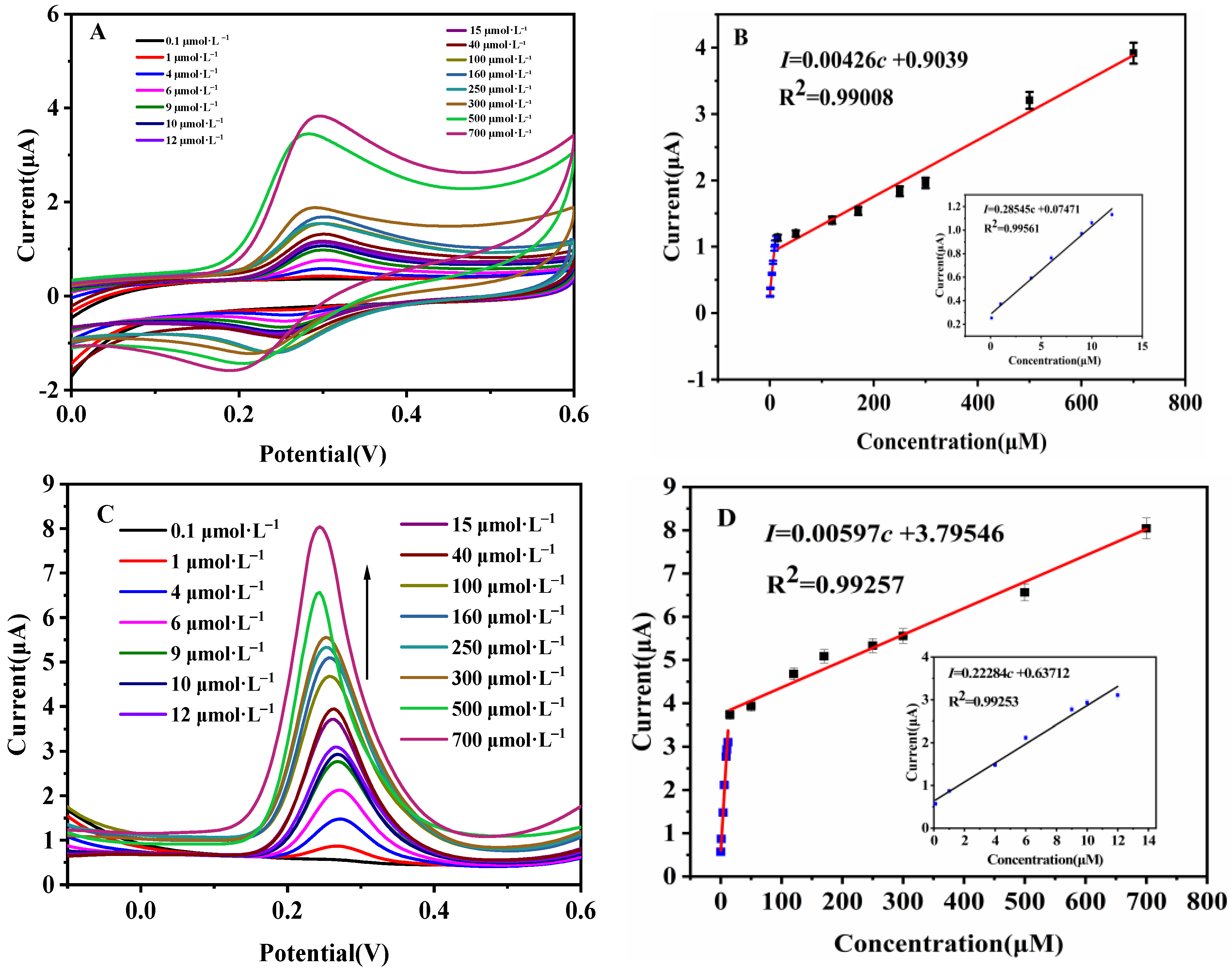
3.4. Qu Analysis
3.5. Heme/GCE Sensor Anti-Interferences, Reproductivity and Stability Study
3.6. Sample Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Zaplatic, E.; Bule, M.; Shah, S.Z.A.; Uddin, M.S.; Niaz, K. Molecular mechanisms underlying protective role of quercetin in attenuating Alzheimer’s disease. Life Sci. 2019, 224, 109–119. [Google Scholar] [CrossRef]
- Patel, R.V.; Mistry, B.M.; Shinde, S.K.; Syed, R.; Singh, V.; Shin, H.S. Therapeutic potential of quercetin as a cardiovascular agent. Eur. J. Med. Chem. 2018, 155, 889–904. [Google Scholar] [CrossRef]
- Singh, P.; Arif, Y.; Bajguz, A.; Hayat, S. The role of quercetin in plants. Plant Physiol. Biochem. 2021, 166, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Zazeri, G.; Ribeiro Povinelli, A.P.; Pavan, N.M.; de Carvalho, D.R.; Cardoso, C.L.; Ximenes, V.F. Experimental studies and computational modeling on cytochrome c reduction by quercetin: The role of oxidability and binding affinity. J. Mol. Struct. 2021, 1244, 130995. [Google Scholar] [CrossRef]
- Shabbir, U.; Rubab, M.; Daliri, E.B.M.; Chelliah, R.; Javed, A.; Oh, D.H. Curcumin, Quercetin, Catechins and Metabolic Diseases: The Role of Gut Microbiota. Nutrients 2021, 13, 206. [Google Scholar] [CrossRef] [PubMed]
- Rauf, A.; Imran, M.; Khan, I.A.; Mujeeb, U.R.; Gilani, S.A.; Mehmood, Z.; Mubarak, M.S. Anticancer potential of quercetin: A comprehensive review. Phytother. Res. 2018, 32, 2109–2130. [Google Scholar] [CrossRef]
- Almeida, A.F.; Borge, G.I.A.; Piskula, M.; Tudose, A.; Tudoreanu, L.; Valentova, K.; Williamson, G.; Santos, C.N. Bioavailability of Quercetin in Humans with a Focus on Interindividual Variation. Compr. Rev. Food Sci. 2018, 17, 714–731. [Google Scholar] [CrossRef] [Green Version]
- Chikara, S.; Nagaprashantha, L.D.; Singhal, J.; Horne, D.; Awasthi, S.; Singhal, S.S. Oxidative stress and dietary phytochemicals: Role in cancer chemoprevention and treatment. Cancer Lett. 2018, 413, 122–134. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, Z.F.; Zhang, D.; Wang, Z.Y.; Wang, L. Quercetin alleviates Cadmium-induced autophagy inhibition via TFEB-dependent lysosomal restoration in primary proximal tubular cells. Ecotoxicol. Environ. Saf. 2021, 208, 111743. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Chen, Z. ZnS quantum dots-based fluorescence spectroscopic technique for the detection of quercetin. Luminescence 2014, 29, 307–313. [Google Scholar] [CrossRef] [PubMed]
- Ravichandran, R.; Rajendran, M.; Devapiriam, D. Antioxidant study of quercetin and their metal complex and determination of stability constant by spectrophotometry method. Food Chem. 2014, 146, 472–478. [Google Scholar] [CrossRef]
- Jing, R.J.; Jiang, X.Y.; Hou, S.R.; Li, X.J.; Yuan, Z.B. Determination of quercetin, luteolin, kaempferol and isoquercitrin in stamen nelumbinis by capillary zone electrophoresis-ultraviolet detection. Chin. J. Anal. Chem. 2007, 35, 1187–1190. [Google Scholar] [CrossRef]
- Zhang, Q.L.; Cui, H. Simultaneous determination of quercetin, kaempferol, and isorhamnetin in phytopharmaceuticals of Hippophae rhamnoides L. by high-performance liquid chromatography with chemiluminescence detection. J. Sep. Sci. 2005, 28, 1171–1178. [Google Scholar] [CrossRef]
- Jin, D.; Hakamata, H.; Takahashi, K.; Kotani, A.; Kusu, F. Determination of quercetin in human plasma after ingestion of commercial canned green tea by semi-micro HPLC with electrochemical detection. Biomed. Chromatogr. 2004, 18, 662–666. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Xue, Q.; Chen, T.; Li, J.; Liu, Y.; Shan, X.; Liu, F.; Jia, J. Recent advances in electrochemical sensors for antibiotics and their applications. Chin. Chem. Lett. 2021, 32, 609–619. [Google Scholar] [CrossRef]
- Kaya, H.O.; Cetin, A.E.; Azimzadeh, M.; Topkaya, S.N. Pathogen detection with electrochemical biosensors: Advantages, challenges and future perspectives. J. Electroanal. Chem. 2021, 882. [Google Scholar] [CrossRef] [PubMed]
- Senocak, A.; Koksoy, B.; Akyuz, D.; Koca, A.; Klyamer, D.; Basova, T.; Demirbas, E.; Durmus, M. Highly selective and ultra-sensitive electrochemical sensor behavior of 3D SWCNT-BODIPY hybrid material for eserine detection. Biosens. Bioelectron. 2019, 128, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, F.; Ortega, G.; Luis Cabrera, J.; Rubianes, M.D.; Rivas, G.A. Quantification of Quercetin Using Glassy Carbon Electrodes Modified with Multiwalled Carbon Nanotubes Dispersed in Polyethylenimine and Polyacrylic Acid. Electroanalysis 2010, 22, 2650–2657. [Google Scholar] [CrossRef]
- Arvand, M.; Chaibakhsh, N.; Daneshvar, S. Amperometric Determination of Quercetin in Some Foods by Magnetic Core/Shell Fe3O4@ZnO Nanoparticles Modified Glassy Carbon Electrode. Food Anal. Methods 2015, 8, 1911–1922. [Google Scholar] [CrossRef]
- Ma, J.J.; Yu, Y.G.; Yin, S.W.; Tang, C.H.; Yang, X.Q. Cellular Uptake and Intracellular Antioxidant Activity of Zein/Chitosan Nanoparticles Incorporated with Quercetin. J. Agric. Food Chem. 2018, 66, 12783–12793. [Google Scholar] [CrossRef]
- Han, G.C.; Li, H.; Ferranco, A.; Tao, Z.; Cheng, Y.; Chen, Z.; Xue, M.; Feng, X.Z.; Kraatz, H.B. The construction of a simple sensor for the simultaneous detection of nitrite and thiosulfate by heme catalysis. RSC Adv. 2020, 10, 35007–35016. [Google Scholar] [CrossRef]
- Han, G.C.; Hou, J.T.; Huang, Z.L.; Feng, X.Z.; Chen, Z.C.; Xiao, W.X.; Li, S. Electrochemical Behaviors of Ferrocene Dicarboxylate and its Application for Heme Detection. Int. J. Electrochem. Sci. 2017, 12, 6245–6254. [Google Scholar] [CrossRef]
- Han, G.C.; Feng, X.Z.; Liang, J.T.; Xiao, W.X.; Chen, Z.C. Interaction Study of Ferrocene Derivatives and Heme by UV-Vis Spectroscopy. Spectrosc Spect Anal 2016, 36, 1585–1591. [Google Scholar] [CrossRef]
- Feng, X.Z.; Su, X.R.; Ferranco, A.; Chen, Z.C.; Han, G.C.; Jiang, Z.L.; Kraatz, H.B. Real-Time Electrochemical Detection of Uric Acid, Dopamine and Ascorbic Acid by Heme Directly Modified Carbon Electrode. J. Biomed. Nanotechnol. 2020, 16, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.Z.; Li, H.; Ferranco, A.; Chen, Z.; Xue, M.; Han, G.C.; Jiang, Z.; Kraatz, H.-B. A Very Simple Method for Detection of Bisphenol A in Environmental Water by Heme Signal Amplification. J. Electrochem. Soc. 2020, 167, 7503–7511. [Google Scholar] [CrossRef]
- Lee, C.S.; Kim, T.H. Large-Scale Preparation of MoS2/Graphene Composites for Electrochemical Detection of Morin. ACS Appl. Nano Mater. 2021, 4, 6668–6677. [Google Scholar] [CrossRef]
- Kathiravan, D.; Huang, B.R.; Saravanan, A.; Prasannan, A.; Hong, P.D. Highly enhanced hydrogen sensing properties of sericin-induced exfoliated MoS2 nanosheets at room temperature. Sens. Actuators B Chem. 2019, 279, 138–147. [Google Scholar] [CrossRef]



| Added Concentration of Qu (μM) | Current I (μA) | Average Found (μM) | Recovery (%) |
|---|---|---|---|
| 0 | 2.14 ± 0.05 | 8.82 ± 0.26 | - |
| 50 | 3.56 ± 0.13 | 45.42 ± 15.67 | 98.55% |
| 250 | 4.53 ± 0.22 | 240.29 ± 35.94 | 99.53% |
| 500 | 5.80 ± 0.02 | 496.47 ± 6.63 | 102.89% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.-G.; Wan, J.-Z.; Lin, Q.-M.; Han, G.-C.; Feng, X.-Z.; Chen, Z. Convenient Heme Nanorod Modified Electrode for Quercetin Sensing by Two Common Electrochemical Methods. Micromachines 2021, 12, 1519. https://doi.org/10.3390/mi12121519
Liu J-G, Wan J-Z, Lin Q-M, Han G-C, Feng X-Z, Chen Z. Convenient Heme Nanorod Modified Electrode for Quercetin Sensing by Two Common Electrochemical Methods. Micromachines. 2021; 12(12):1519. https://doi.org/10.3390/mi12121519
Chicago/Turabian StyleLiu, Jin-Guang, Jia-Zheng Wan, Qing-Min Lin, Guo-Cheng Han, Xiao-Zhen Feng, and Zhencheng Chen. 2021. "Convenient Heme Nanorod Modified Electrode for Quercetin Sensing by Two Common Electrochemical Methods" Micromachines 12, no. 12: 1519. https://doi.org/10.3390/mi12121519






