Fabrication of a Solution-Processed White Light Emitting Diode Containing a Single Dimeric Copper(I) Emitter Featuring Combined TADF and Phosphorescence
Abstract
:1. Introduction
2. Photophysical Background of Cu2Cl2(P∩N)2
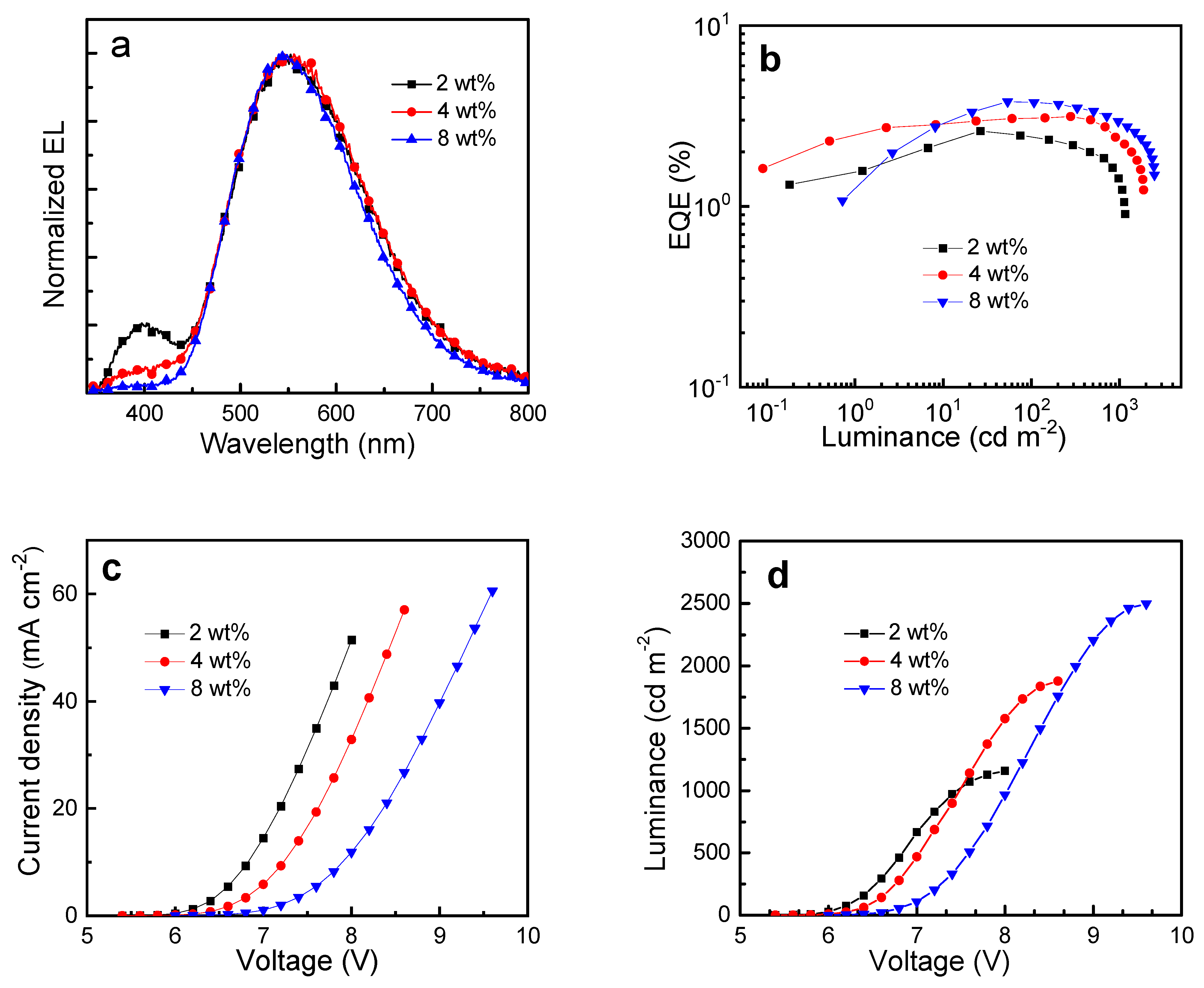
3. Solution-Processed WOLEDs with Cu2Cl2(P∩N)2 as a Single Emitter
4. Conclusions
5. Patents
Author Contributions
Funding
Conflicts of Interest
Appendix A
| Eox and Ered (V) (a) | EHOMO and ELUMO (eV) (b) | |
|---|---|---|
| Cu2Cl2(P∩N)2 | 0.504; −2.488 | −4.91; −1.42 |
References
- Helfrich, W.; Schneider, W.G. Transients of volume-controlled current and of recombination radiation in anthracene. J. Chem. Phys. 1966, 44, 2902–2909. [Google Scholar] [CrossRef]
- Yersin, H. Highly Efficient OLEDs with Phosphorescent Materials; Wiley-VCH Verlag: Weinheim, Germany, 2008. [Google Scholar] [CrossRef]
- Brütting, W.; Adachi, C. Physics of Organic Semiconductors; Wiley-VCH Verlag: Weinheim, Germany, 2012. [Google Scholar]
- Baldo, M.A.; O’Brien, D.F.; You, Y.; Shoustikov, A.; Sibley, S.; Thompson, M.E.; Forrest, S.R. Highly efficient phosphorescent emission from organic electroluminescent devices. Nature 1998, 395, 151–154. [Google Scholar] [CrossRef]
- Adachi, C.; Baldo, M.A.; Thompson, M.E.; Forrest, S.R. Nearly 100% internal phosphorescence efficiancy in an organic light-emitting device. J. Appl. Phys. 2001, 90, 5048–5051. [Google Scholar] [CrossRef] [Green Version]
- Yersin, H. Triplet Emitters for OLED Applications. Mechanisms of Exciton Trapping and Control of Emission Properties. Top. Curr. Chem. 2004, 241, 1–26. [Google Scholar] [CrossRef]
- Minke, C.; Suermann, M.; Bensmann, B.; Hanke-Rauschenbach, R. Is iridium demand a potential bottleneck in the realization of large-scale PEM water electrolysis? Int. J. Hydrog. Energy 2021, 46, 23581–23590. [Google Scholar] [CrossRef]
- Li, X.; Xie, Y.; Li, Z. Diversity of Luminescent Metal Complexes in OLEDs: Beyond Traditional Precious Metals. Chem. Asian J. 2021, 16, 2817–2829. [Google Scholar] [CrossRef]
- Parker, C.A.; Hatchard, C.G. Triplet-singlet emission in fluid solutions. Phosphorescence of eosin. Trans. Faraday Soc. 1961, 57, 1894–1904. [Google Scholar] [CrossRef]
- Yersin, H.; Monkowius, H. Komplexe mit Kleinen Singulett-Triplett Energie-Abständen zur Verwendung in Opto-Elektronischen Bauteilen (Singulett Harvesting Effekt). German Patent DE102008033563 A1, 17 July 2008. [Google Scholar]
- Deaton, J.C.; Switalski, S.C.; Kondakov, D.Y.; Young, R.H.; Pawlik, T.D.; Giesen, D.J.; Harkins, S.B.; Miller, A.J.M.; Mickenberg, S.F.; Peters, J.C. E-type delayed fluorescence of a phosphine-supported Cu2(μ-NAr2)2 diamond core: Harvesting singlet and triplet excitons in OLEDs. J. Am. Chem. Soc. 2010, 132, 9499–9508. [Google Scholar] [CrossRef] [Green Version]
- Czerwieniec, R.; Yu, J.; Yersin, H. Blue-light emission of Cu(I) complexes and singlet harvesting. Inorg. Chem. 2011, 50, 8293–8301. [Google Scholar] [CrossRef]
- Leitl, M.J.; Zink, D.M.; Schinabeck, A.; Baumann, T.; Volz, D.; Yersin, H. Copper(I) Complexes for Thermally Activated Delayed Fluorescence: From Photophysical to Device Properties. Top. Curr. Chem. 2016, 374, 25–65. [Google Scholar] [CrossRef]
- Czerwieniec, R.; Leitl, M.J.; Homeier, H.H.H.; Yersin, H. Cu(I) complexes—Thermally activated delayed fluorescence. Photophysical approach and material design. Coord. Chem. Rev. 2016, 325, 2–28. [Google Scholar] [CrossRef]
- Yersin, H. Highly Efficient OLEDs: Materials Based on Thermally Activated Delayed Fluorescence; WILEY-VCH: Weinheim, Germany, 2019. [Google Scholar]
- Yersin, H.; Czerwieniec, R.; Shafikov, V.; Suleymanova, A.F. TADF Material Design: Photophysical Background and Case Studies Focusing on Cu(I) and Ag(I) Complexes. ChemPhysChem 2017, 18, 3508–3535. [Google Scholar] [CrossRef] [PubMed]
- Armaroli, N.; Bolink, H.J. (Eds.) Photoluminescent Materials and Electroluminescent Devices; Topics in Current Chemistry Collections; Springer: Cham, Switzerland, 2017. [Google Scholar] [CrossRef]
- Hamze, R.; Peltier, J.L.; Sylvinson, D.; Jung, M.; Cardenas, J.; Haiges, R.; Soleilhavoup, M.; Jazzar, R.; Djurovich, P.I.; Bertrand, G.; et al. Eliminating nonradiative decay in Cu(I) emitters: 99% quantum efficiency and microsecond lifetime. Science 2019, 363, 601–606. [Google Scholar] [CrossRef]
- Shi, S.; Jung, M.C.; Coburn, C.; Tadle, A.; Sylvinson, M.R.D.; Djurovich, P.I.; Forrest, S.R.; Thompson, M.E. Highly Efficient Photo- and Electroluminescence from Two-Coordinate Cu(I) Complexes Featuring Nonconventional N-Heterocyclic Carbenes. J. Am. Chem. Soc. 2019, 141, 3576–3588. [Google Scholar] [CrossRef] [PubMed]
- Romanov, A.S.; Jones, S.T.E.; Gu, Q.; Conaghan, P.J.; Drummond, B.H.; Feng, J.; Chotard, F.; Buizza, L.; Foley, M.; Linnolahti, M.; et al. Carbene metal amide photoemitters: Tailoring conformationally flexible amides for full color range emissions including white-emitting OLED. Chem. Sci. 2020, 11, 435–446. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Föller, J.; Ganter, C.; Steffen, A.; Marian, C.M. Computer-Aided Design of Luminescent Linear N-Heterocyclic Carbene Copper(I) Pyridine Complexes. Inorg. Chem. 2019, 58, 5446–5456. [Google Scholar] [CrossRef]
- Dumur, F. Recent advances in organic light-emitting devices comprising copper complexes: A realistic approach for low-cost and highly emissive devices? Org. Electron. 2015, 21, 27–39. [Google Scholar] [CrossRef]
- Osawa, M.; Hoshino, M.; Hashimoto, M.; Kawata, I.; Igawa, S.; Yashima, M. Application of three-coordinate copper(I) complexes with halide ligands in organic light-emitting diodes that exhibit delayed fluorescence. Dalton Trans. 2015, 44, 8369–8378. [Google Scholar] [CrossRef]
- So, G.K.-M.; Cheng, G.; Wang, J.; Chang, X.; Kwok, C.-C.; Zhang, H.; Che, C.-M. Efficient Color-Tunable Copper(I) Complexes and Their Applications in Solution-Processed Organic Light-Emitting Diodes. Chem. Asian J. 2017, 12, 1490–1498. [Google Scholar] [CrossRef] [Green Version]
- Bizzarri, C.; Hundemer, F.; Busch, J.; Bräse, S. Triplet emitters versus TADF emitters in OLEDs: A comparative study. Polyhedron 2018, 140, 51–66. [Google Scholar] [CrossRef]
- Artem’ev, A.V.; Ryzhikov, M.R.; Taidakov, I.V.; Rakhmanova, M.I.; Varaksina, E.A.; Bagryanskaya, I.Y.; Malysheva, S.F.; Belogorlova, N.A. Bright Green-to-Yellow Emitting Cu(I) Complexes Based on Bis(2-Pyridyl)Phosphine Oxides: Synthesis, Structure and Effective Thermally Activated-Delayed Fluorescence. Dalton Trans. 2018, 47, 2701–2710. [Google Scholar] [CrossRef]
- Ohara, H.; Kobayashi, A.; Kato, M. Effects of N-Heteroaromatic Ligands on Highly Luminescent Mononuclear Copper(I)–Halide Complexes. Comptes Rendus Chim. 2015, 18, 766–775. [Google Scholar] [CrossRef]
- Ohara, H.; Kobayashi, A.; Kato, M. Simple and Extremely Efficient Blue Emitters Based on Mononuclear Cu(I)-Halide Complexes with Delayed Fluorescence. Dalton Trans. 2014, 43, 17317–17323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nozaki, K.; Iwamura, M. Highly Emissive d10 Metal Complexes as TADF Emitters with Versatile Structures and Photophysical Properties. In Highly Efficient OLEDs; Yersin, H., Ed.; Wiley-VCH Verlag: Weinheim, Germany, 2018; pp. 61–91. [Google Scholar] [CrossRef]
- Arnosti, N.; Brunner, F.; Susic, I.; Keller, S.; Junquera-Hernández, J.M.; Prescimone, A.; Bolink, H.J.; Sessolo, M.; Ortí, E.; Housecroft, C.E.; et al. Remote Modification of Bidentate Phosphane Ligands Controlling the Photonic Properties in Their Complexes: Enhanced Performance of [Cu(RN-Xantphos)(N^N)][PF6] in Light-Emitting Electrochemical Cells. Adv. Opt. Mater. 2020, 8, 1901689. [Google Scholar] [CrossRef]
- Igawa, S.; Hashimoto, M.; Kawata, I.; Yashima, M.; Hoshino, M.; Osawa, M. Highly Efficient Green Organic Light-Emitting Diodes Containing Luminescent Tetrahedral Copper(I) Complexes. J. Mater. Chem. C 2013, 1, 542–551. [Google Scholar] [CrossRef]
- Osawa, M.; Kawata, I.; Ishii, R.; Igawa, S.; Hashimoto, M.; Hoshino, M. Application of neutral d10 coinage metal complexes with an anionic bidentate ligand in delayed fluorescence-type organic light-emitting diodes. J. Mater. Chem. C 2013, 1, 4375–4383. [Google Scholar] [CrossRef]
- Uoyama, H.; Goushi, K.; Shizu, K.; Nomura, H.; Adachi, C. Highly efficient organic light-emitting diodes from delayed fluorescence. Nature 2012, 492, 234–238. [Google Scholar] [CrossRef]
- Hosokai, T.; Matsuzaki, H.; Nakanotani, H.; Tokumaru, K.; Tsutsui, T.; Furube, A.; Nasu, K.; Nomura, H.; Yahiro, M.; Adachi, C. Evidence and mechanism of efficient thermally activated delayed fluorescence promoted by delocalized excited states. Sci. Adv. 2017, 3, e1603282. [Google Scholar] [CrossRef] [Green Version]
- Noda, H.; Nakanotani, H.; Adachi, C. Excited state engineering for efficient reverse intersystem crossing. Sci. Adv. 2018, 4, eaao6910. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Shi, Y.-Z.; Wang, K.; Zhang, M.; Zheng, C.-J.; Sun, D.-M.; Dai, G.-L.; Fan, X.-C.; Wang, D.-Q.; Liu, W.; et al. Thermally Activated Delayed Fluorescence Carbonyl Derivatives for Organic Light-Emitting Diodes with Extremely Narrow Full Width at Half-Maximum. ACS Appl. Mater. Interfaces 2019, 11, 13472–13480. [Google Scholar] [CrossRef] [PubMed]
- Nakanotan, H.; Tsuchiya, Y.; Adachi, C. Thermally-activated Delayed Fluorescence for Light-emitting Devices. Chem. Lett. 2021, 50, 938–948. [Google Scholar] [CrossRef]
- Chan, C.-Y.; Tanaka, M.; Lee, Y.-T.; Wong, Y.-W.; Nakanotani, H.; Hatakeyama, T.; Adachi, C. Stable pure-blue hyperfluorescence organic light-emitting diodes with high-efficiency and narrow emission. Nat. Photonics 2021, 15, 203–207. [Google Scholar] [CrossRef]
- Sharma, N.; Wong, M.Y.; Samuel, I.D.W.; Zysman-Colman, E. Solution-Processed TADF Materials and Devices Based on Organic Emitters. In Highly Efficient OLEDs—Materials Based on Thermally Activated Delayed Fluorescence; Yersin, H., Ed.; Wiley-VCH: Weinheim, Germany, 2019; pp. 501–541. [Google Scholar]
- Wong, M.Y.; Zysman-Colman, E. Purely Organic Thermally Activated Delayed Fluorescence Materials for Organic Light-Emitting Diodes. Adv. Mater. 2017, 29, 1605444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, X.-K.; Kim, D.; Brédas, J.-L. Thermally Activated Delayed Fluorescence (TADF) Path toward Efficient Electroluminescence in Purely Organic Materials: Molecular Level Insight. Acc. Chem. Res. 2018, 51, 2215–2224. [Google Scholar] [CrossRef] [PubMed]
- Sommer, G.A.; Mataranga-Popa, L.N.; Czerwieniec, R.; Hofbeck, T.; Homeier, H.H.H.; Müller, T.J.J.; Yersin, H. Design of Conformationally Distorted Donor–Acceptor Dyads Showing Efficient Thermally Activated Delayed Fluorescence. J. Phys. Chem. Lett. 2018, 9, 3692–3697. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, T.D.; Brütting, W. Efficiency Enhancement of Organic Light-Emitting Diodes Exhibiting Delayed Fluorescence and Nonisotropic Emitter Orientation. In Highly Efficient OLEDs—Materials Based on Thermally Activated Delayed Fluorescence; Yersin, H., Ed.; Wiley-VCH: Weinheim, Germany, 2019; pp. 199–228. [Google Scholar]
- Liu, Y.; Li, C.; Ren, Z.; Yan, S.; Bryce, M.R. All-organic thermally activated delayed fluorescence materials for organic light-emitting diodes. Nat. Rev. Mater. 2018, 3, 18020. [Google Scholar] [CrossRef]
- Yersin, H.; Mataranga-Popa, L.; Czerwieniec, R.; Dovbii, Y. Design of a New Mechanism beyond Thermally Activated Delayed Fluorescence toward Fourth Generation Organic Light Emitting Diodes. Chem. Mater. 2019, 31, 6110–6116. [Google Scholar] [CrossRef]
- Yersin, H.; Mataranga-Popa, L.; Li, S.-W.; Czerwieniec, R. Design strategies for materials showing thermally activated delayed fluorescence and beyond: Towards the fourth-generation OLED mechanism. J. Soc. Inf. Disp. 2018, 26, 194–199. [Google Scholar] [CrossRef]
- Zink, D.M.; Bächle, M.; Baumann, T.; Nieger, M.; Kühn, M.; Wang, C.; Klopper, W.; Monkowius, U.; Hofbeck, T.; Yersin, H.; et al. Synthesis, structure, and characterization of dinuclear copper(I) halide complexes with P^N ligands featuring exciting photoluminescence properties. Inorg. Chem. 2013, 52, 2292–2305. [Google Scholar] [CrossRef]
- Hofbeck, T.; Monkowius, U.; Yersin, H. Highly efficient luminescence of Cu(I) compounds: Thermally activated delayed fluorescence combined with short-lived phosphorescence. J. Am. Chem. Soc. 2015, 137, 399–404. [Google Scholar] [CrossRef]
- Hofbeck, T.; Niehaus, T.A.; Fleck, M.; Monkowius, U.; Yersin, H. P∩N Bridged Cu(I) Dimers Featuring Both TADF and Phosphorescence. From Overview towards Detailed Case Study of the Excited Singlet and Triplet States. Molecules 2021, 26, 3415. [Google Scholar] [CrossRef]
- Schinabeck, A.; Leitl, M.J.; Yersin, H. Dinuclear Cu(I) Complex with Combined Bright TADF and Phosphorescence. Zero-Field Splitting and Spin-Lattice Relaxation Effects of the Triplet State. J. Phys. Chem. Lett. 2018, 9, 2848–2856. [Google Scholar] [CrossRef]
- Schinabeck, A.; Rau, N.; Klein, M.; Sundermeyer, J.; Yersin, H. Deep blue emitting Cu(I) tripod complexes. Design of high quantum yield materials showing TADF-assisted phosphorescence. Dalton Trans. 2018, 47, 17067–17076. [Google Scholar] [CrossRef]
- Leitl, M.J.; Küchle, F.-R.; Mayer, H.A.; Wesemann, L.; Yersin, H. Brightly blue and green emitting Cu(I) dimers for singlet harvesting in OLEDs. J. Phys. Chem. A 2013, 117, 11823–11836. [Google Scholar] [CrossRef]
- Gneuß, T.; Leitl, M.J.; Finger, L.H.; Rau, N.; Yersin, H.; Sundermeyer, J. A new class of luminescent Cu(I) complexes with tripodal ligands—TADF emitters for the yellow to red color range. Dalton Trans. 2015, 44, 8506–8520. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, X.-L.; Yu, R.; Wu, X.-Y.; Liang, D.; Jia, J.-H.; Lu, C.-Z. A strongly greenish-blue-emitting Cu4Cl4 cluster with an efficient spin-orbit coupling (SOC): Fast phosphorescence versus thermally activated delayed fluorescence. Chem. Commun. 2016, 52, 6288–6291. [Google Scholar] [CrossRef] [PubMed]
- Baranov, A.Y.; Berezin, A.S.; Samsonenko, D.G.; Mazur, A.S.; Tolstoy, P.M.; Plyusnin, V.F.; Kolesnikov, I.E.; Artem’ev, A.V. New Cu(I) halide complexes showing TADF combined with room temperature phosphorescence: The balance tuned by halogens. Dalton Trans. 2020, 49, 3155–3163. [Google Scholar] [CrossRef]
- Li, C.; Li, W.; Henwood, A.F.; Hall, D.; Cordes, D.B.; Slawin, A.M.Z.; Lemaur, V.; Olivier, Y.; Samuel, I.D.W.; Zysman-Colman, E. Luminescent Dinuclear Copper(I) Complexes Bearing an Imidazolylpyrimidine Bridging Ligand. Inorg. Chem. 2020, 59, 14772–14784. [Google Scholar] [CrossRef]
- Hobbollahi, E.; List, M.; Hupp, B.; Mohr, F.; Berger, R.J.F.; Steffen, A.; Monkowius, U. Highly efficient cold-white light emission in a Au2CuCl2(P∩N)2PF6 type salt. Dalton Trans. 2017, 46, 3438–3442. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, K.; Shearer, J.; Catalano, V.J. Subtle Modulation of Cu4X4L2 Phosphine Cluster Cores Leads to Changes in Luminescence. Inorg. Chem. 2015, 54, 6245–6256. [Google Scholar] [CrossRef]
- Artem'ev, A.V.; Shafikov, M.Z.; Schinabeck, A.; Antonova, O.V.; Berezin, A.S.; Bagryanskaya, I.Y.; Plusnin, P.E.; Yersin, H. Sky-blue thermally activated delayed fluorescence (TADF) based on Ag(I) complexes: Strong solvation-induced emission enhancement. Inorg. Chem. Front. 2019, 6, 3168–3176. [Google Scholar] [CrossRef]
- Artem’ev, A.V.; Davydova, M.P.; Berezin, A.S.; Ryzhikov, M.R.; Samsonenko, D.G. Dicopper(I) Paddle-Wheel Complexes with Thermally Activated Delayed Fluorescence Adjusted by Ancillary Ligands. Inorg. Chem. 2020, 59, 10699–10706. [Google Scholar] [CrossRef]
- Davydova, M.P.; Rakhmanova, M.I.; Bagryanskaya, I.Y.; Brylev, K.A.; Artem’ev, A.V. A 1D Coordination Polymer Based on CuI and 2-(Diphenylphosphino)Pyrimidine: Synthesis, Structure and Luminescent Properties. J. Struct. Chem. 2020, 61, 894–898. [Google Scholar] [CrossRef]
- Stoïanov, A.; Gourlaouen, C.; Vela, S.; Daniel, C. Luminescent Dinuclear Copper(I) Complexes as Potential Thermally Activated Delayed Fluorescence (TADF) Emitters: A Theoretical Study. J. Phys. Chem. A 2018, 122, 1413–1421. [Google Scholar] [CrossRef]
- Monkowius, U.; Hofbeck, T.; Yersin, H. Singulett-Harvesting mit Zweikernigen Kupfer(I)-Komplexen für Opto-Elektronische Vorrichtungen. German Patent DE102011080240 A1, 2 August 2013. [Google Scholar]
- Wallesch, M.; Verma, A.; Fléchon, C.; Flügge, H.; Zink, D.M.; Seifermann, S.M.; Navarro, J.M.; Vitova, T.; Göttlicher, J.; Steininger, R.; et al. Towards Printed Organic Light-EmittingDevices: A Solution-Stable, Highly Soluble CuI-NHetPHOS Complex for Inkjet Processing. Chemistry 2016, 22, 16400–16405. [Google Scholar] [CrossRef]
- Busch, J.M.; Koshelev, D.S.; Vashchenko, A.A.; Fuhr, O.; Nieger, M.; Utochnikova, V.V.; Bräse, S. Various Structural Design Modifications: Para-Substituted Diphenylphosphinopyridine Bridged Cu(I) Complexes in Organic Light-Emitting Diodes. Inorg. Chem. 2021, 60, 2315–2332. [Google Scholar] [CrossRef] [PubMed]
- Cheng, G.; Chow, P.-K.; Kui, S.C.F.; Kwok, C.-C.; Che, C.-M. High-Efficiency polymer light-emitting devices with robust phosphorescent platinum(II) emitters containing tetradentate dianionic O^N^C^N ligands. Adv. Mater. 2013, 25, 6765–6770. [Google Scholar] [CrossRef] [PubMed]
- Yersin, H.; Rausch, A.F.; Czerwieniec, R.; Hofbeck, T.; Fischer, T. The triplet state of organo-transition metal compounds. Triplet harvesting and singlet harvesting for efficient OLEDs. Coord. Chem. Rev. 2011, 255, 2622–2652. [Google Scholar] [CrossRef]
- Siddique, Z.A.; Yamamoto, Y.; Ohno, T.; Nozaki, K. Structure-dependent photophysical properties of singlet and triplet metal-to-ligand charge transfer states in copper(I) bis(diimine) compounds. Inorg. Chem. 2003, 42, 6366–6378. [Google Scholar] [CrossRef]
- Iwamura, M.; Takeuchi, S.; Tahara, T. Real-time observation of the photoinduced structural change of bis(2,9-dimethyl-1,10-phenanthroline)copper(I) by femtosecond fluorescence spectroscopy: A realistic potential curve of the Jahn-Teller distortion. J. Am. Chem. Soc. 2007, 129, 5248–5256. [Google Scholar] [CrossRef]
- Chen, L.X.; Shaw, G.B.; Novozhilova, I.; Liu, T.; Jennings, G.; Attenkofer, K.; Meyer, G.J.; Coppens, P. MLCT state structure and dynamics of a copper(I) diimine complex characterized by pump-probe X-ray and laser spectroscopies and DFT calculations. J. Am. Chem. Soc. 2003, 125, 7022–7034. [Google Scholar] [CrossRef]
- Turro, N.J. Modern Molecular Photochemistry of Organic Molecules; Benjamin/Cummings: Melon Park, CA, USA, 1978. [Google Scholar]
- Zhang, Q.; Komino, T.; Huang, S.; Matsunami, S.; Goushi, K.; Adachi, C. Triplet Exciton Confinement in Green Organic Light-Emitting Diodes Containing Luminescent Charge-Transfer Cu(I) Complexes. Adv. Funct. Mater. 2012, 22, 2327–2336. [Google Scholar] [CrossRef]
- Teng, T.; Xiong, J.; Cheng, G.; Zhou, C.; Lv, X.; Li, K. Solution-Processed OLEDs Based on Thermally Activated Delayed Fluorescence Copper(I) Complexes with Intraligand Charge-Transfer Excited State. Molecules 2021, 26, 1125. [Google Scholar] [CrossRef]
- Klein, M.; Rau, N.; Wende, M.; Sundermeyer, J.; Cheng, G.; Che, C.-M.; Schinabeck, A.; Yersin, H. Cu(I) and Ag(I) Complexes with a New Type of Rigid Tridentate N,P,P-Ligand for Thermally Activated Delayed Fluorescence and OLEDs with High External Quantum Efficiency. Chem. Mater. 2020, 32, 10365–10382. [Google Scholar] [CrossRef]
- Son, K.S.; Yahiro, M.; Imai, T.; Yoshizaki, H.; Adachi, C. Analyzing Bipolar Carrier Transport Characteristics of Diarylamino-Substituted Heterocyclic Compounds in Organic Light-Emitting Diodes by Probing Electroluminescence Spectra. Chem. Mater. 2008, 20, 4439–4446. [Google Scholar] [CrossRef]
- Anthopoulos, T.D.; Markham, J.P.J.; Namdas, E.B.; Samuel, I.D.W. Highly effcient single-layer dendrimer light-emitting diodes with balanced charge transport. Appl. Phys. Lett. 2003, 82, 4824–4826. [Google Scholar] [CrossRef] [Green Version]
- Sharma, G.; Hashmi, S.Z.; Kuma, U.; Kattayat, S.; Ahmad, M.A.; Kumar, S.; Dalela, S.; Alvi, P.A. Optical and electronic characteristics of ITO/NPB/Alq3:DCJTB/ Alq3/Ag heterostructure based organic light emitting diode. Optik 2020, 223, 165572. [Google Scholar] [CrossRef]
- Sworakowski, J.; Lipiński, J.; Janus, K. On the reliability of determination of energies of HOMO and LUMO levels in organic semiconductors from electrochemical measurements. A simple picture based on the electrostatic model. Org. Electron. 2016, 33, 300–310. [Google Scholar] [CrossRef]
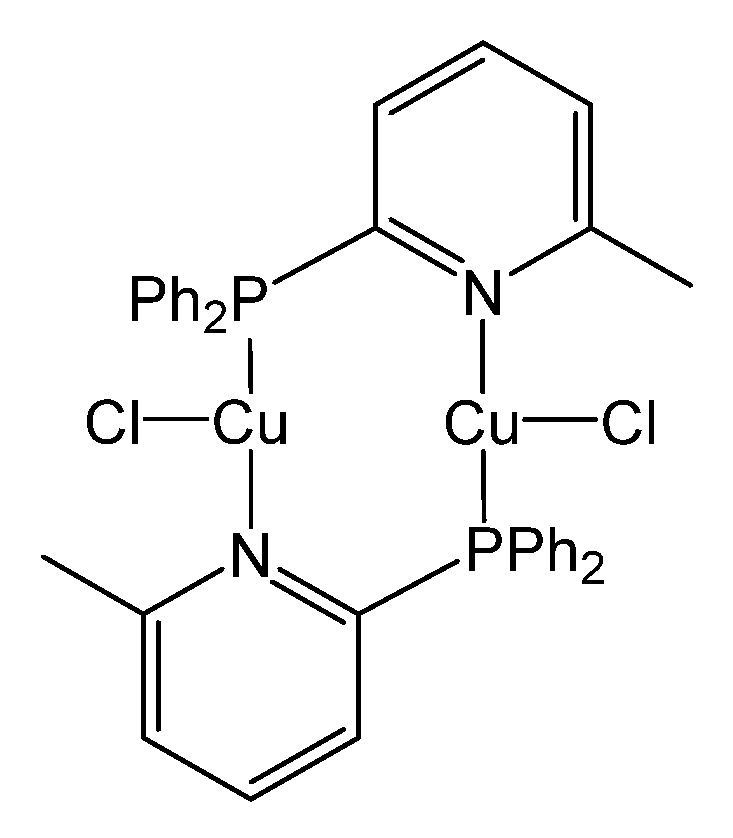

| Photophys. Data | Neat Powder (a) | Doped in PYD2 (b) | Doped in mCP (b),(c) | Doped in PVK (b),(c) | Doped in TCTA (b),(c) | Doped in CBP (b),(c) |
|---|---|---|---|---|---|---|
| λmax (d) | 485 nm | 544 nm | 535 nm | 545 nm | 542 nm | 537 nm |
| φPL (e) | 92% | 27% | 20% | 11% | 10% | 13% |
| τ (e) | 8.3 µs | 3.1 µs | 5.5 µs | 3.2 µs | 4.3 µs | 2.8 µs |
| ∆(S1 − T1) (f) | 930 cm−1 (115 meV) |
| Concentration (wt %) | L (a) (cd m−2) | CE (b) (cd A−1) | PE (c) (lm W−1) | EQE (d) (%) | CIE (e) (x, y) | FWHM (f) (nm) | CRI (g) | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Max | at 1000 cd m−2 | Max | at 1000 cd m−2 | Max | at 1000 cd m−2 | |||||
| 2 | 1160 | 6.48 | 3.54 | 3.39 | 1.48 | 2.61 | 1.42 | 0.38, 0.45 | 162 | 72 |
| 4 | 1880 | 8.39 | 6.24 | 4.01 | 2.71 | 3.14 | 2.31 | 0.38, 0.48 | 159 | 69 |
| 8 | 2500 | 10.5 | 8.15 | 4.25 | 3.20 | 3.80 | 2.95 | 0.38, 0.49 | 153 | 64 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, G.; Zhou, D.; Monkowius, U.; Yersin, H. Fabrication of a Solution-Processed White Light Emitting Diode Containing a Single Dimeric Copper(I) Emitter Featuring Combined TADF and Phosphorescence. Micromachines 2021, 12, 1500. https://doi.org/10.3390/mi12121500
Cheng G, Zhou D, Monkowius U, Yersin H. Fabrication of a Solution-Processed White Light Emitting Diode Containing a Single Dimeric Copper(I) Emitter Featuring Combined TADF and Phosphorescence. Micromachines. 2021; 12(12):1500. https://doi.org/10.3390/mi12121500
Chicago/Turabian StyleCheng, Gang, Dongling Zhou, Uwe Monkowius, and Hartmut Yersin. 2021. "Fabrication of a Solution-Processed White Light Emitting Diode Containing a Single Dimeric Copper(I) Emitter Featuring Combined TADF and Phosphorescence" Micromachines 12, no. 12: 1500. https://doi.org/10.3390/mi12121500






