Advanced Top-Down Fabrication for a Fused Silica Nanofluidic Device
Abstract
:1. Introduction
2. Materials and Methods
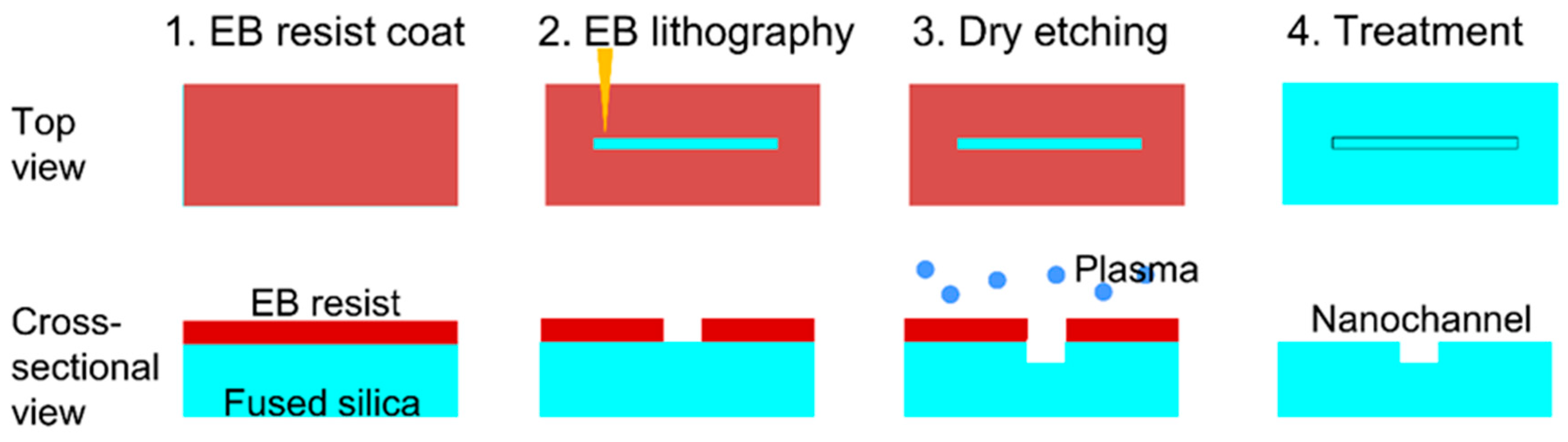
2.1. Nanochannel Fabrication
2.2. Streaming Current Measurements
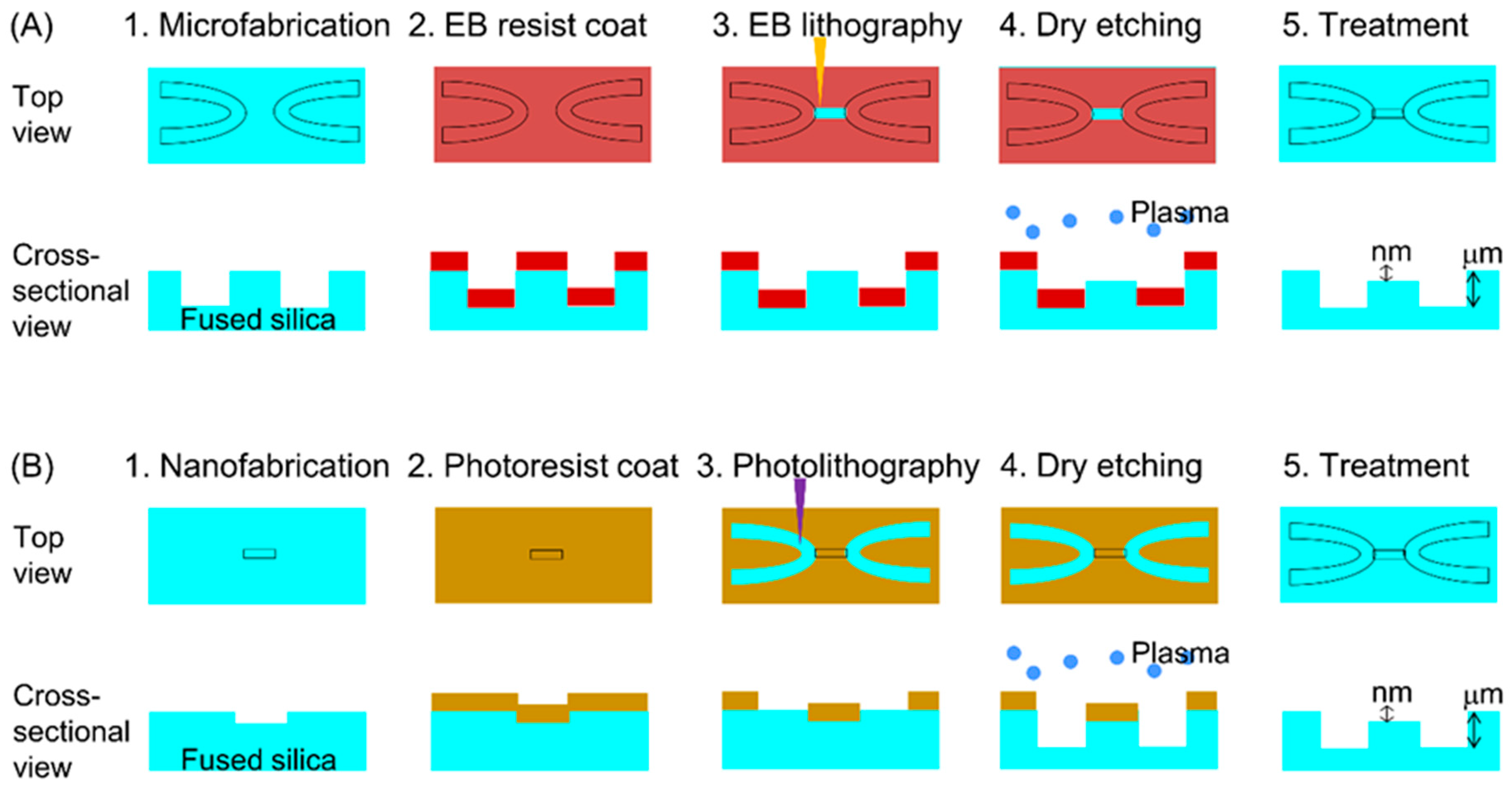
2.3. Micro-Nano Interface Fabrication
3. Results and Discussion
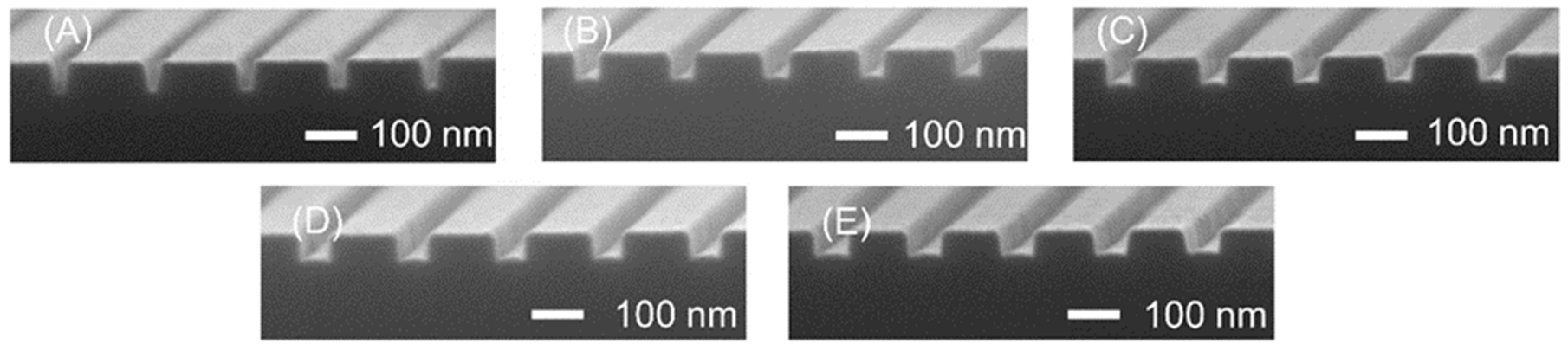
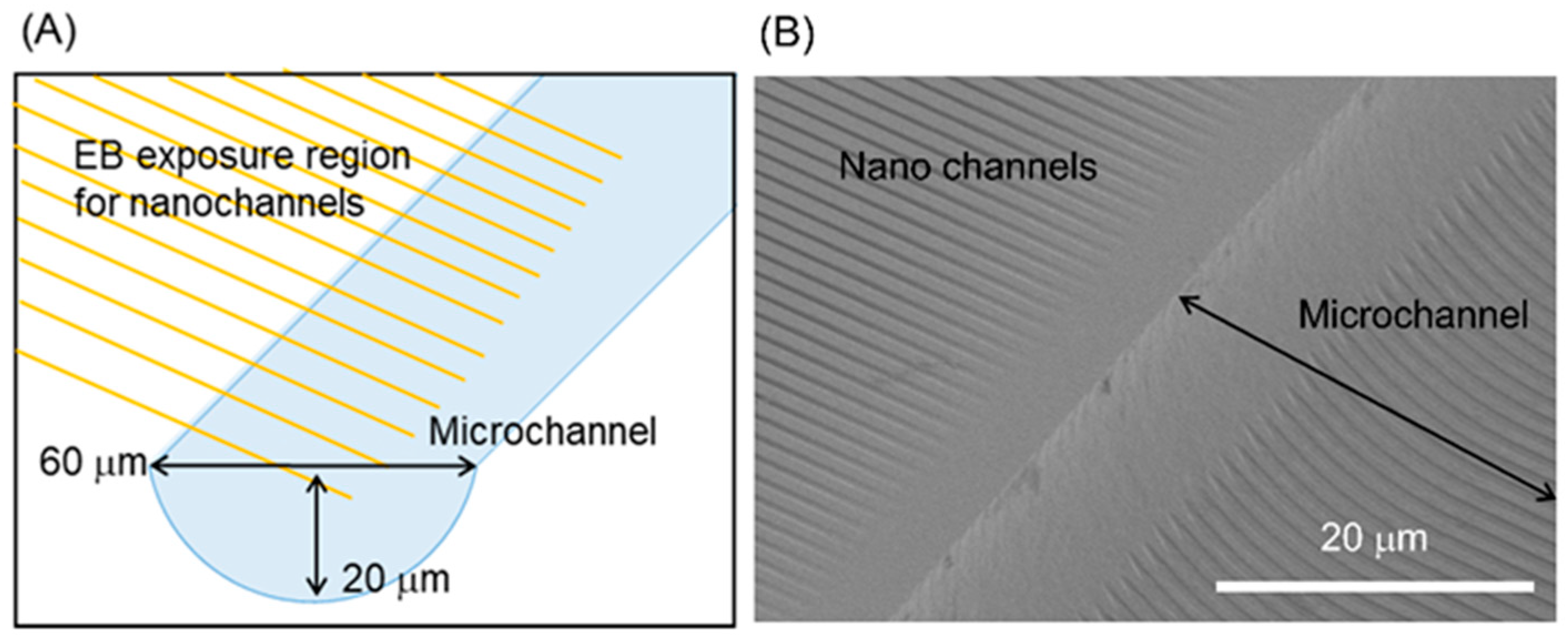
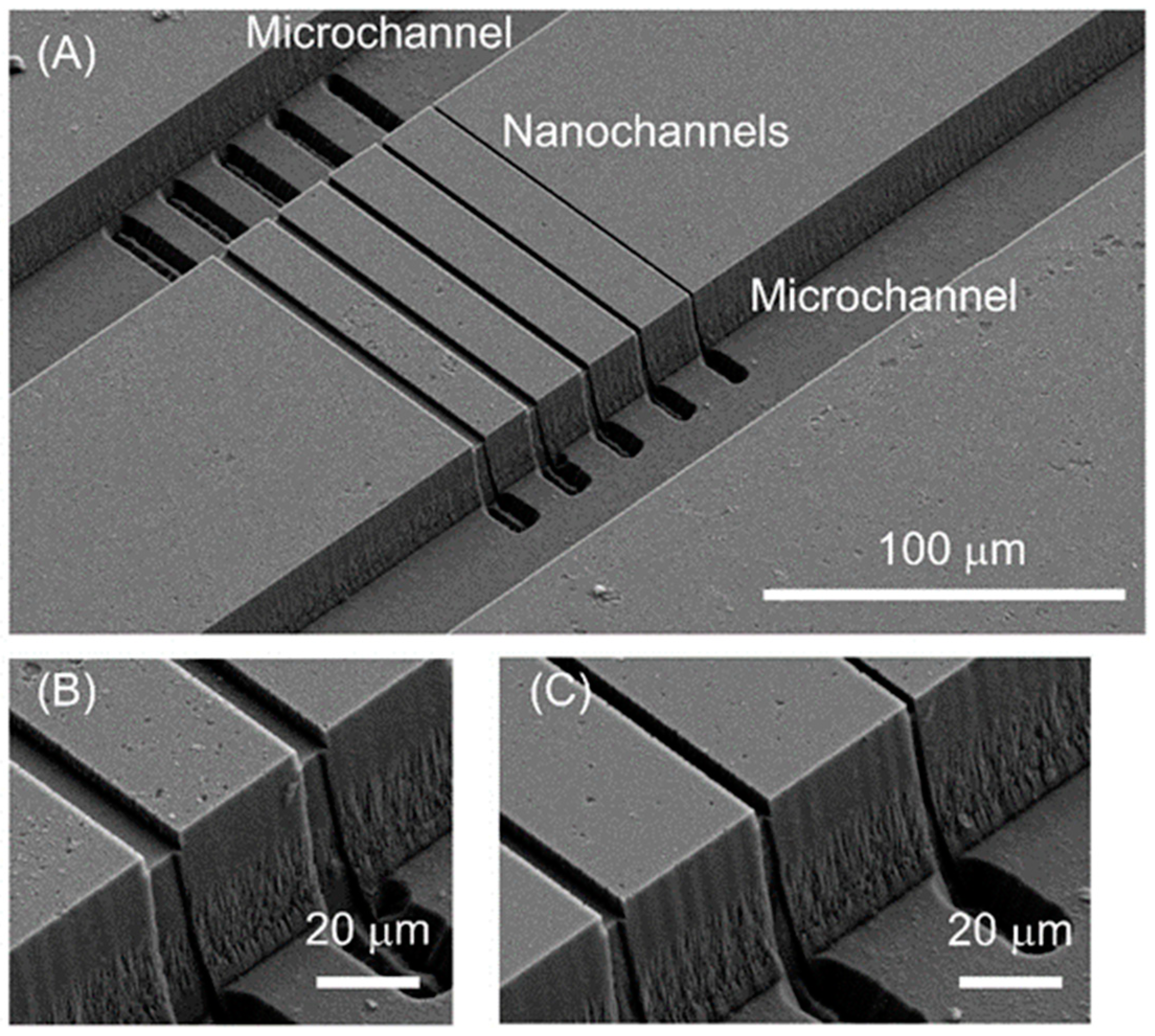
3.1. Nanochannel Fabrication
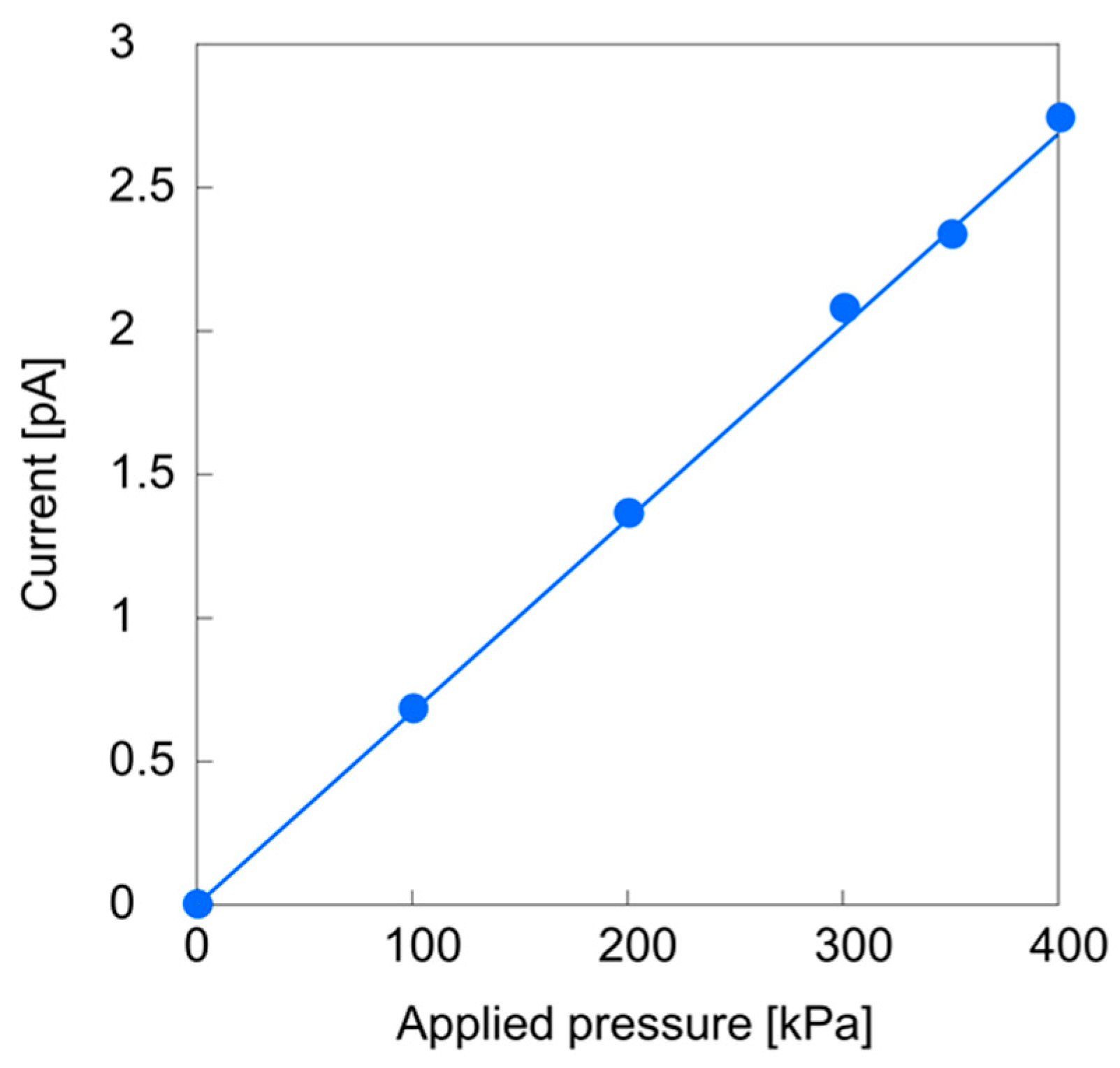
3.2. Streaming Current Measurements
3.3. Micro-Nano Interface Fabrication
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mawatari, K.; Kazoe, Y.; Shimizu, H.; Pihosh, Y.; Kitamori, T. Extended-Nanofluidics: Fundamental Technologies, Unique Liquid Properties, and Application in Chemical and Bio Analysis Methods and Devices. Anal. Chem. 2014, 86, 4068–4077. [Google Scholar] [CrossRef] [PubMed]
- Morikawa, K.; Tsukahara, T. Investigation of Unique Protonic and Hydrodynamic Behavior of Aqueous Solutions Confined in Extended Nanospaces. Isr. J. Chem. 2014, 54, 1564–1572. [Google Scholar] [CrossRef]
- Siwy, Z.S.; Howorka, S. Engineered voltage-responsive nanopores. Chem. Soc. Rev. 2010, 39, 1115–1132. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Friedman, A.K.; Baker, L.A. Nanopore Sensing. Anal. Chem. 2017, 89, 157–188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, X.; Yang, Q.; Ding, L.; Su, B. Ultrathin Silica Membranes with Highly Ordered and Perpendicular Nanochannels for Precise and Fast Molecular Separation. ACS Nano 2015, 9, 11266–11277. [Google Scholar] [CrossRef]
- Bocquet, L.; Tabeling, P. Physics and technological aspects of nanofluidics. Lab Chip 2014, 14, 3143–3158. [Google Scholar] [CrossRef]
- Siria, A.; Poncharal, P.; Biance, A.-L.; Fulcrand, R.; Blase, X.; Purcell, S.T.; Bocquet, L. Giant osmotic energy conversion measured in a single transmembrane boron nitride nanotube. Nat. Cell Biol. 2013, 494, 455–458. [Google Scholar] [CrossRef]
- Strogatz, S.H.; Abrams, D.M.; McRobie, A.; Eckhardt, B.; Ott, E. Theoretical mechanics: Crowd synchrony on the Millennium Bridge. Nat. Cell Biol. 2005, 438, 43–44. [Google Scholar] [CrossRef]
- Whitby, M.; Cagnon, L.; Thanou, M.; Quirke, N. Enhanced Fluid Flow through Nanoscale Carbon Pipes. Nano Lett. 2008, 8, 2632–2637. [Google Scholar] [CrossRef]
- Han, C.; Hou, X.; Zhang, H.; Guo, W.; Li, H.; Jiang, L. Enantioselective Recognition in Biomimetic Single Artificial Nanochannels. J. Am. Chem. Soc. 2011, 133, 7644–7647. [Google Scholar] [CrossRef]
- Kocer, A.; Tauk, L.; Déjardin, P. Nanopore sensors: From hybrid to abiotic systems. Biosens. Bioelectron. 2012, 38, 1–10. [Google Scholar] [CrossRef]
- Zhang, H.; Hou, X.; Hou, J.; Zeng, L.; Tian, Y.; Li, L.; Jiang, L. Synthetic Asymmetric-Shaped Nanodevices with Symmetric pH-Gating Characteristics. Adv. Funct. Mater. 2015, 25, 1102–1110. [Google Scholar] [CrossRef]
- Mijatovic, D.; Eijkel, J.C.T.; Berg, A.V.D. Technologies for nanofluidic systems: Top-down vs. bottom-up—A review. Lab Chip 2005, 5, 492–500. [Google Scholar] [CrossRef] [PubMed]
- Bocquet, L.; Charlaix, E. Nanofluidics, from bulk to interfaces. Chem. Soc. Rev. 2010, 39, 1073–1095. [Google Scholar] [CrossRef] [Green Version]
- Napoli, M.; Eijkel, J.C.T.; Pennathur, S. Nanofluidic technology for biomolecule applications: A critical review. Lab Chip 2010, 10, 957–985. [Google Scholar] [CrossRef] [PubMed]
- Le, T.H.H.; Shimizu, H.; Morikawa, K. Advances in Label-Free Detections for Nanofluidic Analytical Devices. Micromachines 2020, 11, 885. [Google Scholar] [CrossRef] [PubMed]
- Han, J. Separation of Long DNA Molecules in a Microfabricated Entropic Trap Array. Science 2000, 288, 1026–1029. [Google Scholar] [CrossRef]
- Reccius, C.H.; Mannion, J.T.; Cross, J.D.; Barton, R.A. Compression and Free Expansion of Single DNA Molecules in Nanochannels. Phys. Rev. Lett. 2005, 95, 268101. [Google Scholar] [CrossRef] [Green Version]
- Reisner, W.; Beech, J.P.; Larsen, N.B.; Flyvbjerg, H.; Kristensen, A.; Tegenfeldt, J.O. Nanoconfinement-Enhanced Conformational Response of Single DNA Molecules to Changes in Ionic Environment. Phys. Rev. Lett. 2007, 99, 058302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pu, Q.; Yun, J.; Temkin, A.H.; Liu, S. Ion-Enrichment and Ion-Depletion Effect of Nanochannel Structures. Nano Lett. 2004, 4, 1099–1103. [Google Scholar] [CrossRef]
- Kim, S.J.; Wang, Y.-C.; Lee, J.H.; Jang, H.; Han, J. Concentration Polarization and Nonlinear Electrokinetic Flow near a Nanofluidic Channel. Phys. Rev. Lett. 2007, 99, 044501. [Google Scholar] [CrossRef] [Green Version]
- Kwak, R.; Pham, V.S.; Kim, B.; Chen, L.; Han, J. Enhanced Salt Removal by Unipolar Ion Conduction in Ion Concentration Polarization Desalination. Sci. Rep. 2016, 6, 25349. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, Z.; Bu, Y.; Yobas, L. Conductance Interplay in Ion Concentration Polarization across 1D Nanochannels: Microchannel Surface Shunt and Nanochannel Conductance. Anal. Chem. 2019, 92, 1252–1259. [Google Scholar] [CrossRef]
- Karnik, R.; Duan, C.; Castelino, K.; Daiguji, A.H.; Majumdar, A. Rectification of Ionic Current in a Nanofluidic Diode. Nano Lett. 2007, 7, 547–551. [Google Scholar] [CrossRef]
- Hou, X.; Yang, F.; Li, L.; Song, Y.; Jiang, L.; Zhu, D. A Biomimetic Asymmetric Responsive Single Nanochannel. J. Am. Chem. Soc. 2010, 132, 11736–11742. [Google Scholar] [CrossRef]
- Perry, J.M.; Zhou, K.; Harms, Z.D.; Jacobson, S.C. Ion Transport in Nanofluidic Funnels. ACS Nano 2010, 4, 3897–3902. [Google Scholar] [CrossRef]
- Karnik, R.; Castelino, K.; Fan, R.; Yang, P.; Majumdar, A. Effects of Biological Reactions and Modifications on Conductance of Nanofluidic Channels. Nano Lett. 2005, 5, 1638–1642. [Google Scholar] [CrossRef]
- Schoch, R.B.; Cheow, L.F.; Han, J. Electrical Detection of Fast Reaction Kinetics in Nanochannels with an Induced Flow. Nano Lett. 2007, 7, 3895–3900. [Google Scholar] [CrossRef] [Green Version]
- Durand, N.F.Y.; Renaud, P. Label-free determination of protein–surface interaction kinetics by ionic conductance inside a nanochannel. Lab Chip 2009, 9, 319–324. [Google Scholar] [CrossRef] [PubMed]
- Tokeshi, M.; Minagawa, T.; Uchiyama, K.; Hibara, A.; Sato, K.; Hisamoto, H.; Kitamori, T. Continuous-Flow Chemical Processing on a Microchip by Combining Microunit Operations and a Multiphase Flow Network tion of complicated chemical processing on a microchip. Anal. Chem. 2002, 74, 1565–1571. [Google Scholar] [CrossRef]
- Shirai, K.; Mawatari, K.; Ohta, R.; Shimizu, H.; Kitamori, T. A single-molecule ELISA device utilizing nanofluidics. Analyst 2018, 143, 943–948. [Google Scholar] [CrossRef]
- Ishibashi, R.; Mawatari, K.; Kitamori, T. Highly Efficient and Ultra-small Volume Separation by Pressure-Driven Liquid Chromatography in Extended Nanochannels. Small 2012, 8, 1237–1242. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, K.; Morikawa, K.; Imanaka, H.; Imamura, K.; Kitamori, T. Picoliter enzyme reactor on a nanofluidic device exceeding the bulk reaction rate. Analyst 2020, 145, 5801–5807. [Google Scholar] [CrossRef]
- Nakao, T.; Kazoe, Y.; Mori, E.; Morikawa, K.; Fukasawa, T.; Yoshizaki, A.; Kitamori, T. Cytokine analysis on a countable number of molecules from living single cells on nanofluidic devices. Analyst 2019, 144, 7200–7208. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Mawatari, K.; Morikawa, K.; Kitamori, T. Living Single Cell Analysis Platform Utilizing Microchannel, Single Cell Chamber, and Extended-nano Channel. Anal. Sci. 2016, 32, 75–78. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Kazoe, Y.; Mawatari, K.; Sugii, Y.; Kitamori, T. Viscosity and Wetting Property of Water Confined in Extended Nanospace Simultaneously Measured from Highly-Pressurized Meniscus Motion. J. Phys. Chem. Lett. 2012, 3, 2447–2452. [Google Scholar] [CrossRef]
- Morikawa, K.; Mawatari, K.; Kato, M.; Tsukahara, T.; Kitamori, T. Streaming potential/current measurement system for investigation of liquids confined in extended-nanospace. Lab Chip 2010, 10, 871–875. [Google Scholar] [CrossRef]
- Morikawa, K.; Matsushita, K.; Tsukahara, T. Rapid Plasma Etching for Fabricating Fused Silica Microchannels. Anal. Sci. 2017, 33, 1453–1456. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abad, E.; Merino, S.; Retolaza, A.; Juarros, A. Design and fabrication using nanoimprint lithography of a nanofluidic device for DNA stretching applications. Microelectron. Eng. 2008, 85, 818–821. [Google Scholar] [CrossRef]
- Peng, R.; Li, D. Electroosmotic flow in single PDMS nanochannels. Nanoscale 2016, 8, 12237–12246. [Google Scholar] [CrossRef] [Green Version]
- Peng, R.; Li, D. Fabrication of polydimethylsiloxane (PDMS) nanofluidic chips with controllable channel size and spacing. Lab Chip 2016, 16, 3767–3776. [Google Scholar] [CrossRef]
- Park, Y.-S.; Oh, J.M.; Cho, Y.-K. Non-lithographic nanofluidic channels with precisely controlled circular cross sections. RSC Adv. 2018, 8, 19651–19658. [Google Scholar] [CrossRef] [Green Version]
- Fernandez-Cuesta, I.; Palmarelli, A.L.; Liang, X.; Zhang, J.; Dhuey, S.; Olynick, D.L.; Cabrini, S. Fabrication of fluidic devices with 30 nm nanochannels by direct imprinting. J. Vac. Sci. Technol. B 2011, 29, 06F801. [Google Scholar] [CrossRef] [Green Version]
- Jeong, J.W.; Yang, S.R.; Hur, Y.H.; Kim, S.W.; Baek, K.M.; Yim, S.; Jang, H.-I.; Park, J.H.; Lee, S.Y.; Park, C.-O.; et al. High-resolution nanotransfer printing applicable to diverse surfaces via interface-targeted adhesion switching. Nat. Commun. 2014, 5, 5387. [Google Scholar] [CrossRef] [Green Version]
- Cao, H.; Yu, Z.; Wang, J.; Tegenfeldt, J.O.; Austin, R.H.; Chen, E.; Wu, W.; Chou, S.Y. Fabrication of 10 nm enclosed nanofluidic channels. Appl. Phys. Lett. 2002, 81, 174–176. [Google Scholar] [CrossRef] [Green Version]
- Nam, S.-W.; Lee, M.-H.; Lee, S.-H.; Lee, D.-J.; Rossnagel, S.M.; Kim, K.-B. Sub-10-nm Nanochannels by Self-Sealing and Self-Limiting Atomic Layer Deposition. Nano Lett. 2010, 10, 3324–3329. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yobas, L. Slowing DNA Translocation in a Nanofluidic Field-Effect Transistor. ACS Nano 2016, 10, 3985–3994. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.-H.; Lee, H.; Jin, T.; Park, S.; Yoon, B.J.; Sung, G.Y.; Kim, K.-B.; Kim, S.J. Sub-10 nm transparent all-around-gated ambipolar ionic field effect transistor. Nanoscale 2015, 7, 936–946. [Google Scholar] [CrossRef] [Green Version]
- Menard, L.D.; Ramsey, J.M. Fabrication of Sub-5 nm Nanochannels in Insulating Substrates Using Focused Ion Beam Milling. Nano Lett. 2011, 11, 512–517. [Google Scholar] [CrossRef] [Green Version]
- Menard, L.; Zhou, J.; Woodson, M.; Mair, C.; Alarie, J.; Ramsey, J. Focused Ion Beam Milled Nanochannels for Confinement Enabled DNA Single-Molecule Studies. Microsc. Microanal. 2012, 18, 606–607. [Google Scholar] [CrossRef]
- Fang, F.; He, Y.-Q.; Tian, L.; Li, Y.-Y.; Wu, Z.-Y. Making of a single solid-state nanopore on the wall of fused silica capillary. R. Soc. Open Sci. 2018, 5, 171633. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Mei, Y.; Xia, R. A New Wetting Mechanism Based upon Triple Contact Line Pinning. Langmuir 2011, 27, 196–200. [Google Scholar] [CrossRef] [PubMed]
- Forsberg, P.S.H.; Priest, C.; Brinkmann, M.; Sedev, R.; Ralston, J. Contact Line Pinning on Microstructured Surfaces for Liquids in the Wenzel State. Langmuir 2010, 26, 860–865. [Google Scholar] [CrossRef]
- Semprebon, C.; Forsberg, P.; Priest, C.; Brinkmann, M. Pinning and wicking in regular pillar arrays. Soft Matter 2014, 10, 5739–5748. [Google Scholar] [CrossRef]
- Gumuscu, B.; Bomer, J.G.; Berg, A.V.D.; Eijkel, J.C.T. Large scale patterning of hydrogel microarrays using capillary pinning. Lab Chip 2015, 15, 664–667. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sano, H.; Kazoe, Y.; Morikawa, K.; Kitamori, T. Implementation of a nanochannel open/close valve into a glass nanofluidic device. Microfluid. Nanofluidics 2020, 24, 1–11. [Google Scholar] [CrossRef]

| KMPR Flat | KMPR Channel | KMPR HDMS Flat | KMPR HDMS Channel |
|---|---|---|---|
| 105° ± 12° | 134° ± 19° | 91° ± 5° | 85° ± 5° |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morikawa, K.; Kazoe, Y.; Takagi, Y.; Tsuyama, Y.; Pihosh, Y.; Tsukahara, T.; Kitamori, T. Advanced Top-Down Fabrication for a Fused Silica Nanofluidic Device. Micromachines 2020, 11, 995. https://doi.org/10.3390/mi11110995
Morikawa K, Kazoe Y, Takagi Y, Tsuyama Y, Pihosh Y, Tsukahara T, Kitamori T. Advanced Top-Down Fabrication for a Fused Silica Nanofluidic Device. Micromachines. 2020; 11(11):995. https://doi.org/10.3390/mi11110995
Chicago/Turabian StyleMorikawa, Kyojiro, Yutaka Kazoe, Yuto Takagi, Yoshiyuki Tsuyama, Yuriy Pihosh, Takehiko Tsukahara, and Takehiko Kitamori. 2020. "Advanced Top-Down Fabrication for a Fused Silica Nanofluidic Device" Micromachines 11, no. 11: 995. https://doi.org/10.3390/mi11110995





