Microfluidic Passive Flow Regulatory Device with an Integrated Check Valve for Enhanced Flow Control
Abstract
:1. Introduction
2. Materials and Methods
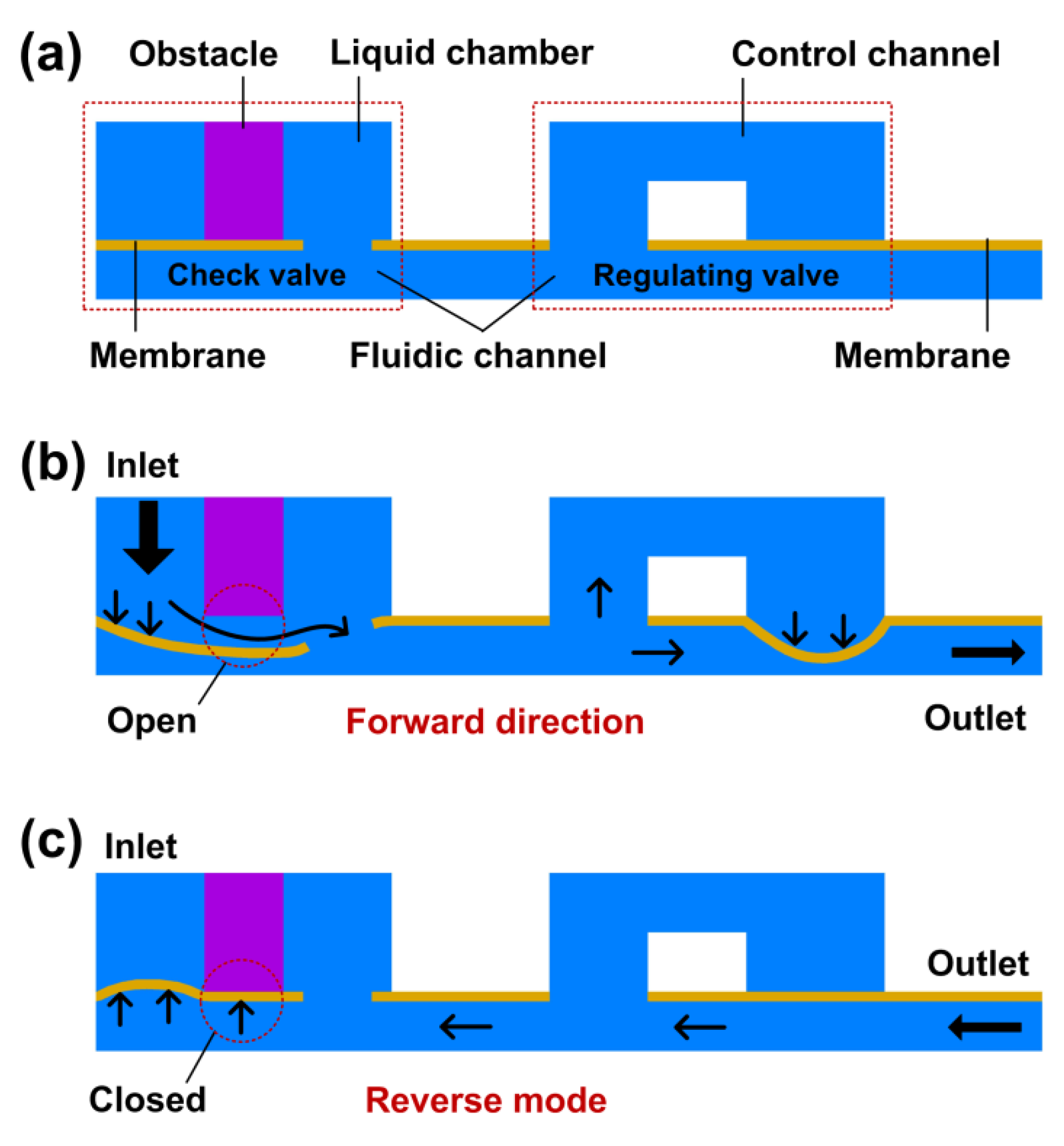
2.1. The Working Principle of the Device
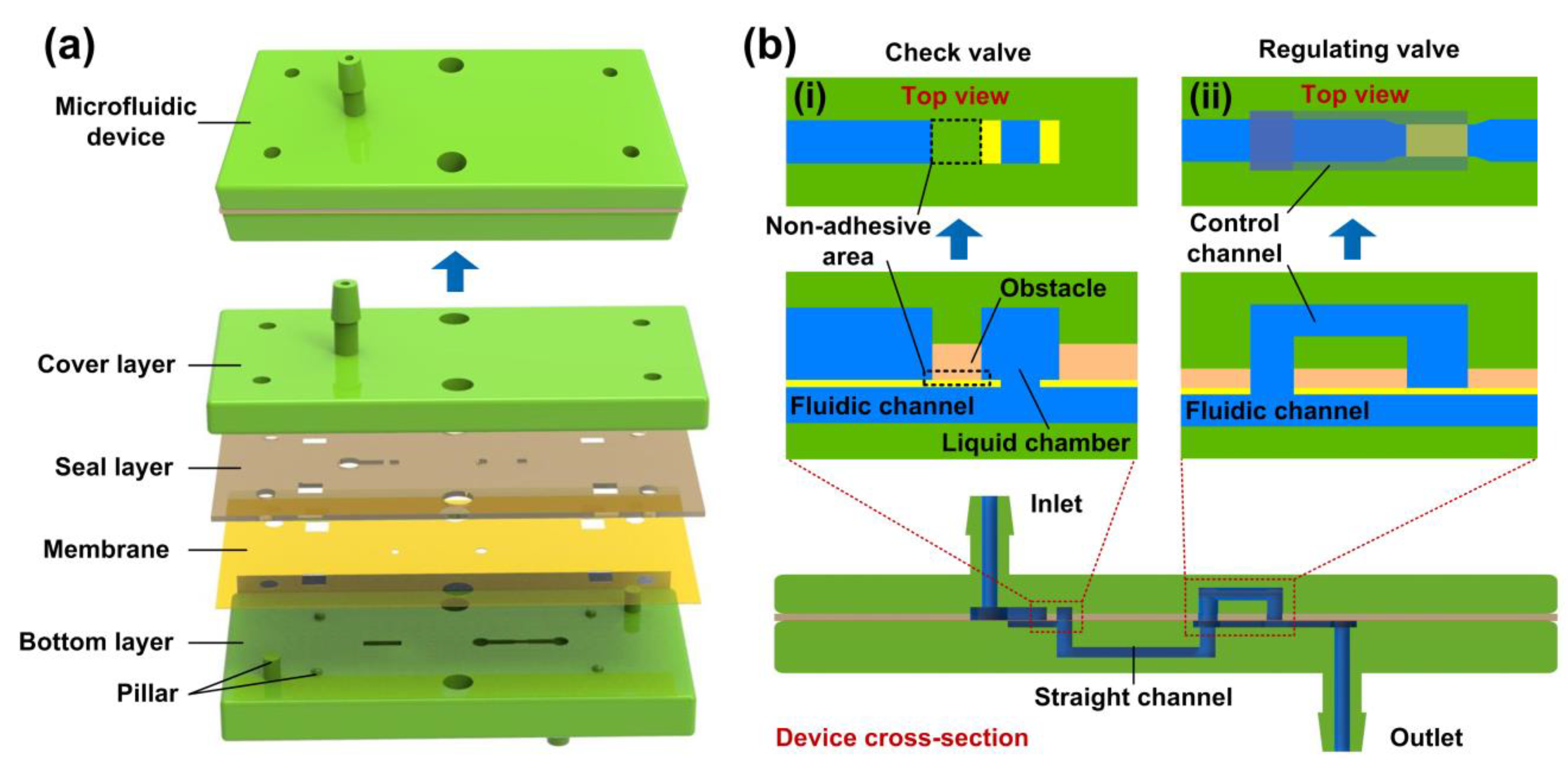
2.2. Device Design
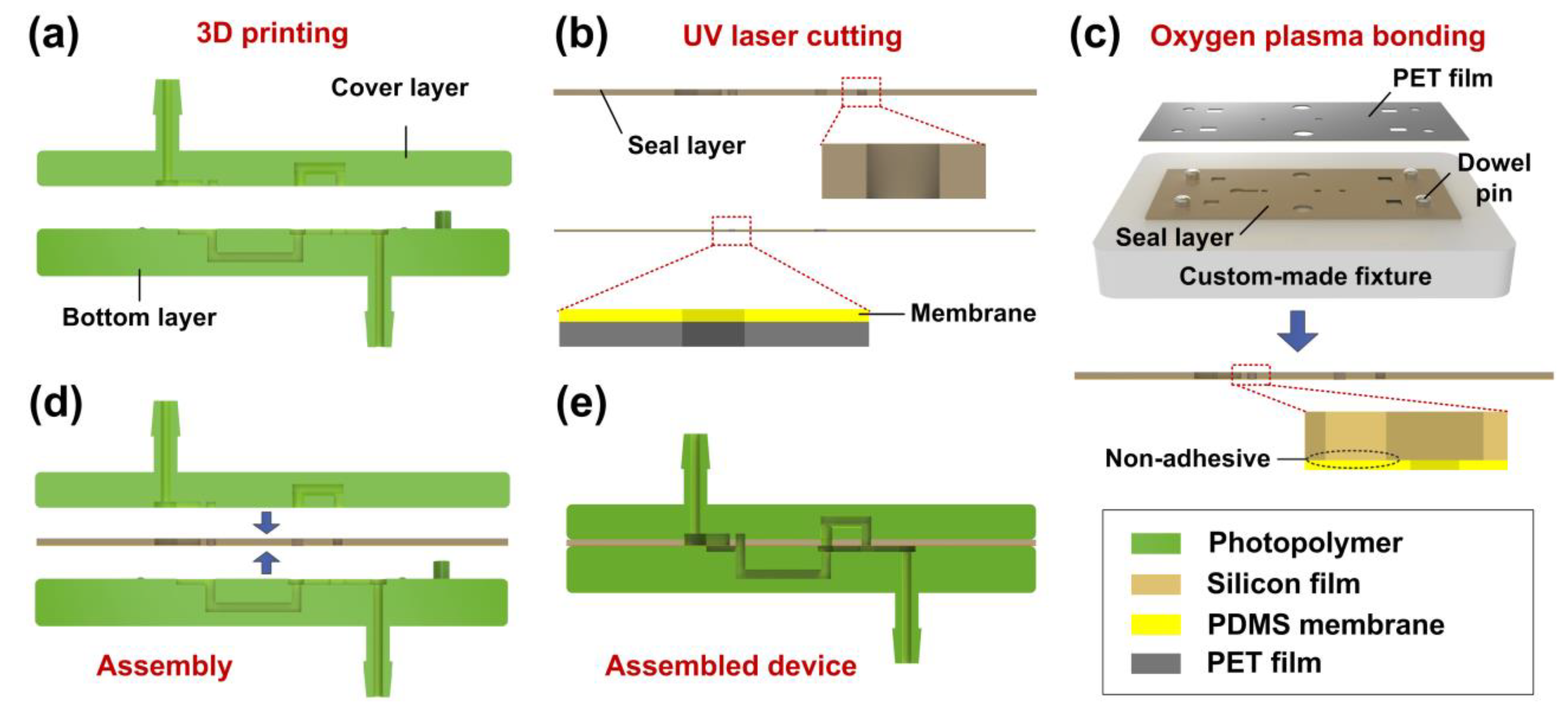
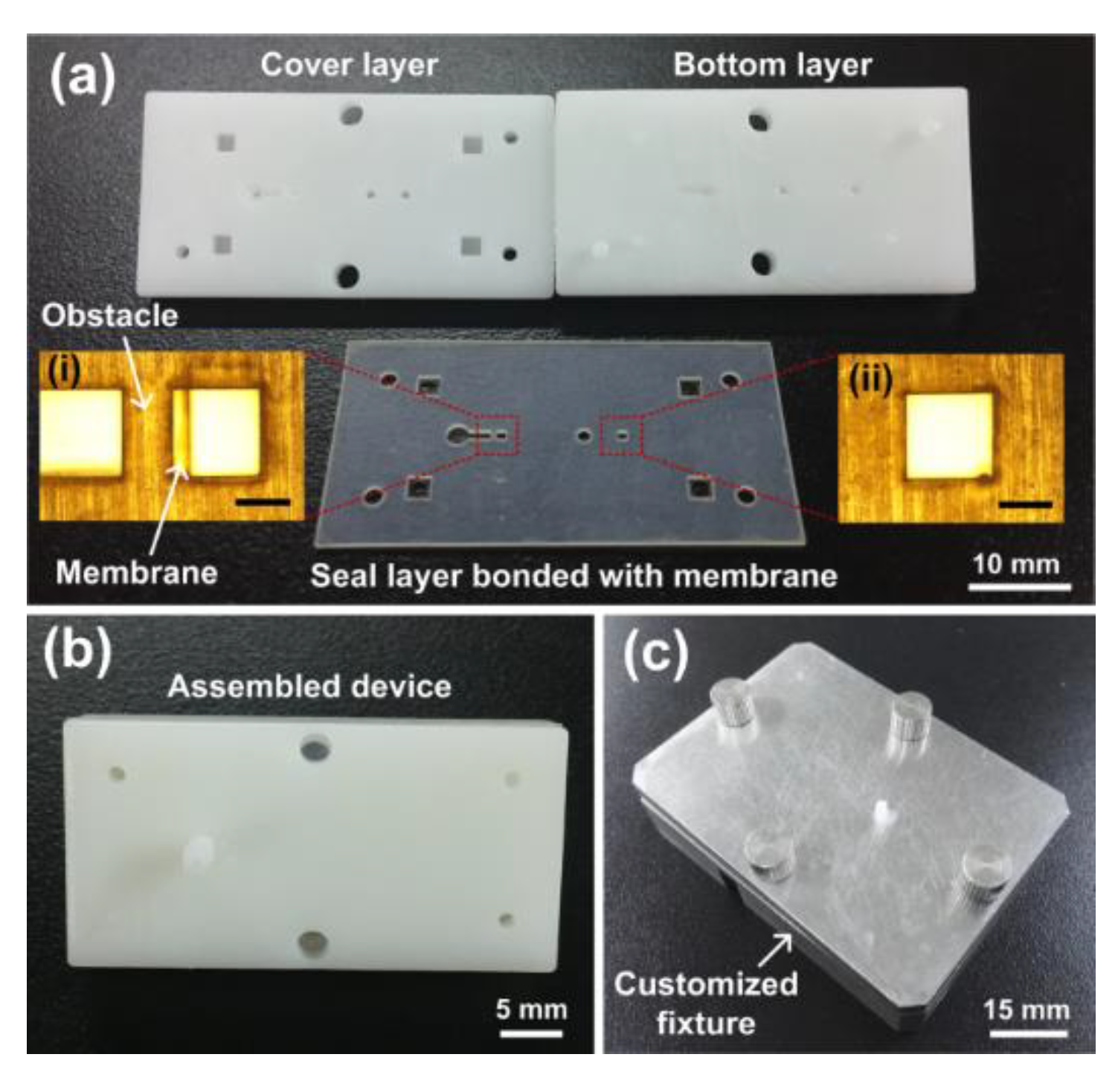
2.3. Device Fabrication
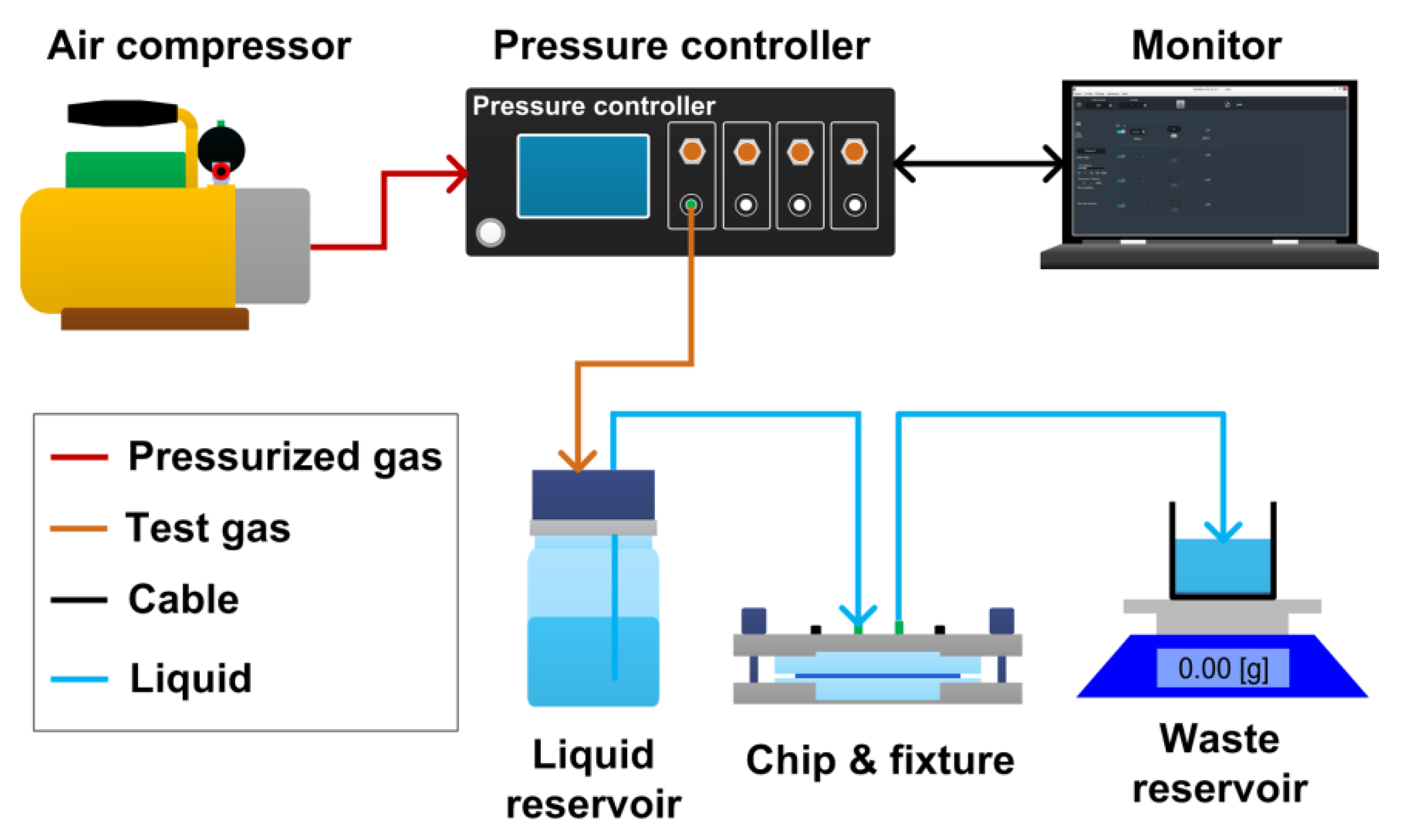
2.4. Experimental Setup
3. Results and discussion
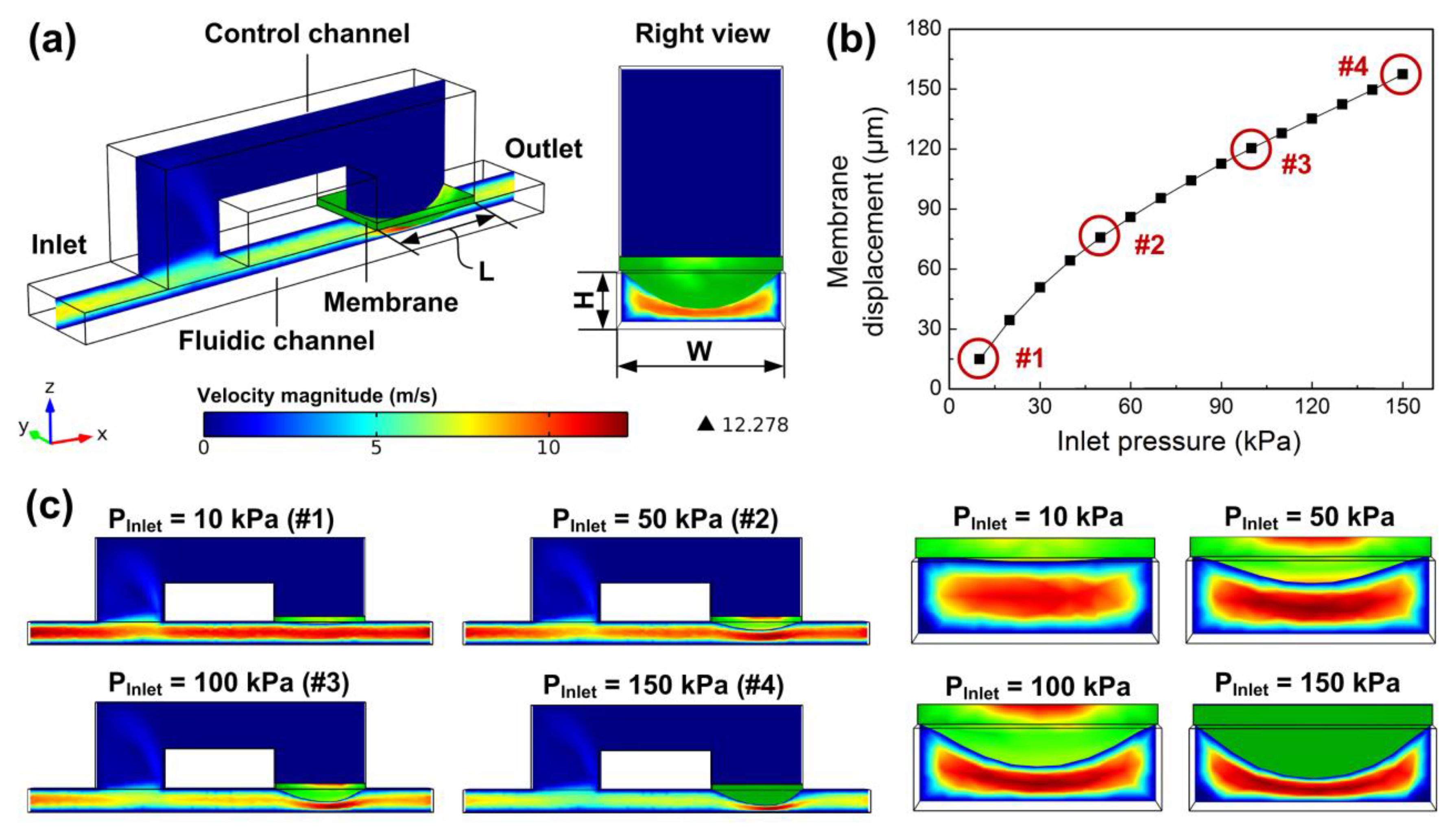
3.1. Fluid-Structure Interaction (FSI) Modeling
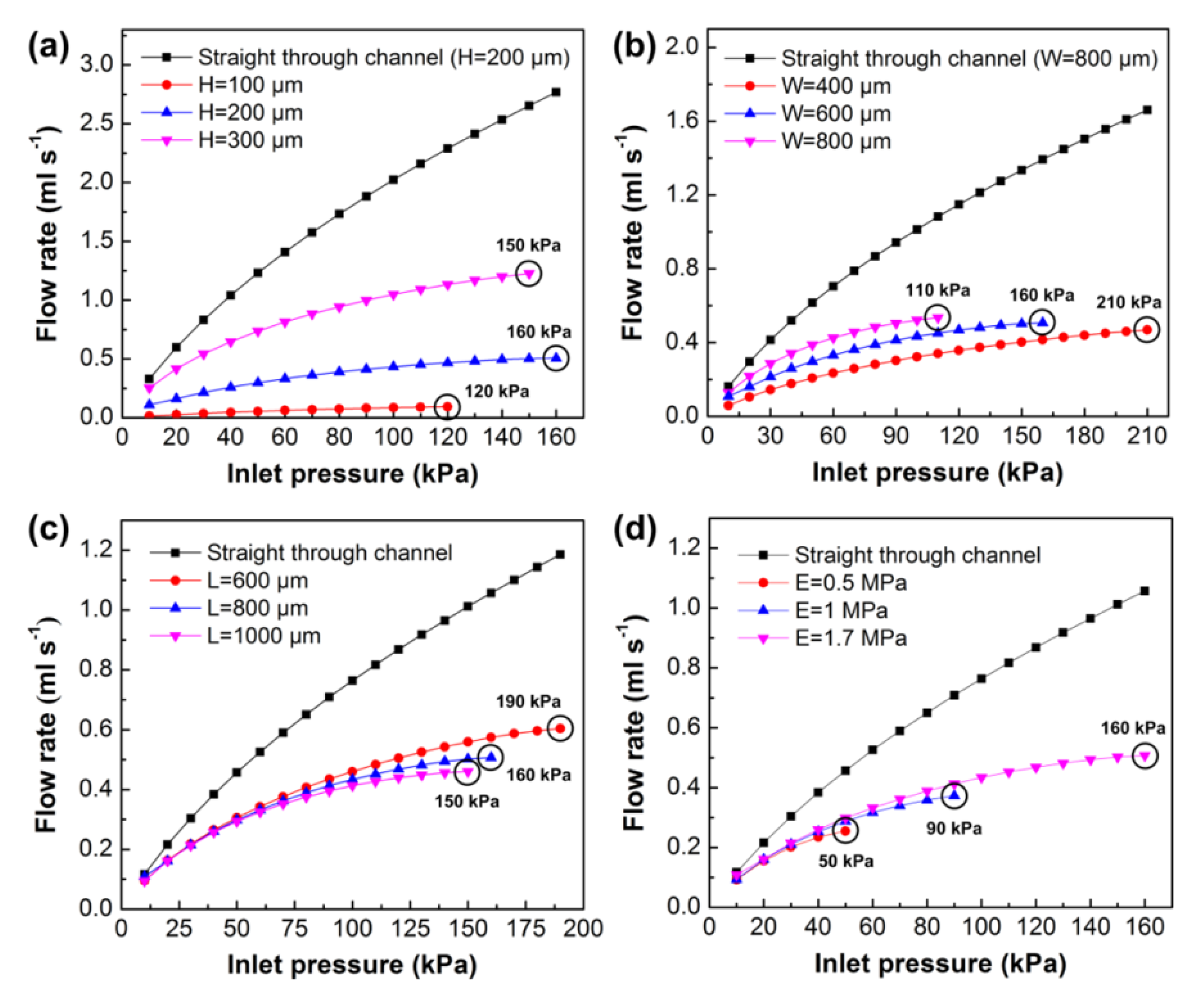
3.2. Numerical Simulation Analysis
3.3. Experimental Examination
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rho, H.S.; Yang, Y.; Hanke, A.T.; Ottens, M.; Terstappen, L.W.; Gardeniers, H. Programmable v-type valve for cell and particle manipulation in microfluidic devices. Lab Chip 2016, 16, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhu, Z.; Xiang, N.; Long, F.; Ni, Z. Automated microfluidic instrument for label-free and high-throughput cell separation. Anal. Chem. 2018, 90, 4212–4220. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Kim, J. A microfluidic-based dynamic microarray system with single-layer pneumatic valves for immobilization and selective retrieval of single microbeads. Microfluid. Nanofluid. 2013, 16, 623–633. [Google Scholar] [CrossRef]
- Wang, J.-H.; Lee, G.-B. Formation of tunable, emulsion micro-droplets utilizing flow-focusing channels and a normally-closed micro-valve. Micromachines 2013, 4, 306–320. [Google Scholar] [CrossRef]
- Ishida, T.; McLaughlin, D.; Tanaka, Y.; Omata, T. First-come-first-store microfluidic device of droplets using hydrophobic passive microvalves. Sens. Actuators B 2018, 254, 1005–1010. [Google Scholar] [CrossRef]
- Gong, H.; Woolley, A.T.; Nordin, G.P. High density 3D printed microfluidic valves, pumps, and multiplexers. Lab Chip 2016, 16, 2450–2458. [Google Scholar] [CrossRef] [PubMed]
- Hwang, A.A.; Lu, J.; Tamanoi, F.; Zink, J.I. Functional nanovalves on protein-coated nanoparticles for in vitro and in vivo controlled drug delivery. Small 2015, 11, 319–328. [Google Scholar] [CrossRef] [PubMed]
- Cousseau, P.; Hirschi, R.; Frehner, B.; Gamper, S.; Maillefer, D. Improved micro-flow regulator for drug delivery systems. In Proceedings of the 14th IEEE International Conference on Micro Electro Mechanical Systems (Cat. No.01CH37090), Interlaken, Switzerland, 25 January 2001; pp. 527–530. [Google Scholar]
- Au, A.K.; Lai, H.; Utela, B.R.; Folch, A. Microvalves and micropumps for BioMEMS. Micromachines 2011, 2, 179–220. [Google Scholar] [CrossRef]
- Pourmand, A.; Shaegh, S.A.M.; Ghavifekr, H.B.; Najafi Aghdam, E.; Dokmeci, M.R.; Khademhosseini, A.; Zhang, Y.S. Fabrication of whole-thermoplastic normally closed microvalve, micro check valve, and micropump. Sens. Actuators B 2018, 262, 625–636. [Google Scholar] [CrossRef]
- Kawai, K.; Arima, K.; Morita, M.; Shoji, S. Microfluidic valve array control system integrating a fluid demultiplexer circuit. J. Micromech. Microeng. 2015, 25, 065016. [Google Scholar] [CrossRef]
- Kaminaga, M.; Ishida, T.; Omata, T. Fabrication of pneumatic microvalve for tall microchannel using inclined lithography. Micromachines 2016, 7, 224. [Google Scholar] [CrossRef] [PubMed]
- Pugliese, M.; Ferrara, F.; Bramanti, A.P.; Gigli, G.; Maiorano, V. In-plane cost-effective magnetically actuated valve for microfluidic applications. Smart Mater. Struct. 2017, 26, 045033. [Google Scholar] [CrossRef]
- Harper, J.C.; Andrews, J.M.; Ben, C.; Hunt, A.C.; Murton, J.K.; Carson, B.D.; Bachand, G.D.; Lovchik, J.A.; Arndt, W.D.; Finley, M.R.; et al. Magnetic-adhesive based valves for microfluidic devices used in low-resource settings. Lab Chip 2016, 16, 4142–4151. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.; Nair, A.R.; Thakur, R.; Fridman, G. Normally closed plunger-membrane microvalve self-actuated electrically using a shape memory alloy wire. Microfluid. Nanofluid. 2018, 22, 29. [Google Scholar] [CrossRef]
- Potkay, J.A.; Wise, K.D. A hybrid thermopneumatic and electrostatic microvalve with integrated position sensing. Micromachines 2012, 3, 379–395. [Google Scholar] [CrossRef]
- Vahid, B.; Boris, S. Flow control using a thermally actuated microfluidic relay valve. J. Microelectromech. Syst. 2010, 19, 1079–1087. [Google Scholar]
- Yalikun, Y.; Tanaka, Y. Large-scale integration of all-glass valves on a microfluidic device. Micromachines 2016, 7, 83. [Google Scholar] [CrossRef]
- Thorsen, T.; Maerkl, S.J.; Quake, S.R. Microfluidic large-scale integration. Science 2002, 298, 580–584. [Google Scholar] [CrossRef]
- Araci, I.E.; Quake, S.R. Microfluidic very large scale integration (mVLSI) with integrated micromechanical valves. Lab Chip 2012, 12, 2803–2806. [Google Scholar] [CrossRef]
- Oskooei, A.; Abolhasani, M.; Gunther, A. Bubble gate for in-plane flow control. Lab Chip 2013, 13, 2519–2527. [Google Scholar] [CrossRef]
- Safavieh, R.; Juncker, D. Capillarics: pre-programmed, self-powered microfluidic circuits built from capillary elements. Lab Chip 2013, 13, 4180–4189. [Google Scholar] [CrossRef] [PubMed]
- Thurgood, P.; Zhu, J.Y.; Nguyen, N.; Nahavandi, S.; Jex, A.R.; Pirogova, E.; Baratchi, S.; Khoshmanesh, K. A self-sufficient pressure pump using latex balloons for microfluidic applications. Lab Chip 2018, 18, 2730–2740. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.J.; Ye, W.; Kartalov, E.P. Quantitative modeling of the behaviour of microfluidic autoregulatory devices. Lab Chip 2012, 12, 1890–1896. [Google Scholar] [CrossRef] [PubMed]
- Cornaggia, L.; Conti, L.; Hannebelle, M.; Gamper, S.; Dumont-Fillon, D.; Lintel, H.; Renaud, P.; Chappel, E. Passive flow control valve for protein delivery. Cogent Eng. 2017, 4, 1413923. [Google Scholar] [CrossRef]
- Kartalov, E.P.; Walker, C.; Taylor, C.R.; Anderson, W.F.; Scherer, A. Microfluidic vias enable nested bioarrays and autoregulatory devices in Newtonian fluids. Proc. Natl. Acad. Sci. 2006, 103, 12280–12284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, B.; Lin, Q. Planar micro-check valves exploiting large polymer compliance. Sens. Actuators A 2007, 134, 186–193. [Google Scholar] [CrossRef]
- Yang, B.; Lin, Q. A planar compliance-based self-adaptive microfluid variable resistor. J. Microelectromech. Syst. 2007, 16, 411–419. [Google Scholar] [CrossRef]
- Unger, M.A.; Chou, H.-P.; Thorsen, T.; Scherer, A.; Quake, S.R. Monolithic microfabricated valves and pumps by multilayer soft lithography. Science 2000, 288, 113–116. [Google Scholar] [CrossRef]
- Zhang, X.; Xiang, N.; Tang, W.; Huang, D.; Wang, X.; Yi, H.; Ni, Z. A passive flow regulator with low threshold pressure for high-throughput inertial isolation of microbeads. Lab Chip 2015, 15, 3473–3480. [Google Scholar] [CrossRef]
- Zhang, X.; Zhu, Z.; Ni, Z.; Xiang, N.; Yi, H. Inexpensive, rapid fabrication of polymer-film microfluidic autoregulatory valve for disposable microfluidics. Biomed. Microdevices 2017, 19, 21. [Google Scholar] [CrossRef]
- Doh, I.; Cho, Y.H. Passive flow-rate regulators using pressure-dependent autonomous deflection of parallel membrane valves. Lab Chip 2009, 9, 2070–2075. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, X.; Chen, K.; Cheng, J.; Xiang, N.; Ni, Z. Passive flow regulator for precise high-throughput flow rate control in microfluidic environments. RSC Adv. 2016, 6, 31639–31646. [Google Scholar] [CrossRef]
- Natarajan, G.P.; Kim, S.-J.; Kim, C.-W. Analysis of membrane behavior of a normally closed microvalve using a fluid-structure interaction model. Micromachines 2017, 8, 355. [Google Scholar] [CrossRef]
- Zhang, X.; Huang, D.; Tang, W.; Jiang, D.; Chen, K.; Yi, H.; Xiang, N.; Ni, Z. A low cost and quasi-commercial polymer film chip for high-throughput inertial cell isolation. RSC Adv. 2016, 6, 9734–9742. [Google Scholar] [CrossRef]
- Chen, S.; Liu, Y.; Shen, Y.; Wang, J.; Yang, Z. The structure of wheel check valve influence on air block phenomenon of piezoelectric micro-pump. Micromachines 2015, 6, 1745–1754. [Google Scholar] [CrossRef]
- Hyeon, J.; So, H. Microfabricaton of microfluidic check valves using comb-shaped moving plug for suppression of backflow in microchannel. Biomed. Microdevices 2019, 21, 19. [Google Scholar] [CrossRef]
- Nguyen, N.-T.; Truong, T.-Q.; Wong, K.-K.; Ho, S.-S.; Low, C.L.-N. Micro check valves for integration into polymeric microfluidic devices. J. Micromech. Microeng. 2004, 14, 69–75. [Google Scholar] [CrossRef]
- Tanaka, Y.; Sato, K.; Kitamori, T. Assembly and simple demonstration of a micropump installing PDMS-based thin membranes as flexible micro check valves. J. Biomed. Nanotechnol. 2009, 5, 516–520. [Google Scholar] [CrossRef] [PubMed]
- Jeon, N.L.; Chiu, D.T.; Wargo, C.J.; Wu, H.; Choi, I.S.; Anderson, J.R.; Whitesides, G.M. Design and fabrication of integrated passive valves and pumps for flexible polymer 3-dimensional microfluidic systems. Biomed. Microdevices 2002, 4, 117–121. [Google Scholar] [CrossRef]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Zhang, Z. Microfluidic Passive Flow Regulatory Device with an Integrated Check Valve for Enhanced Flow Control. Micromachines 2019, 10, 653. https://doi.org/10.3390/mi10100653
Zhang X, Zhang Z. Microfluidic Passive Flow Regulatory Device with an Integrated Check Valve for Enhanced Flow Control. Micromachines. 2019; 10(10):653. https://doi.org/10.3390/mi10100653
Chicago/Turabian StyleZhang, Xinjie, and Zhenyu Zhang. 2019. "Microfluidic Passive Flow Regulatory Device with an Integrated Check Valve for Enhanced Flow Control" Micromachines 10, no. 10: 653. https://doi.org/10.3390/mi10100653



