Reducing the Nano-Scale Aggregation of Perylene Diimide Based Acceptor by Conjugating a Bridge with a Large Volume
Abstract
:1. Introduction
2. Results and Discussion
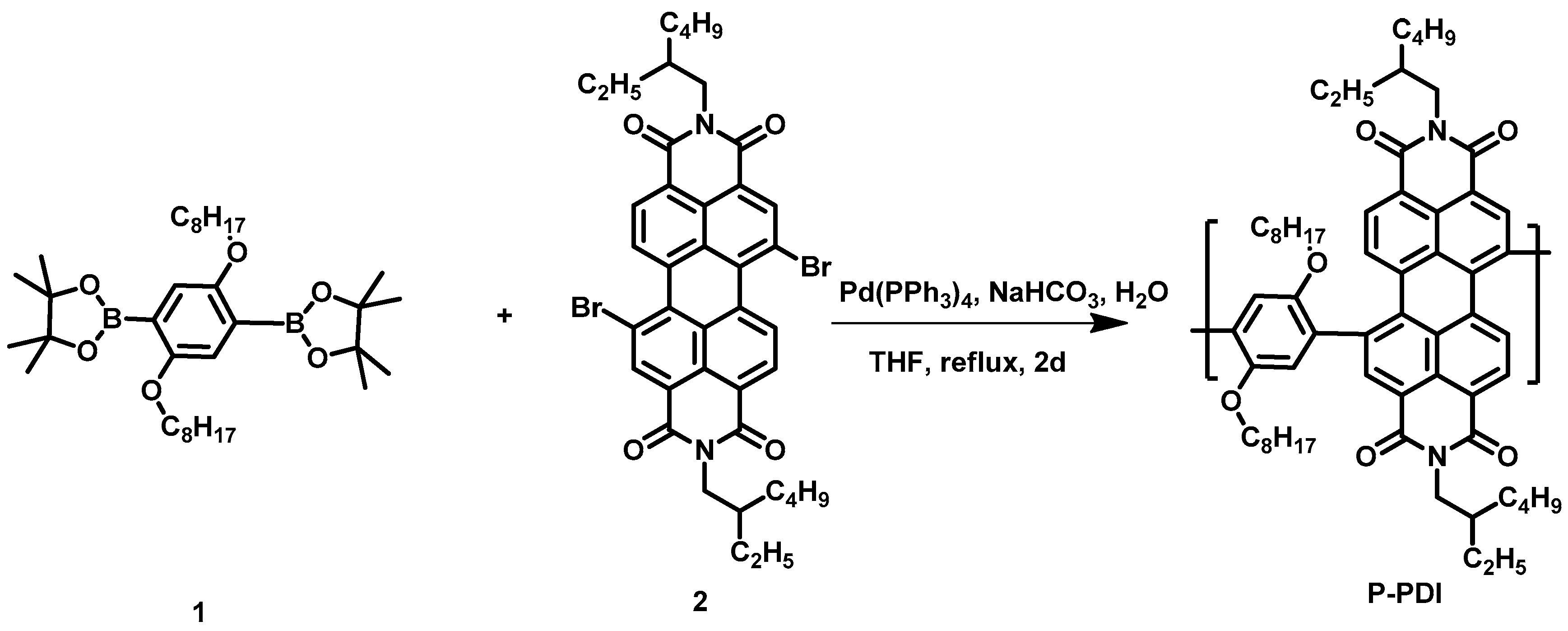
2.1. Materials Synthesis and Characterization
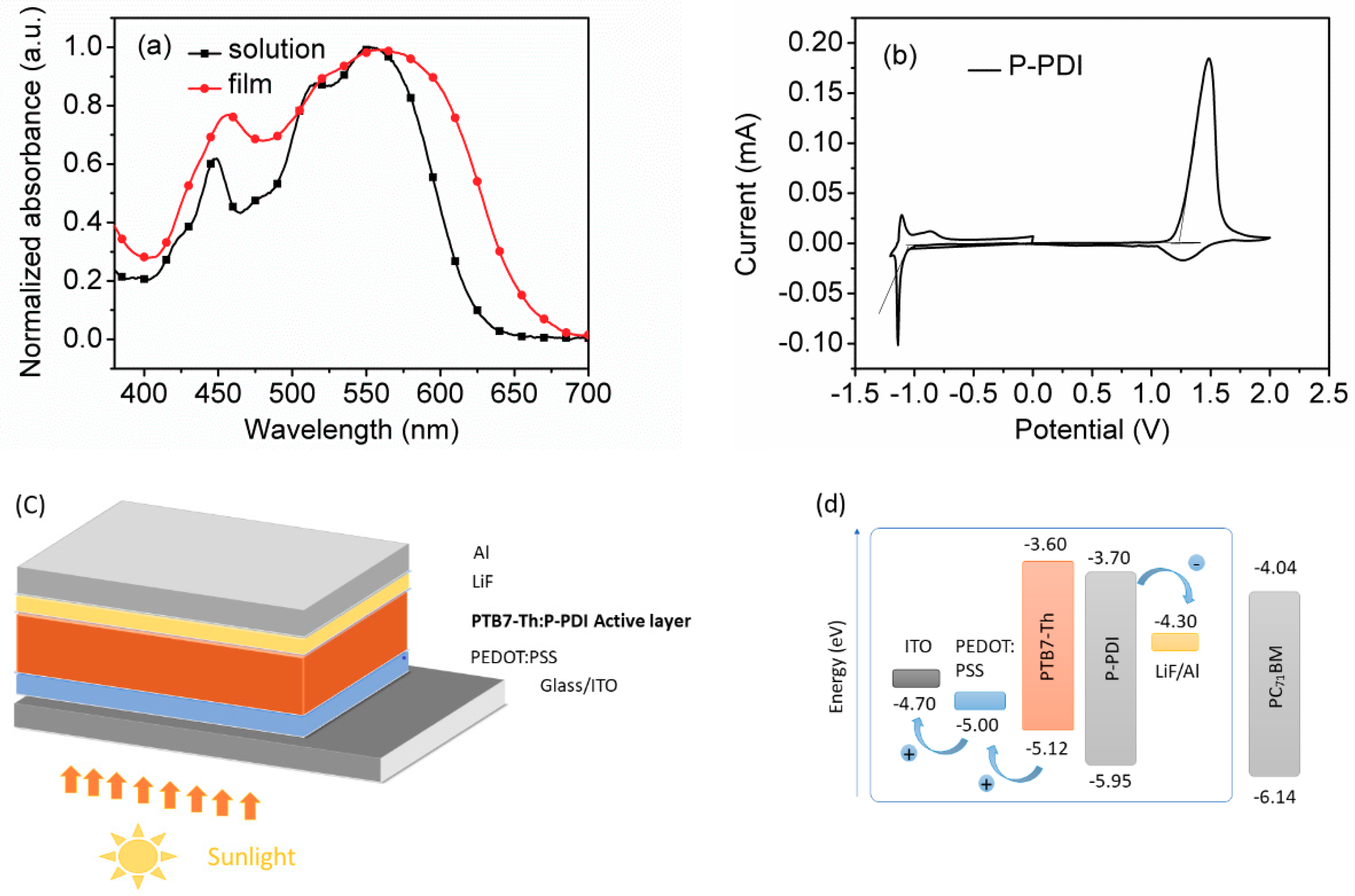
2.2. Optical Properties
2.3. Electrochemical Properties
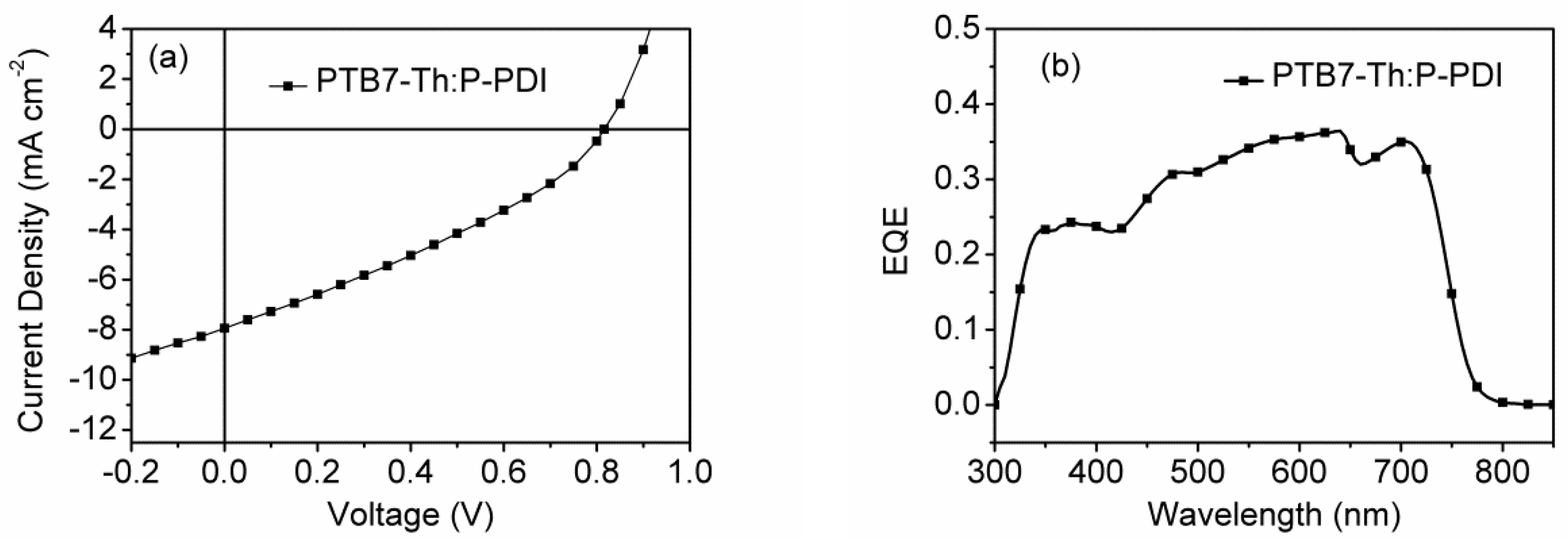
2.4. Photovoltaic Properties
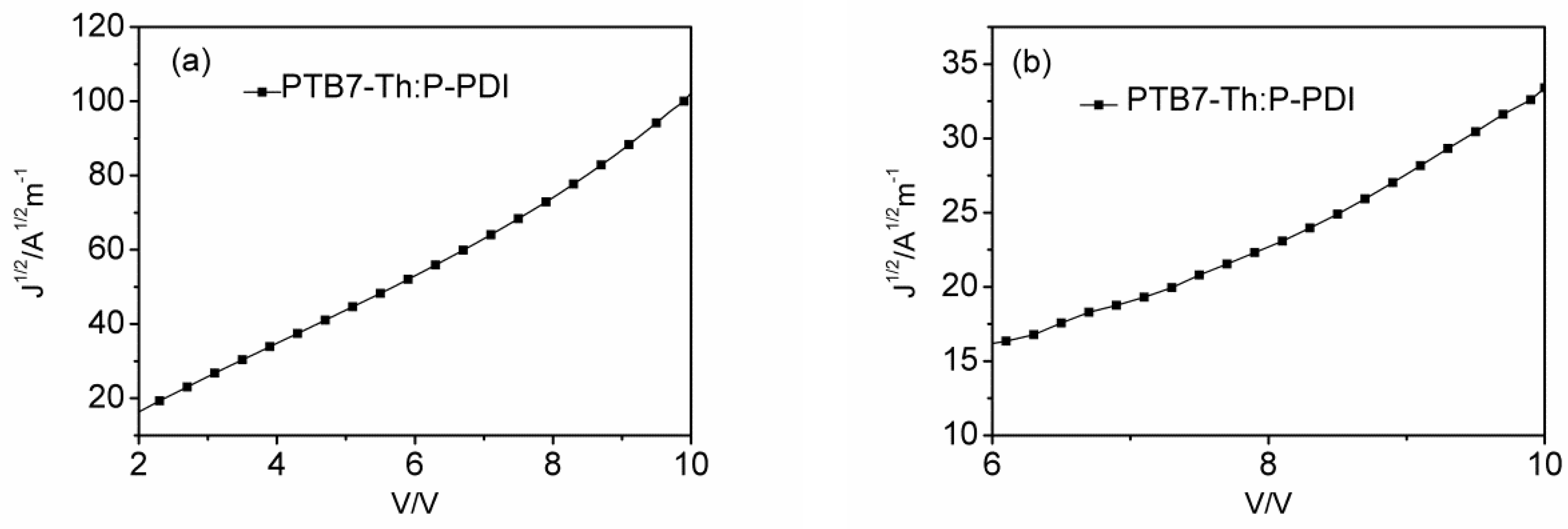
2.5. Transport Properties
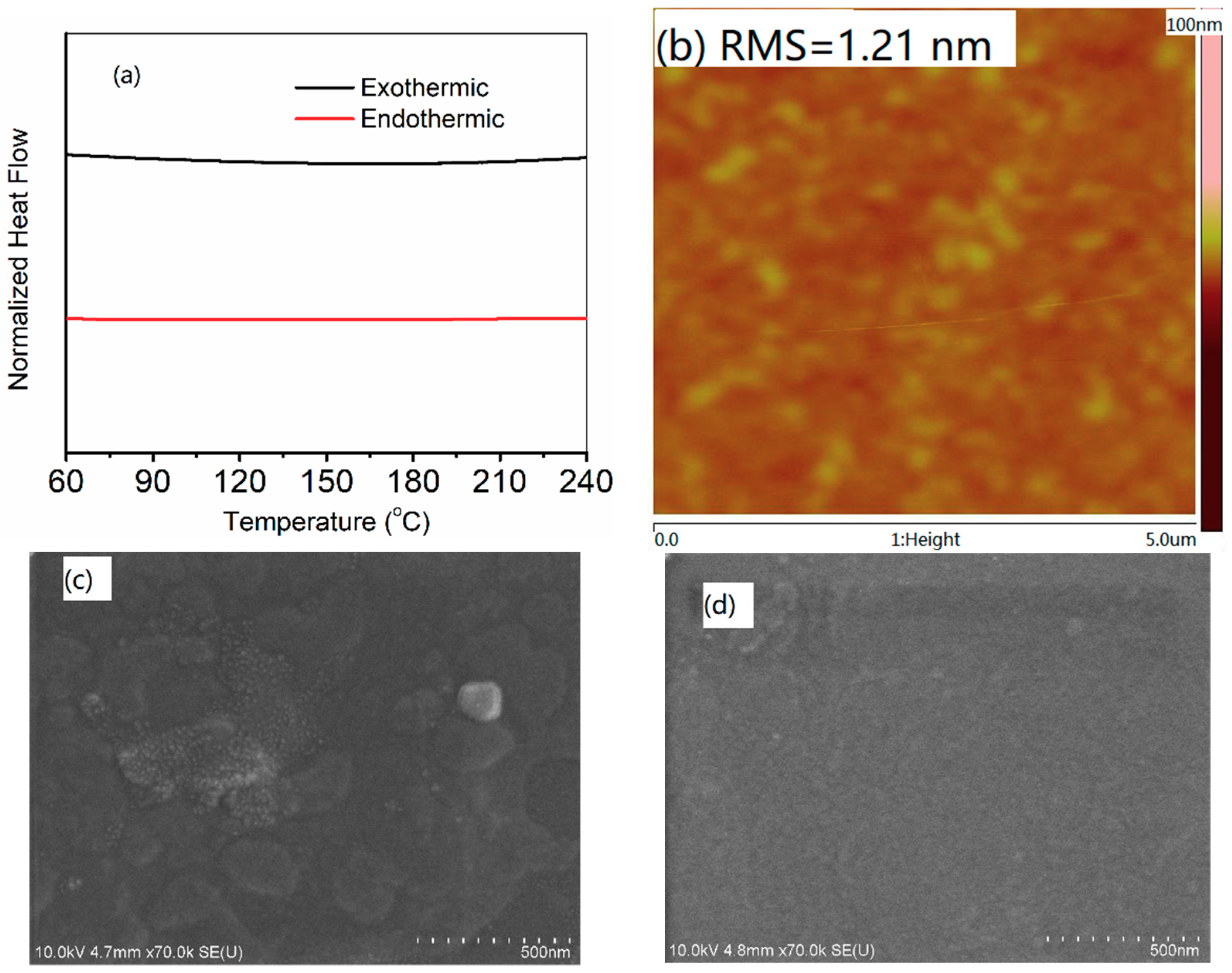
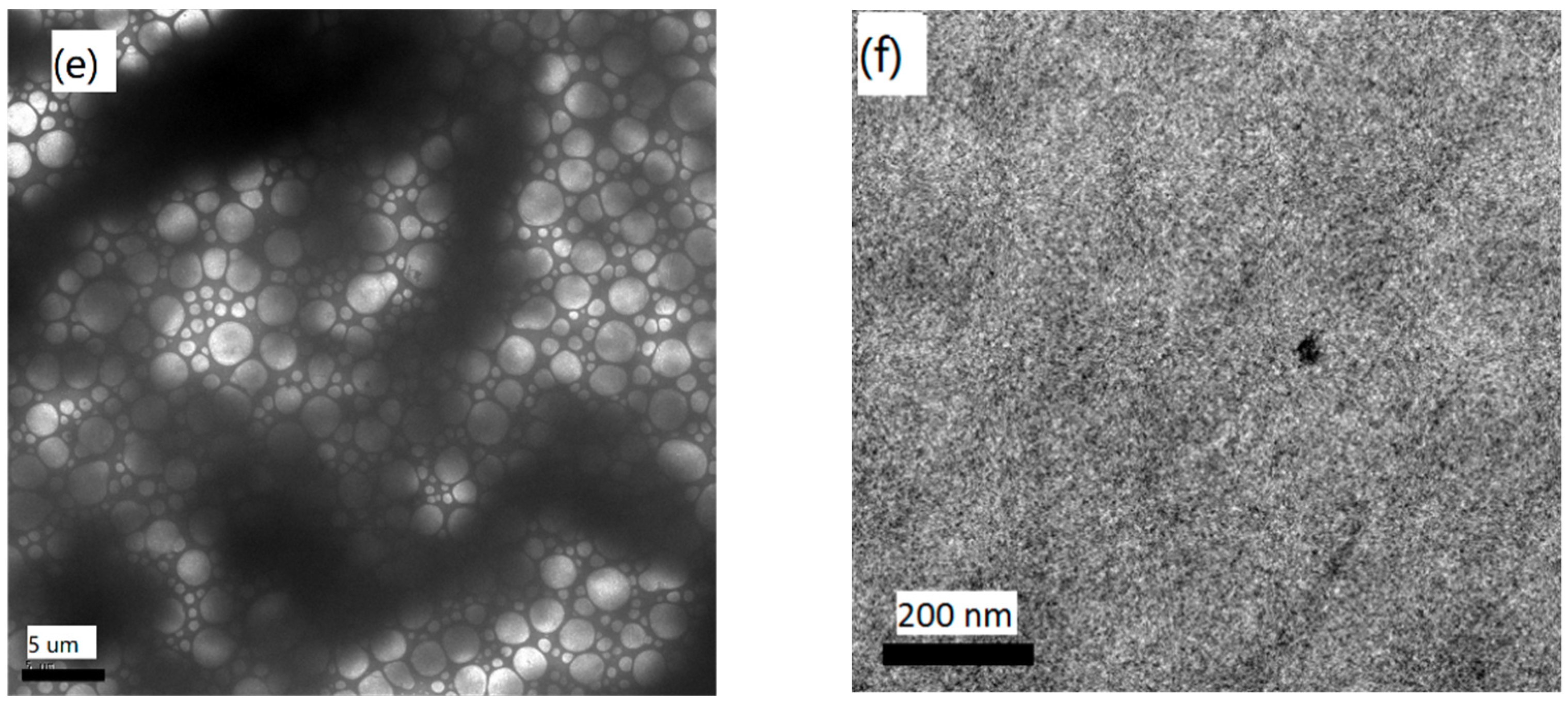
2.6. Film Morphology
3. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A. Experiment Part
Appendix A.1. Materials
Appendix A.2. Polymer Solar Cells Fabrication and Characterization
Appendix A.3. Space-Charge Limited Current Measurement
Appendix A.4. The Synthesis Route of P-PDI
References
- Thompson, B.C.; Fréchet, J.M. Organic photovoltaics—Polymer-fullerene composite solar cells. Angew. Chem. Int. Ed. 2008, 47, 58–77. [Google Scholar] [CrossRef] [PubMed]
- Park, S.H.; Roy, A.; Beaupré, S.; Cho, S.; Coates, N.; Moon, J.S.; Heeger, A.J. Bulk heterojunction solar cells with internal quantum efficiency approaching 100%. Nat. Photonics 2009, 3, 297. [Google Scholar] [CrossRef]
- Günes, S.; Neugebauer, H.; Sariciftci, N.S. Conjugated polymer-based organic solar cells. Chem. Rev. 2007, 107, 1324–1338. [Google Scholar] [CrossRef] [PubMed]
- Brabec, C.J.; Sariciftci, N.S.; Hummelen, J.C. Plastic solar cells. Adv. Funct. Mater. 2001, 11, 15–26. [Google Scholar] [CrossRef]
- Zhao, J.; Li, Y.; Yang, G.; Jiang, K.; Lin, H.; Ade, H.; Yan, H. Efficient organic solar cells processed from hydrocarbon solvents. Nat. Energy 2016, 1, 15027. [Google Scholar] [CrossRef]
- Lee, M.M.; Teuscher, J.; Miyasaka, T.; Murakami, T.N.; Snaith, H.J. Efficient Hybrid Solar Cells Based on Meso-Superstructured Organometal Halide Perovskites. Science 2012, 338, 643–647. [Google Scholar] [CrossRef] [Green Version]
- Burschka, J.; Pellet, N.; Moon, S.J.; Humphry-Baker, R.; Gao, P.; Nazeeruddin, M.K.; Grätzel, M. Sequential deposition as a route to high-performance perovskite-sensitized solar cells. Nature 2013, 499, 316. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Zhan, X. Non-fullerene acceptors for organic photovoltaics: An emerging horizon. Mater. Horiz. 2014, 1, 470–488. [Google Scholar] [CrossRef]
- Facchetti, A. Polymer donor–polymer acceptor (all-polymer) solar cells. Mater. Today 2013, 16, 123–132. [Google Scholar] [CrossRef]
- Nielsen, C.B.; Holliday, S.; Chen, H.-Y.; Cryer, S.J.; McCulloch, I. Non-fullerene electron acceptors for use in organic solar cells. Acc. Chem. Res. 2015, 48, 2803–2812. [Google Scholar] [CrossRef]
- Chochos, C.L.; Tagmatarchis, N.; Gregoriou, V.G. Rational design on n-type organic materials for high performance organic photovoltaics. RSC Adv. 2013, 3, 7160–7181. [Google Scholar] [CrossRef]
- McAfee, S.M.; Topple, J.M.; Hill, I.G.; Welch, G.C. Key components to the recent performance increases of solution processed non-fullerene small molecule acceptors. J. Mater. Chem. A 2015, 3, 16393–16408. [Google Scholar] [CrossRef]
- Cui, Y.; Yao, H.; Zhang, J.; Zhang, T.; Wang, Y.; Hong, L.; Xian, K.; Xu, B.; Zhang, S.; Peng, J.; et al. Over 16% efficiency organic photovoltaic cells enabled by a chlorinated acceptor with increased open-circuit voltages. Nat. Commun. 2019, 10, 2515. [Google Scholar] [CrossRef] [PubMed]
- Zhong, H.; Wu, C.H.; Li, C.Z.; Carpenter, J.; Chueh, C.C.; Chen, J.Y.; Ade, H.; Jen, A.K.Y. Rigidifying Nonplanar Perylene Diimides by Ring Fusion Toward Geometry-Tunable Acceptors for High-Performance Fullerene-Free Solar Cells. Adv. Mater. 2016, 28, 951–958. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yao, J.; Zhan, C. A selenophenyl bridged perylene diimide dimer as an efficient solution-processable small molecule acceptor. Chem. Commun. 2015, 51, 1058–1061. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.H.; Chueh, C.C.; Xi, Y.Y.; Zhong, H.L.; Gao, G.P.; Wang, Z.H.; Pozzo, L.D.; Wen, T.C.; Jen, A.K.Y. Influence of Molecular Geometry of Perylene Diimide Dimers and Polymers on Bulk Heterojunction Morphology Toward High-Performance Nonfullerene Polymer Solar Cells. Adv. Funct. Mater. 2015, 25, 5326–5332. [Google Scholar] [CrossRef]
- Lin, Y.; Wang, Y.; Wang, J.; Hou, J.; Li, Y.; Zhu, D.; Zhan, X. A Star-Shaped Perylene Diimide Electron Acceptor for High-Performance Organic Solar Cells. Adv. Mater. 2014, 26, 5137–5142. [Google Scholar] [CrossRef] [PubMed]
- Liao, S.; Jhuo, H.; Cheng, Y.; Chen, S. Fullerene Derivative—Doped Zinc Oxide Nanofilm as the Cathode of Inverted Polymer Solar Cells with Low—Bandgap Polymer (PTB7-Th) for High Performance. Adv. Mater. 2013, 25, 4766–4771. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, X.; Xiao, H.; Li, G.; Liu, Y.; Li, C.; Huang, H.; Chen, X.; Bo, Z. 1,8-Naphthalimide Based Planar Small Molecular Acceptor for Organic Solar Cells. ACS Appl. Mater. Interfaces 2016, 8, 5475–5483. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, X.; Li, G.; Xiao, H.; Li, W.; Xie, S.; Li, C.; Bo, Z. A nonfullerene acceptor for wide band gap polymer based organic solar cells. Chem. Commun. 2016, 52, 469–472. [Google Scholar] [CrossRef]
- Qi, B.; Wang, J. Fill factor in organic solar cells. Phys. Chem. Chem. Phys. 2013, 15, 8972–8982. [Google Scholar] [CrossRef]
- Vollbrecht, J. Excimers in organic electronics. N. J. Chem. 2018, 42, 11249–11254. [Google Scholar] [CrossRef]
- Vollbrecht, J.; Brus, V.; Ko, S.; Lee, J.; Karki, A.; Cao, D.; Cho, K.; Bazam, G.; Nguyen, T. Quantifying the Nongeminate Recombination Dynamics in Nonfullerene Bulk Heterojunction Organic Solar Cells. Adv. Energy Mater. 2019, 9, 1901438. [Google Scholar] [CrossRef]
- Kwon, O.K.; Park, J.-H.; Park, S.K.; Park, S.Y. Soluble Dicyanodistyrylbenzene-Based Non-Fullerene Electron Acceptors with Optimized Aggregation Behavior for High-Efficiency Organic Solar Cells. Adv. Energy Mater. 2015, 5, 1400929. [Google Scholar] [CrossRef]
- Chen, Y.; Tang, A.; Zhang, X.; Lu, Z.; Huang, J.; Zhan, C.; Yao, J. A new solution-processed diketopyrrolopyrrole donor for non-fullerene small-molecule solar cells. J. Mater. Chem. A 2014, 2, 1869–1876. [Google Scholar] [CrossRef]
- Lin, Y.; Wang, J.; Zhang, Z.G.; Bai, H.; Li, Y.; Zhu, D.; Zhan, X. An electron acceptor challenging fullerenes for efficient polymer solar cells. Adv. Mater. 2015, 27, 1170–1174. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, J.; Lu, H.; Wu, J.; Li, G.; Li, C.; Li, S.; Bo, Z. 1,8-Naphthalimide based small molecular acceptor for polymer solar cells with high open circuit voltage. J. Mater. Chem. C 2015, 3, 6979–6985. [Google Scholar] [CrossRef]
- Lu, Z.; Jiang, B.; Zhang, X.; Tang, A.; Chen, L.; Zhan, C.; Yao, J. Perylene–Diimide Based Non-Fullerene Solar Cells with 4.34% Efficiency through Engineering Surface Donor/Acceptor Compositions. Chem. Mater. 2014, 26, 2907–2914. [Google Scholar] [CrossRef]

| Active Layer | Voc (V) | Jsc (mA·cm−2) | FF | Power Conversation Efficiency (PCE) (%) Best/Ave a | Film Thickness (nm) |
|---|---|---|---|---|---|
| PTB7-Th:P-PDI | 0.82 | 7.92 | 0.34 | 2.21/2.10 | 102 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, J.-Y.; Xia, X.-D.; Zhang, J. Reducing the Nano-Scale Aggregation of Perylene Diimide Based Acceptor by Conjugating a Bridge with a Large Volume. Micromachines 2019, 10, 640. https://doi.org/10.3390/mi10100640
Chen J-Y, Xia X-D, Zhang J. Reducing the Nano-Scale Aggregation of Perylene Diimide Based Acceptor by Conjugating a Bridge with a Large Volume. Micromachines. 2019; 10(10):640. https://doi.org/10.3390/mi10100640
Chicago/Turabian StyleChen, Jun-Yi, Xu-Dong Xia, and Jicheng Zhang. 2019. "Reducing the Nano-Scale Aggregation of Perylene Diimide Based Acceptor by Conjugating a Bridge with a Large Volume" Micromachines 10, no. 10: 640. https://doi.org/10.3390/mi10100640






