Snake Venomics and Antivenomics of Bothrops diporus, a Medically Important Pitviper in Northeastern Argentina
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of the Venom Proteome of B. diporus. Comparison with the Toxin Composition of Venoms from Species of the B. neuwiedi Complex
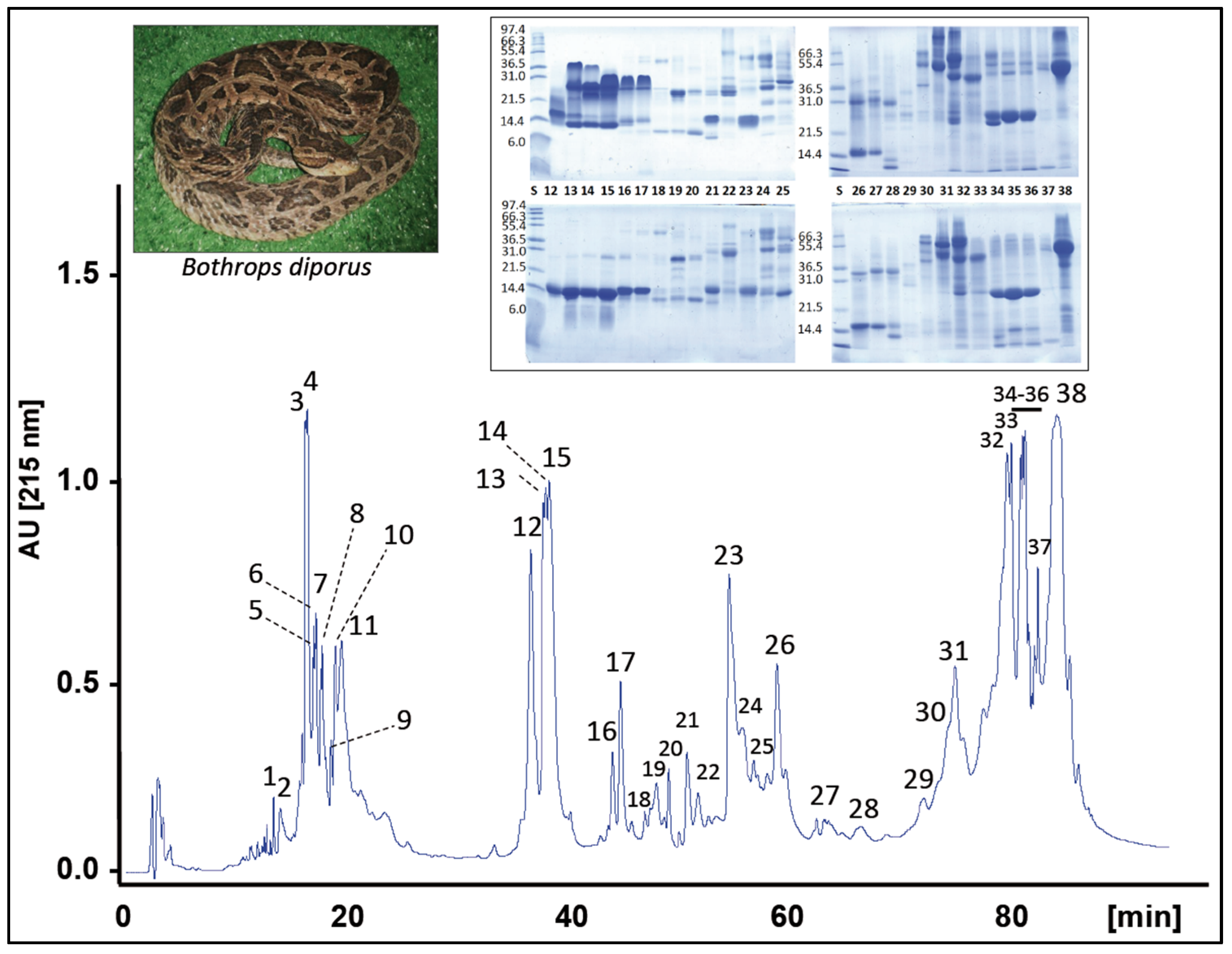
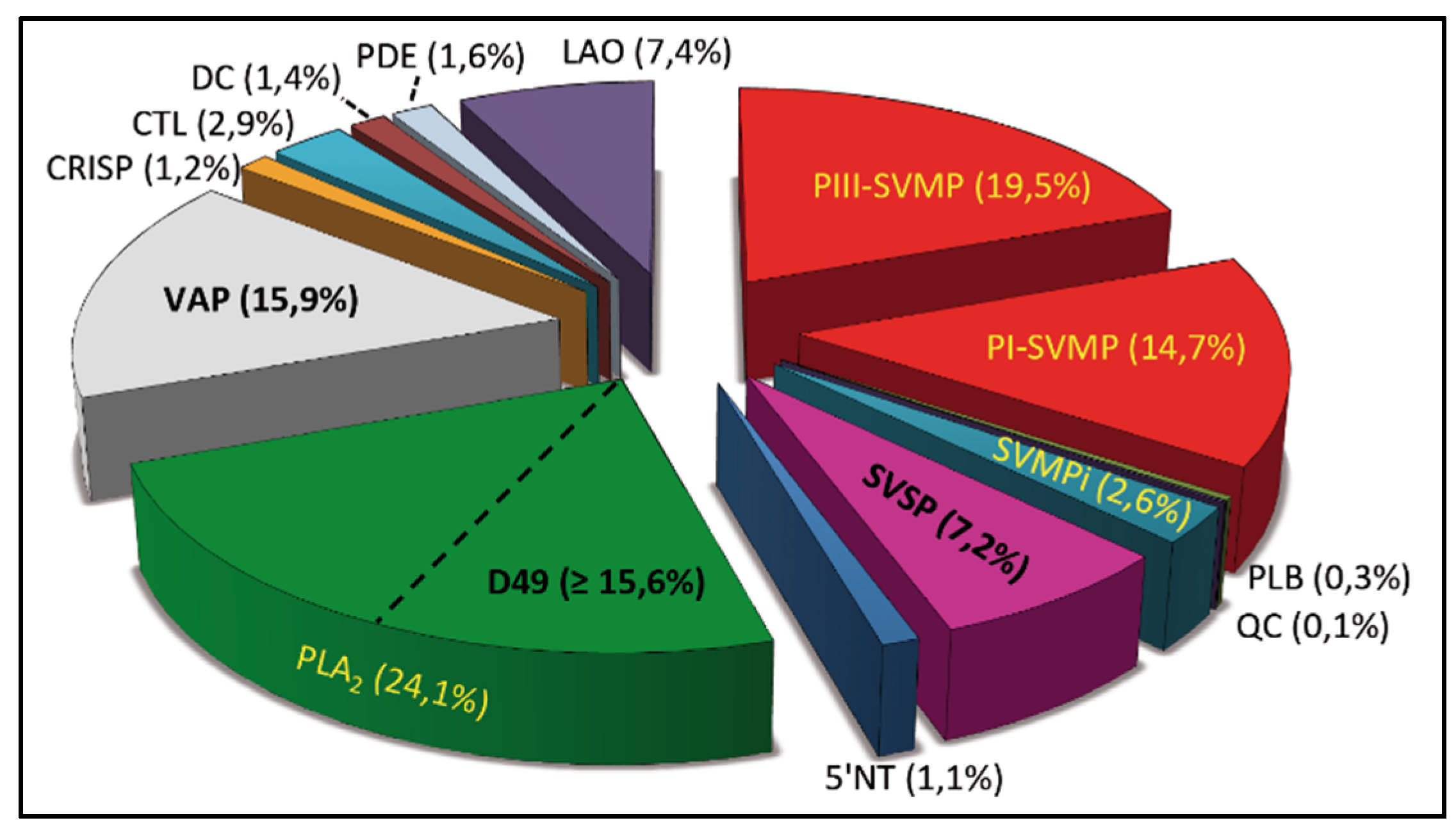
2.2. Correlations between Major Venom Proteins and Venom Toxicity
2.3. Minor Venom Proteins
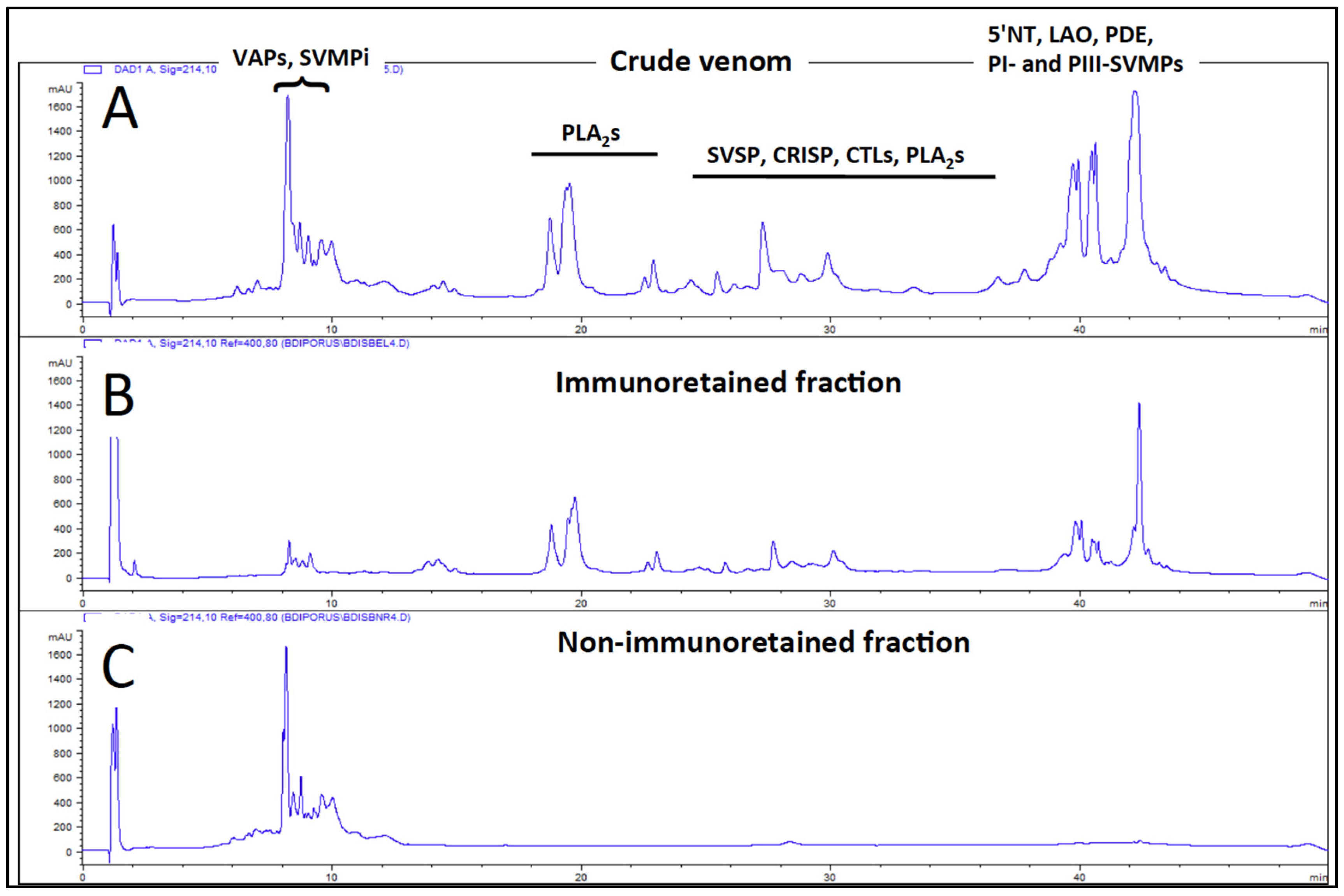
2.4. Antivenomics
3. Concluding Remarks
4. Experimental Section
4.1. Venom
4.2. Isolation and Characterization of Venom Proteins
4.3. Characterization of the Venom Peptidome and Proteome
4.4. Antivenomics
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Cope, E.D. Catalogues of the reptiles obtained during the explorations of the Parana Paraguay, Vermejo and Uruguay rivers by Capt. Thos. J. Page, U.S.N.; and of those procured by Lieut. N. Michier, U.S.Top Eng., Commander of the Expedition conducting the survey of the Atrato River. I. The Paraguay collection. Proc. Acad. Natl. Sci. Phila. 1862, 14, 346–359. [Google Scholar]
- Silva, V.X. The Bothrops neuwiedi complex. In The Venomous Reptiles of the Western Hemisphere; Campbell, J.A., Lamar, W.W., Eds.; Cornell University Press: Ithaca, NY, USA, 2004; Volume 2, pp. 410–422. [Google Scholar]
- Silva, V.X.; Rodrigues, M.F. Taxonomic revision of the Bothrops neuwiedi complex (Serpentes, Viperidae) with description of a new species. Phyllomedusa 2008, 7, 45–90. [Google Scholar] [CrossRef]
- Fenwick, A.M.; Evans, J.A.; Parkinson, C.L. Morphological and molecular evidence for phylogeny and classification of south american pitvipers, genera Bothrops, Bothriopsis, and Bothrocophias (Serpentes: Viperidae). Zool. J. Linnean Soc. 2009, 156, 617–640. [Google Scholar] [CrossRef]
- Carrasco, P.A.; Mattoni, C.I.; Leynaud, G.C.; Scrocchi, G.J. Morphology, phylogeny and taxonomy of south american bothropoid pitvipers (serpentes, viperidae). Zool. Scr. 2012, 41, 109–124. [Google Scholar] [CrossRef]
- Ocampo, M.; Fernandez, G.P. Bothrops diporus (Cope, 1862). Nuevo registro para Bolivia y ampliación en su distribución norteña. Cuad. Herpetol. 2014, 28, 47–48. [Google Scholar]
- Esteso, S.C. Ofidismo en la República Argentina; Editorial Arpón: Córdoba, Argentina, 1985. [Google Scholar]
- De Roodt, A.R.C.N. Aspectos epidemiológicos del ofidismo en argentina con énfasis en la región nordeste. In La Problemática del Ofidismo en la Región Nordeste de Argentina. Una Mirada Científica Integradora; Peichoto, M.E., Salomón, O.D, Eds.; Instituto Nacional de Medicina Tropical: Puerto Iguazú, Argentina, 2014; pp. 121–154. [Google Scholar]
- Giraudo, A.R. Diversidad e historia natural de serpientes de interés sanitario del nordeste argentino. In La Problemática del Ofidismo en la Región Nordeste de Argentina. Una Mirada Científica Integradora.; Peichoto, M.E., Salomón, O.D., Eds.; Instituto Nacional de Medicina Tropical: Puerto Iguazú, Argentina, 2014; pp. 9–68. [Google Scholar]
- De Oliveira, V.C.; Lanari, L.C.; Hajos, S.E.; de Roodt, A.R. Toxicity of Bothrops neuwiedi complex (“yarará chica”) venom from different regions of Argentina (Serpentes, Viperidae). Toxicon 2011, 57, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Quartino, P.J.Y.; Barra, J.L.; Fidelio, G.D. Cloning and functional expression of secreted PLA2 from Bothrops diporus (yarara chica). Biochem. Biophys. Res. Commun. 2012, 427, 321–325. [Google Scholar] [CrossRef] [PubMed]
- Yunes Quartino, P.J.; Portela, M.; Lima, A.; Durán, R.; Lomonte, B.; Fidelio, G.D. A constant area monolayer method to assess optimal lipid packing for lipolysis tested with several secreted PLA2. Biochim. Biophys. Acta 2015, 1848, 2216–2224. [Google Scholar] [CrossRef] [PubMed]
- Daniele, J.; Bianco, I.; Fidelio, G. Kinetic and pharmacological characterization of PLA2 from Bothrops neuwiedii venom. Arch. Biochem. Biophys. 1995, 318, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Daniele, J.; Bianco, I.; Delgado, C.; Carrillo, D.B.; Fidelio, G. A new PLA2 isoform isolated from Bothrops neuwiedii (yarará chica) venom with novel kinetic and chromatographic properties. Toxicon 1997, 35, 1205–1215. [Google Scholar] [CrossRef]
- Vidal, J.; Cattaneo, P.; Stoppani, A. Some characteristic properties of phospholipases A2 from Bothrops neuwiedii venom. Arch. Biochem. Biophys. 1972, 151, 168–179. [Google Scholar] [CrossRef]
- Geoghegan, P.; Angulo, Y.; Cangelosi, A.; Dı́az, M.; Lomonte, B. Characterization of a basic phospholipase A2-homologue myotoxin isolated from the venom of the snake Bothrops neuwiedii (yarara chica) from Argentina. Toxicon 1999, 37, 1735–1746. [Google Scholar] [CrossRef]
- Gutiérrez, J.M.; León, G. Snake antivenoms. Technological, clinical and public health issues. In Animal Toxins: State of the Art. Perspectives in Health and Biotechnology; De Lima, M.E., Pimenta, A.M.C., Martin-Euclaire, M.F., Zingali, R.B., Rochat, H., Eds.; Editora UFMG: Belo Horizonte, Brazil, 2009; pp. 393–421. [Google Scholar]
- Ministerio de Salud. Guía de Prevención, Diagnóstico, Tratamiento y Vigilancia Epidemiológica de los Envenenamientos Ofídicos; Ministerio de Salud: Buenos Aires, Argentina, 2007; pp. 1–48. [Google Scholar]
- Gutiérrez, J.M.; Higashi, H.G.; Wen, F.H.; Burnouf, T. Strengthening antivenom production in Central and South American public laboratories: Report of a workshop. Toxicon 2007, 53, 625–630. [Google Scholar] [CrossRef] [PubMed]
- Segura, A.; Castillo, M.C.; Núñez, V.; Yarlequé, A.; Gonçalves, L.R.; Villalta, M.; Bonilla, C.; Herrera, M.; Vargas, M.; Fernández, M.; et al. Preclinical assessment of the neutralizing capacity of antivenoms produced in six Latin American countries against medically-relevant Bothrops snake venoms. Toxicon 2010, 56, 980–989. [Google Scholar] [CrossRef] [PubMed]
- Eichberg, S.; Sanz, L.; Calvete, J.J.; Pla, D. Constructing comprehensive venom proteome reference maps for integrative venomics. Expert Rev. Proteom. 2015, 12, 557–573. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, R.S.; Boldrini-França, J.; Fonseca, F.P.; de la Torre, P.; Henrique-Silva, F.; Sanz, L.; Calvete, J.J.; Rodrigues, V.M. Combined snake venomics and venom gland transcriptomic analysis of Bothropoides pauloensis. J. Proteom. 2012, 75, 2707–2720. [Google Scholar] [CrossRef] [PubMed]
- Sousa, L.F.; Nicolau, C.A.; Peixoto, P.S.; Bernardoni, J.L.; Oliveira, S.S.; Portes-Junior, J.A.; Mourão, R.H.; Lima-dos-Santos, I.; Sano-Martins, I.S.; Chalkidis, H.M.; et al. Comparison of phylogeny, venom composition and neutralization by antivenom in diverse species of Bothrops complex. PLoS Negl. Trop. Dis. 2013, 7, e2442. [Google Scholar] [CrossRef] [PubMed]
- Gay, C.C.; Maruñak, S.L.; Teibler, G.P.; Leiva, A.C.L.; Acosta, O. Effect of monospecific antibodies against baltergin in myotoxicity induced by Bothrops alternatus venom from northeast of Argentina. Role of metalloproteinases in muscle damage. Toxicon 2013, 63, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Acosta de Pérez, O.; Koscinczuk, P.; Teibler, P.; Sánchez Negrette, M.; Ruíz, R.; Bogarín, G. Actividades hemorrágica y edematizante y alteraciones histológicas en almohadilla plantar del ratón inducidas por venenos de serpientes de los géneros Bothrops y Crotalus de Argentina. Toxicon 1998, 36, 1165–1172. [Google Scholar] [CrossRef]
- Gutiérrez, J.M.; Lomonte, B. Local pathological effects induced by Bothrops snake venoms. Mem. Inst. Butantan 1995, 33, 1405–1474. [Google Scholar]
- Nishioka, S.A.; Silvera, P.V.P. A clinical and epidemiologic study of 292 cases of lance-headed viper bite in a Brazilian teaching hospital. Am. J. Trop. Med. Hyg. 1992, 47, 805–810. [Google Scholar]
- Warrell, D.A. Snakebites in central and south america: Epidemiology, clinical features, and clinical management. In The Venomous Reptiles in the Western Hemisphere; Campbell, J., Lamar, W., Eds.; Cornell University Press: Comstock, Ithaca, NY, USA, 2004; Volume II, pp. 709–761. [Google Scholar]
- Gutiérrez, J.M.; Lomonte, B. Phospholipase A2 myotoxins from Bothrops snake venoms. Toxicon 1995, 33, 1405–1424. [Google Scholar] [CrossRef]
- Lomonte, B.; Angulo, Y.; Sasa, M.; Gutiérrez, J.M. The phospholipase A2 homologues of snake venoms: Biological activities and their possible adaptive roles. Protein Pept. Lett. 2009, 16, 860–876. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, J.M.; Ownby, C.L. Skeletal muscle degeneration induced by venom phospholipases A2: Insights into the mechanisms of local and systemic myotoxicity. Toxicon 2003, 42, 915–931. [Google Scholar] [CrossRef] [PubMed]
- Fox, J.W.; Serrano, S.M. Structural considerations of the snake venom metalloproteinases, key members of the M12 reprolysin family of metalloproteinases. Toxicon 2005, 45, 969–985. [Google Scholar] [CrossRef] [PubMed]
- Escalante, T.; Rucavado, A.; Fox, J.W.; Gutiérrez, J.M. Key events in microvascular damage induced by snake venom hemorrhagic metalloproteinases. J. Proteom. 2011, 74, 1781–1794. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, V.M.; Soares, A.M.; Guerra-Sá, R.; Rodrigues, V.; Fontes, M.R.; Giglio, J.R. Structural and functional characterization of neuwiedase, a nonhemorrhagic fibrin(ogen)olytic metalloprotease from Bothrops neuwiedi snake venom. Arch. Biochem. Biophys. 2000, 381, 213–224. [Google Scholar] [CrossRef] [PubMed]
- Naves de Souza, D.L.; Gomes, M.S.R.; Ferreira, F.B.; Rodrigues, R.S.; Achê, D.C.; Richardson, M.; Borges, M.H.; Rodrigues, V.M. Biochemical and enzymatic characterization of BpMP-I, a fibrinogenolytic metalloproteinase isolated from Bothropoides pauloensis snake venom. Comp. Biochem. Physiol. B 2012, 161, 102–119. [Google Scholar] [CrossRef] [PubMed]
- Costa, F.L.; Rodrigues, R.S.; Izidoro, L.F.; Menaldo, D.L.; Hamaguchi, A.; Homsi-Brandeburgo, M.I.; Fuly, A.L.; Soares, S.G.; Selistre-de-Araujo, H.S.; Barraviera, B. Biochemical and functional properties of a thrombin-like enzyme isolated from Bothrops pauloensis snake venom. Toxicon 2009, 54, 725–735. [Google Scholar] [CrossRef] [PubMed]
- Markland, F.S. Snake venoms and the hemostatic system. Toxicon 1998, 36, 1749–1800. [Google Scholar] [CrossRef]
- Kini, R.M. Anticoagulant proteins from snake venoms: Structure, function and mechanism. Biochem. J. 2006, 397, 377–387. [Google Scholar] [CrossRef] [PubMed]
- Clemetson, K.J. Snaclecs (snake C-type lectins) that inhibit or activate platelets by binding to receptors. Toxicon 2010, 56, 1236–1246. [Google Scholar] [CrossRef] [PubMed]
- Arlinghaus, F.T.; Eble, J.A. C-type lectin-like proteins from snake venoms. Toxicon 2012, 60, 512–519. [Google Scholar] [CrossRef] [PubMed]
- Du, X.-Y.; Clemetson, K.J. Snake venom L-amino acid oxidases. Toxicon 2002, 40, 659–665. [Google Scholar]
- Rodrigues, R.S.; da Silva, J.F.; Boldrini-França, J.; Fonseca, F.P.; Otaviano, A.R.; Henrique Silva, F.; Hamaguchi, A.; Magro, A.J.; Braz, A.S.; dos Santos, J.I.; et al. Structural and functional properties of Bp-LAAO, a new L-amino acid oxidase isolated from Bothrops pauloensis snake venom. Biochimie 2009, 91, 490–501. [Google Scholar] [CrossRef] [PubMed]
- Menin, L.; Perchuć, A.; Favreau, P.; Perret, F.; Michalet, S.; Schöni, R.; Wilmer, M.; Stöcklin, R. High throughput screening of bradykinin-potentiating peptides in Bothrops moojeni snake venom using precursor ion mass spectrometry. Toxicon 2008, 51, 1288–1302. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, S.H.; Bartelt, D.C.; Greene, L.J. Isolation of bradykinin-potentiating peptides from Bothrops jararaca venom. Biochemistry 1970, 9, 2583–2593. [Google Scholar] [CrossRef] [PubMed]
- Greene, L.-J.; Camargo, A.C.; Krieger, E.M.; Stewart, J.M.; Ferreira, S.H. Inhibition of the conversion of angiotensin I to II and potentiation of bradykinin by small peptides present in Bothrops jararaca venom. Circ. Res. 1972, 31 (Suppl. 2), 62–71. [Google Scholar] [PubMed]
- Luft, F.C. The Bothrops legacy: Vasoactive peptides from Brazil. Renin Rep. 2008, 10, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Pimenta, D.C.; Prezoto, B.C.; Konno, K.; Melo, R.L.; Furtado, M.F.; Camargo, A.C.; Serrano, S.M. Mass spectrometric analysis of the individual variability of Bothrops jararaca venom peptide fraction. Evidence for sex-based variation among the bradykinin-potentiating peptides. Rapid Commun. Mass Spectrom. 2007, 21, 1034–1042. [Google Scholar] [CrossRef] [PubMed]
- Ianzer, D.; Konno, K.; Marques-Porto, R.; Vieira Portaro, F.C.; Stöcklin, R.; Martins de Camargo, A.C.; Pimenta, D.C. Identification of five new bradykinin potentiating peptides (BPPs) from Bothrops jararaca crude venom by using electrospray ionization tandem mass spectrometry after a two-step liquid chromatography. Peptides 2004, 25, 1085–1092. [Google Scholar] [CrossRef] [PubMed]
- Pla, D.; Sanz, L.; Molina-Sánchez, P.; Zorita, V.; Madrigal, M.; Flores-Díaz, M.; Alape-Girón, A.; Núñez, V.; Andrés, V.; Gutiérrez, J.M.; et al. Snake venomics of Lachesis muta rhombeata and genus-wide antivenomics assessment of the paraspecific immunoreactivity of two antivenoms evidence the high compositional and immunological conservation across Lachesis. J. Proteom. 2013, 89, 112–123. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, L.F.; Galle, A.; Raida, M.; Schrader, M.; Lebrun, I.; Habermehl, G. Isolation: Analysis and properties of three bradykinin–potentiating peptides (BPP-II, BPP-III, and BPP-V) from Bothrops neuwiedi venom. J. Protein Chem. 1998, 17, 285–289. [Google Scholar] [CrossRef] [PubMed]
- Pawlak, J.; Kini, R.M. Snake venom glutaminyl cyclase. Toxicon 2006, 48, 278–286. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.F.; Hung, C.C.; Wu, S.H.; Chiou, S.H. Characterization of three endogenous peptide inhibitors for multiple metalloproteinases with fibrinogenolytic activity from the venom of Taiwan habu (Trimeresurus mucrosquamatus). Biochem. Biophys. Res. Commun. 1998, 248, 562–568. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.F.; Chiou, S.H.; Ko, T.P.; Wang, A.H. Determinants of the inhibition of a Taiwan habu venom metalloproteinase by its endogenous inhibitors revealed by X-ray crystallography and synthetic inhibitor analogues. Eur. J. Biochem. 2002, 269, 3047–3056. [Google Scholar] [CrossRef] [PubMed]
- Wagstaff, S.C.; Favreau, P.; Cheneval, O.; Laing, G.D.; Wilkinson, M.C.; Miller, R.L.; Stöcklin, R.; Harrison, R.A. Molecular characterisation of endogenous snake venom metalloproteinase inhibitors. Biochem. Biophys. Res. Commun. 2008, 365, 650–656. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, Y.; Morita, T. Structure and function of snake venom cysteine-rich secretory proteins. Toxicon 2004, 44, 227–231. [Google Scholar] [CrossRef] [PubMed]
- Matsunaga, Y.; Yamazaki, Y.; Hyodo, F.; Sugiyama, Y.; Nozaki, M.; Morita, T. Structural divergence of cysteine-rich secretory proteins in snake venoms. J. Biochem. 2009, 145, 365–375. [Google Scholar] [CrossRef] [PubMed]
- Doery, H.M.; Pearson, J.E. Phospholipase B in snake venoms and bee venom. Biochem. J. 1964, 92, 599–602. [Google Scholar] [CrossRef] [PubMed]
- Takasaki, C.; Tamiya, N. Isolation and properties of lysophospholipases from the venom of an Australian elapid snake, Pseudechis australis. Biochem. J. 1982, 203, 269–276. [Google Scholar] [CrossRef] [PubMed]
- Bernheimer, A.W.; Weinstein, S.A.; Linder, R. Isoelectric analysis of some Australian elapid snake venoms with special reference to phospholipase B and hemolysis. Toxicon 1986, 24, 841–849. [Google Scholar] [CrossRef]
- Bernheimer, A.W.; Linder, R.; Weinstein, S.A.; Kim, K.S. Isolation and characterization of a phospholipase B from venom of Collett’s snake, Pseudechis colletti. Toxicon 1987, 25, 547–554. [Google Scholar] [CrossRef]
- Gullan, J.M.; Jackson, E.M. 5-Nucleotidase. Biochem. J. 1938, 32, 597–601. [Google Scholar] [CrossRef]
- Dhananjaya, B.L.; D’Souza, C.J. The pharmacological role of nucleotidases in snake venoms. Cell Biochem. Funct. 2010, 28, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Burnstock, G.; Verkhratsky, A. Evolutionary origins of the purinergic signalling system. Acta Physiol. 2009, 195, 415–447. [Google Scholar] [CrossRef] [PubMed]
- Aird, S.D. Ophidian envenomation strategies and the role of purines. Toxicon 2002, 40, 335–393. [Google Scholar] [CrossRef]
- Cintra-Francischinelli, M.; Caccin, P.; Chiavegato, A.; Pizzo, P.; Carmignoto, G.; Angulo, Y.; Lomonte, B.; Gutiérrez, J.M.; Montecucco, C. Bothrops snake myotoxins induce a large efflux of ATP and potassium with spreading of cell damage and pain. Proc. Natl. Acad. Sci. USA 2010, 107, 14140–14145. [Google Scholar] [CrossRef] [PubMed]
- Caccin, P.; Pellegatti, P.; Fernández, J.; Vono, M.; Cintra-Francischinelli, M.; Lomonte, B.; Gutiérrez, J.M.; Di Virgilio, F.; Montecucco, C. Why myotoxin-containing snake venoms possess powerful nucleotidases? Biochem. Biophys. Res. Commun. 2013, 430, 1289–1293. [Google Scholar] [CrossRef] [PubMed]
- Pla, D.; Gutierrez, J.M.; Calvete, J.J. Second generation snake antivenomics: Comparing immunoaffinity and immunodepletion protocols. Toxicon 2012, 60, 688–699. [Google Scholar] [CrossRef] [PubMed]
- Calvete, J.J.; Sanz, L.; Pla, D.; Lomonte, B.; Gutiérrez, J.M. Omics meets biology: Application to the design and preclinical assessment of antivenoms. Toxins 2014, 6, 3388–3405. [Google Scholar] [CrossRef] [PubMed]
- Lomonte, B.; Escolano, J.; Fernández, J.; Sanz, L.; Angulo, Y.; Gutiérrez, J.M.; Calvete, J.J. Snake venomics and antivenomics of the arboreal neotropical pitvipers Bothriechis lateralis and Bothriechis schlegelii. J. Proteome Res. 2008, 7, 2445–2457. [Google Scholar] [CrossRef] [PubMed]
- Fernández, J.; Lomonte, B.; Sanz, L.; Angulo, Y.; Gutiérrez, J.M.; Calvete, J.J. Snake venomics of Bothriechis nigroviridis reveals extreme variability among palm pitviper venoms: Different evolutionary solutions for the same trophic purpose. J. Proteome Res. 2010, 9, 4234–4241. [Google Scholar] [CrossRef] [PubMed]
- Lomonte, B.; Tsai, W.C.; Bonilla, F.; Solórzano, A.; Solano, G.; Angulo, Y.; Gutiérrez, J.M.; Calvete, J.J. Snake venomics and toxicological profiling of the arboreal pitviper Bothriechis supraciliaris from Costa Rica. Toxicon 2012, 59, 592–599. [Google Scholar] [CrossRef] [PubMed]
- Lomonte, B.; Mora-Obando, D.; Fernández, J.; Sanz, L.; Pla, D.; Gutiérrez, J.M.; Calvete, J.J. First crotoxin-like phospholipase A2 complex from a New World non-rattlesnake species: Nigroviriditoxin, from the arboreal Neotropical snake Bothriechis nigroviridis. Toxicon 2015, 93, 144–154. [Google Scholar] [CrossRef] [PubMed]
- Bogarín, G.; Morais, J.F.; Yamaguchi, I.K.; Stephano, M.A.; Marcelino, J.R.; Nishikawa, A.K.; Guidolin, R.; Rojas, G.; Higashi, H.G.; Gutiérrez, J.M. Neutralization of crotaline snake venoms from Central and South America by antivenoms produced in Brazil and Costa Rica. Toxicon 2000, 38, 1429–1441. [Google Scholar] [CrossRef]
- Alape-Girón, A.; Sanz, L.; Escolano, J.; Flores-Díaz, M.; Madrigal, M.; Sasa, M.; Calvete, J.J. Snake venomics of the lancehead pitviper Bothrops asper: Geographic, individual, and ontogenetic variations. J. Proteome Res. 2008, 7, 3556–3571. [Google Scholar] [CrossRef] [PubMed]
- Calvete, J.J.; Borges, A.; Segura, A.; Flores-Díaz, M.; Alape-Girón, A.; Gutiérrez, J.M.; Diez, N.; de Sousa, L.; Kiriakos, D.; Sánchez, E.; et al. Snake venomics and antivenomics of Bothrops colombiensis, a medically important pitviper of the Bothrops atrox-asper complex endemic to Venezuela: Contributing to its taxonomy and snakebite management. J. Proteom. 2009, 72, 227–240. [Google Scholar] [CrossRef] [PubMed]
- Núñez, V.; Cid, P.; Sanz, L.; de La Torre, P.; Angulo, Y.; Lomonte, B.; Gutiérrez, J.M.; Calvete, J.J. Snake venomics and antivenomics of Bothrops atrox venoms from Colombia and the Amazon regions of Brazil, Perú and Ecuador suggest the occurrence of geographic variation of venom phenotype by a trend towards paedomorphism. J. Proteom. 2009, 73, 57–78. [Google Scholar] [CrossRef] [PubMed]
- Valente, R.H.; Guimarães, P.R.; Junqueira, M.; Neves-Ferreira, A.G.; Soares, M.R.; Chapeaurouge, A.; Trugilho, M.R.; León, I.R.; Rocha, S.L.; Oliveira-Carvalho, A.L.; et al. Bothrops insularis venomics: A proteomic analysis supported by transcriptomic-generated sequence data. J. Proteomics 2009, 72, 241–255. [Google Scholar] [CrossRef] [PubMed]
- Kohlhoff, M.; Borges, M.H.; Yarlequé, A.; Cabezas, C.; Richardson, M.; Sánchez, E.F. Exploring the proteomes of the venoms of the Peruvian pit vipers Bothrops atrox, B. barnetti and B. pictus. J. Proteom. 2012, 75, 2181–2195. [Google Scholar] [CrossRef] [PubMed]
- Bernardes, C.P.; Menaldo, D.L.; Camacho, E.; Rosa, J.C.; Escalante, T.; Rucavado, A.; Lomonte, B.; Gutiérrez, J.M.; Sampaio, S.V. Proteomic analysis of Bothrops pirajai snake venom and characterization of BpirMP, a new P-I metalloproteinase. J. Proteom. 2013, 80, 250–267. [Google Scholar] [CrossRef] [PubMed]
- Fernández Culma, M.; Pereañez, J.A.; Núñez, V.; Lomonte, B. Snake venomics of Bothrops punctatus, a semiarboreal pitviper species from Antioquia, Colombia. Peer J. 2014, 2, e246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jorge, R.J.; Monteiro, H.S.; Gonçalves-Machado, L.; Guarnieri, M.C.; Ximenes, R.M.; Borges-Nojosa, D.M.; Luna, K.P.; Zingali, R.B.; Corrêa-Netto, C.; Gutiérrez, J.M.; et al. Venomics and antivenomics of Bothrops erythromelas from five geographic populations within the Caatinga ecoregion of northeastern Brazil. J. Proteom. 2015, 114, 93–114. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves-Machado, L.; Pla, D.; Sanz, L.; Jorge, R.J.; Leitão-De-Araújo, M.; Alves, M.L.; Alvares, D.J.; de Miranda, J.; Nowatzki, J.; Morais-Zani, K.; et al. Combined venomics, venom gland transcriptomics, bioactivities, and antivenomics of two Bothrops jararaca populations from geographic isolated regions within the Brazilian Atlantic rainforest. J. Proteom. 2015. [Google Scholar] [CrossRef] [PubMed]
- Machado, T.; Silva, V.X.; Silva, M.J. Phylogenetic relationships within Bothrops neuwiedi group (Serpentes, Squamata): Geographically highly-structured lineages, evidence of introgressive hybridization and Neogene/Quaternary diversification. Mol. Phylogenet. Evol. 2014, 71, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Raw, I.; Guidolin, R.; Higashi, H.G.; Kelen, E.M.A. Antivenins in Brazil: Preparation. In Handbook of Natural Toxins; Tu, A., Ed.; Marcel Dekker: New York, NY, USA, 1991; pp. 557–811. [Google Scholar]
- Johnstone, A.; Thorpe, R. Immunochemistry in Practice, 2nd ed.; Blackwell Scientific Publications: Oxford, UK, 1987. [Google Scholar]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gay, C.; Sanz, L.; Calvete, J.J.; Pla, D. Snake Venomics and Antivenomics of Bothrops diporus, a Medically Important Pitviper in Northeastern Argentina. Toxins 2016, 8, 9. https://doi.org/10.3390/toxins8010009
Gay C, Sanz L, Calvete JJ, Pla D. Snake Venomics and Antivenomics of Bothrops diporus, a Medically Important Pitviper in Northeastern Argentina. Toxins. 2016; 8(1):9. https://doi.org/10.3390/toxins8010009
Chicago/Turabian StyleGay, Carolina, Libia Sanz, Juan J. Calvete, and Davinia Pla. 2016. "Snake Venomics and Antivenomics of Bothrops diporus, a Medically Important Pitviper in Northeastern Argentina" Toxins 8, no. 1: 9. https://doi.org/10.3390/toxins8010009





