Rumen and Serum Metabolomes in Response to Endophyte-Infected Tall Fescue Seed and Isoflavone Supplementation in Beef Steers
Abstract
:1. Introduction
2. Results
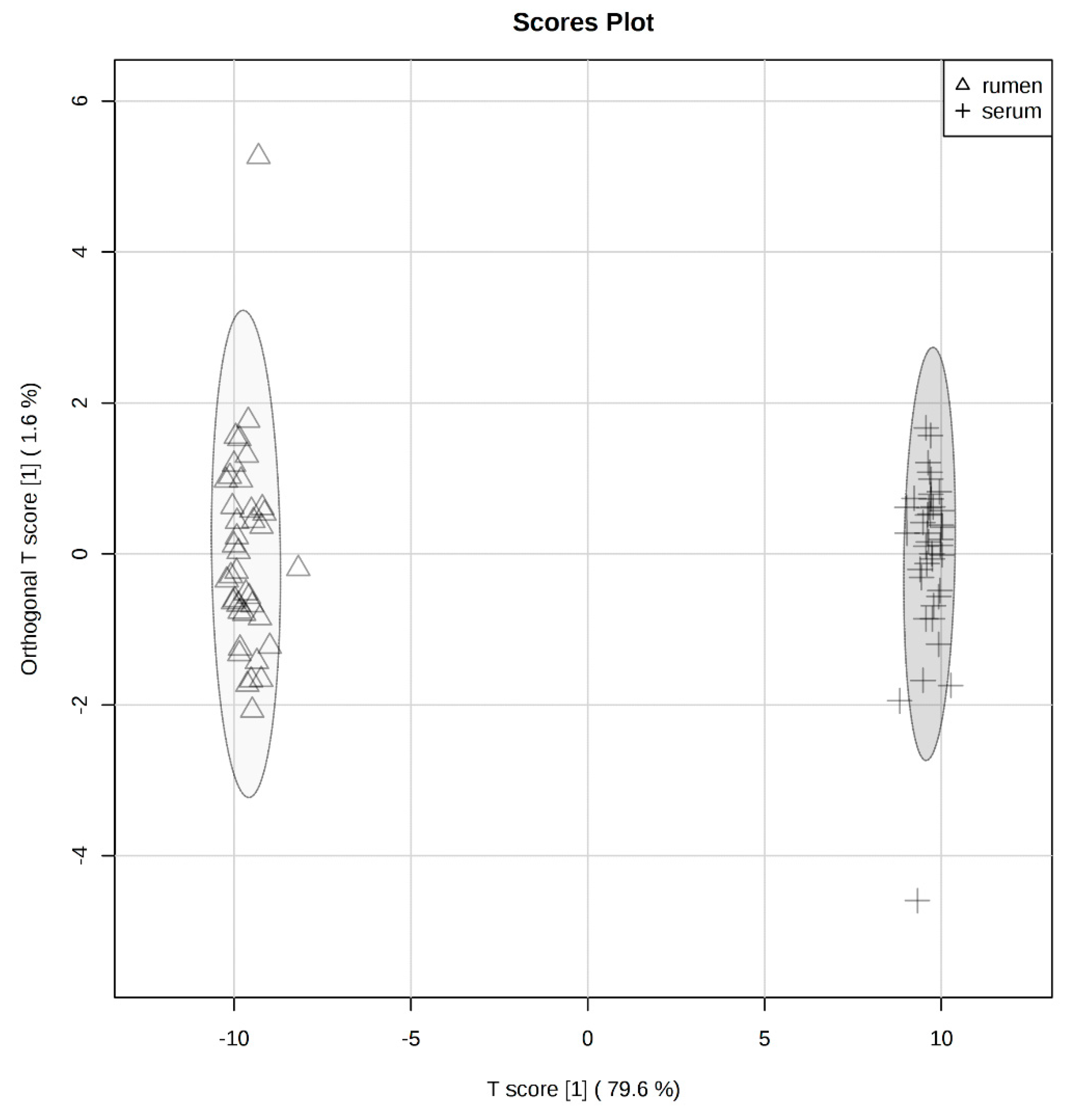
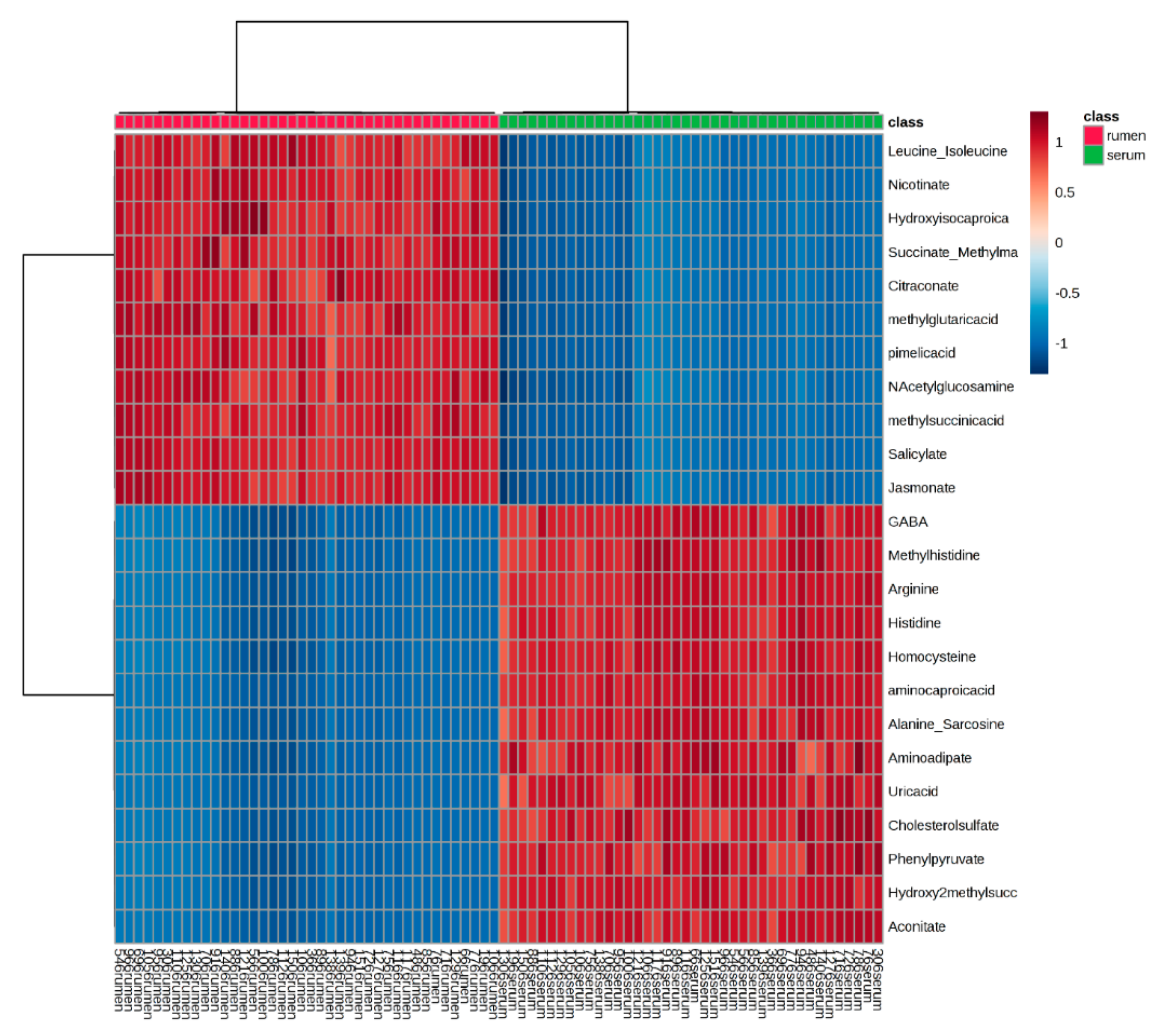
2.1. Global Rumen Fluid and Serum Metabolome Comparison
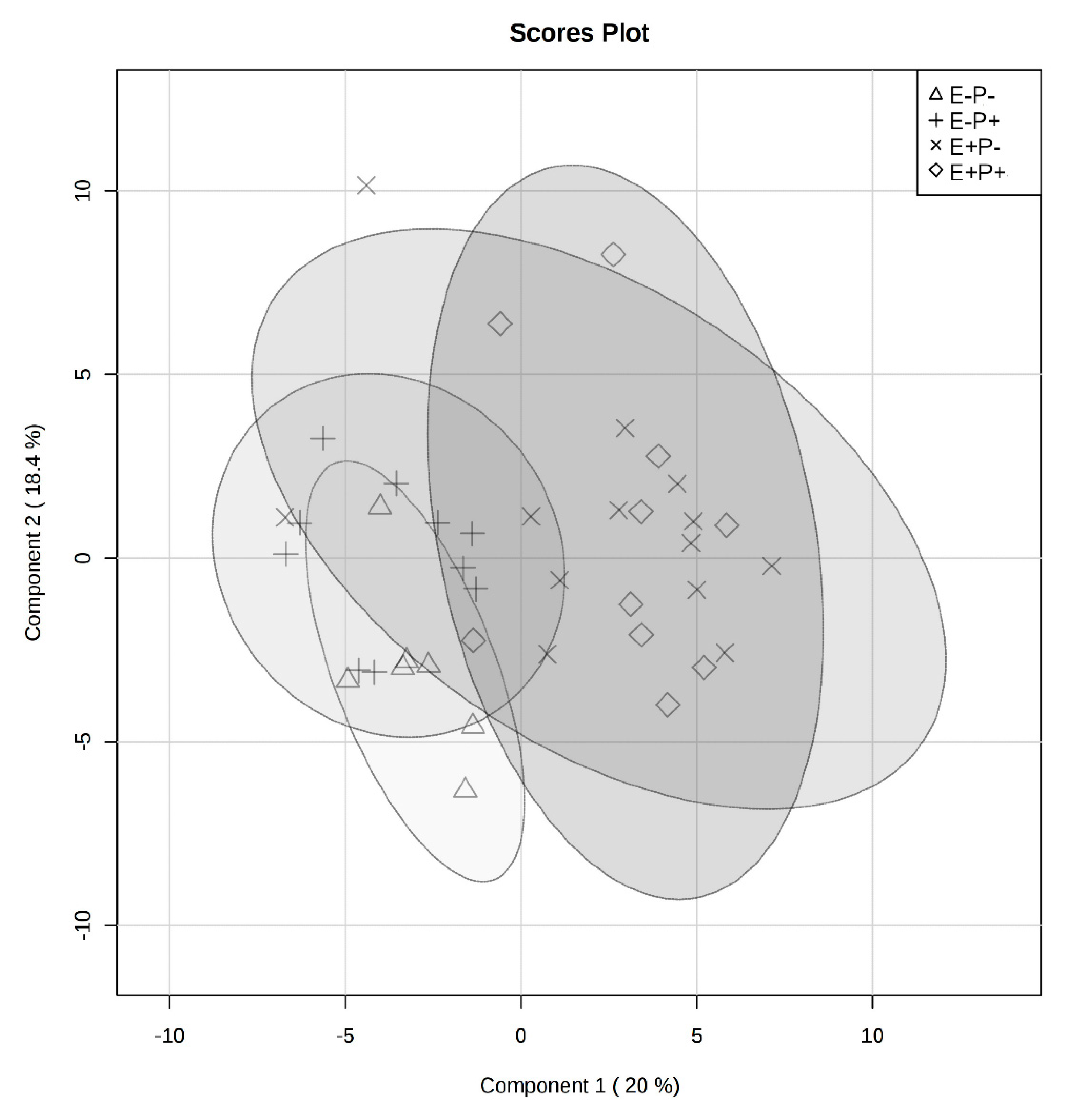
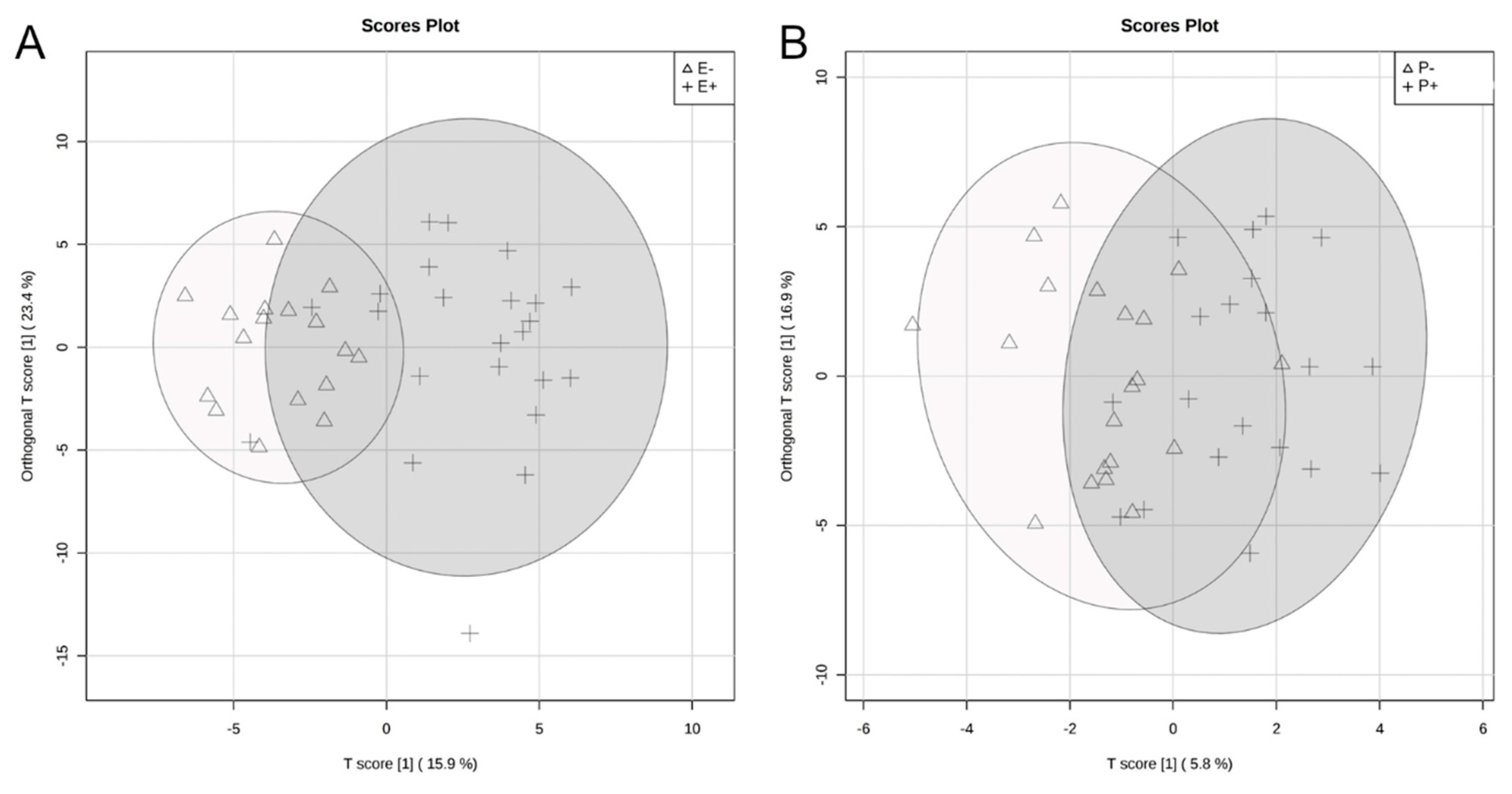
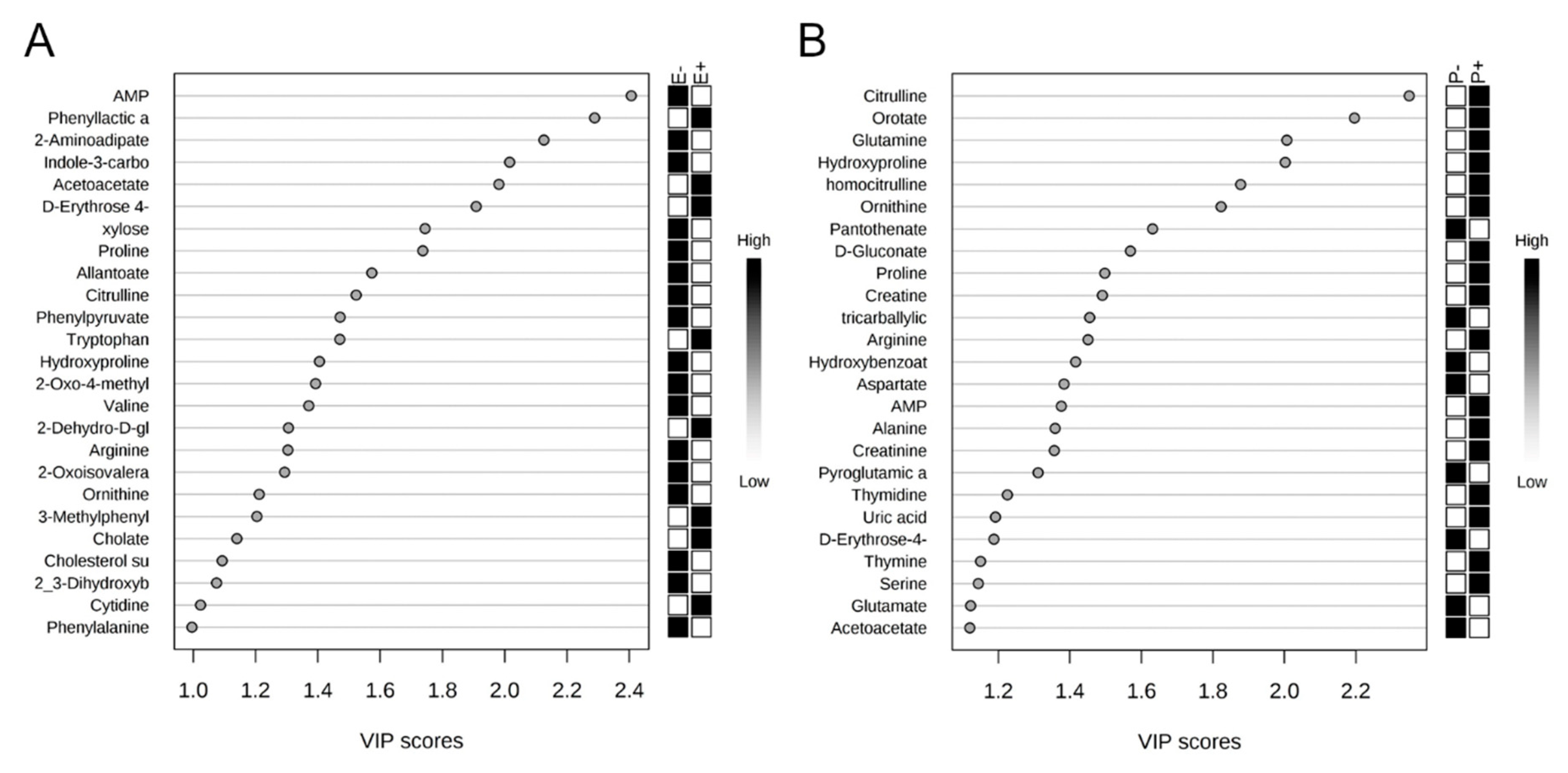
2.2. Rumen Fluid Metabolome
2.3. Serum Metabolome
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Experimental Design and Sample Collection
5.2. Metabolite Extraction and Identification
5.3. Metabolite Identification
5.4. Data Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Poole, R.K.; Poole, D.H. Impact of Ergot Alkaloids on Female Reproduction in Domestic Livestock Species. Toxins 2019, 11, 364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moore, J.D.; Carlisle, A.E.; Nelson, J.A.; McCulley, R.L. Fungal endophyte infection increases tall fescue’s survival, growth, and flowering in a reconstructed prairie. Restor. Ecol. 2019, 27, 1000–1007. [Google Scholar] [CrossRef]
- Klotz, J.L. Activities and Effects of Ergot Alkaloids on Livestock Physiology and Production. Toxins 2015, 7, 2801–2821. [Google Scholar] [CrossRef] [PubMed]
- Strickland, J.R.; Aiken, G.E.; Klotz, J.L. Ergot Alkaloid Induced Blood Vessel Dysfunction Contributes to Fescue Toxicosis. Forage Grazinglands 2009, 7, 1–7. [Google Scholar] [CrossRef]
- Egert, A.M.; Kim, D.H.; Schrick, F.N.; Harmon, D.L.; Klotz, J.L. Dietary exposure to ergot alkaloids decreases contractility of bovine mesenteric vasculature. J. Anim. Sci. 2014, 92, 1768–1779. [Google Scholar] [CrossRef] [Green Version]
- Poole, D.H.; Lyons, S.E.; Poole, R.K.; Poore, M.H. Ergot alkaloids induce vasoconstriction of bovine uterine and ovarian blood vessels. J. Anim. Sci. 2018, 96, 4812–4822. [Google Scholar] [CrossRef] [Green Version]
- Al-Haidary, A.; Spiers, D.; Rottinghaus, G.E.; Garner, G.B.; Ellersieck, M.R. Thermoregulatory ability of beef heifers following intake of endophyte-infected tall fescue during controlled heat challenge. J. Anim. Sci. 2001, 79, 1780–1788. [Google Scholar] [CrossRef]
- Stuedemann, J.A.; Hoveland, C.S. Fescue Endophyte: History and Impact on Animal Agriculture. J. Prod. Agric. 1988, 1, 39–44. [Google Scholar] [CrossRef]
- Roberts, C.A.; Andrae, J. Tall Fescue Toxicosis and Management. Crop Manag. 2004, 3, 1–18. [Google Scholar] [CrossRef]
- Aiken, G.E.; Flythe, M.D.; Kagan, I.A.; Ji, H.; Bush, L.P. Mitigation of Ergot Vasoconstriction by Clover Isoflavones in Goats (Capra hircus). Front. Vet. Sci. 2016, 3, 17. [Google Scholar] [CrossRef] [Green Version]
- Harlow, B.E.; Flythe, M.D.; Kagan, I.A.; Aiken, G.E. Biochanin A (an Isoflavone Produced by Red Clover) Promotes Weight Gain of Steers Grazed in Mixed Grass Pastures and Fed Dried-Distillers’ Grains. Crop Sci. 2017, 57, 506–514. [Google Scholar] [CrossRef]
- Melchior, E.A.; Smith, J.K.; Schneider, L.G.; Mulliniks, J.T.; Bates, G.E.; Flythe, M.D.; Klotz, J.L.; Ji, H.; Goodman, J.P.; Lee, A.R.; et al. Effects of endophyte-infected tall fescue seed and red clover isoflavones on rumen microbial populations and physiological parameters of beef cattle. Transl. Anim. Sci. 2018, 3, 315–328. [Google Scholar] [CrossRef] [PubMed]
- Hoveland, C.S. Importance and economic significance of the Acremonium endophytes to performance of animals and grass plant. Agric. Ecosyst. Environ. 1993, 44, 3–12. [Google Scholar] [CrossRef]
- Kallenbach, R.L. BILL E. KUNKLE INTERDISCIPLINARY BEEF SYMPOSIUM: Coping with tall fescue toxicosis: Solutions and realities. J. Anim. Sci. 2015, 93, 5487–5495. [Google Scholar] [CrossRef] [PubMed]
- Bergman, E.N. Energy contributions of volatile fatty acids from the gastrointestinal tract in various species. Physiol. Rev. 1990, 70, 567–590. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fontanesi, L. Metabolomics and livestock genomics: Insights into a phenotyping frontier and its applications in animal breeding. Anim. Front. 2016, 6, 73–79. [Google Scholar] [CrossRef] [Green Version]
- Clemmons, B.A.; Mihelic, R.I.; Beckford, R.C.; Powers, J.B.; Melchior, E.A.; McFarlane, Z.D.; Cope, E.R.; Embree, M.M.; Mulliniks, J.T.; Campagna, S.R.; et al. Serum metabolites associated with feed efficiency in black angus steers. Metabolomics 2017, 13, 147. [Google Scholar] [CrossRef]
- Wu, X.; Sun, H.-Z.; Xue, M.; Wang, D.; Guan, L.L.; Liu, J. Serum metabolome profiling revealed potential biomarkers for milk protein yield in dairy cows. J. Proteom. 2018, 184, 54–61. [Google Scholar] [CrossRef]
- Aich, P.; Jalal, S.; Czuba, C.; Schatte, G.; Herzog, K.; Olson, D.J.; Ross, A.R.; Potter, A.A.; Babiuk, L.A.; Griebel, P.J. Comparative Approaches to the Investigation of Responses to Stress and Viral Infection in Cattle. OMICS A J. Integr. Biol. 2007, 11, 413–434. [Google Scholar] [CrossRef]
- Zandkarimi, F.; Vanegas, J.; Fern, X.; Maier, C.S.; Bobe, G. Metabotypes with elevated protein and lipid catabolism and inflammation precede clinical mastitis in prepartal transition dairy cows. J. Dairy Sci. 2018, 101, 5531–5548. [Google Scholar] [CrossRef]
- Liao, Y.; Hu, R.; Wang, Z.; Peng, Q.; Dong, X.; Zhang, X.; Zou, H.; Pu, Q.; Xue, B.; Wang, L. Metabolomics Profiling of Serum and Urine in Three Beef Cattle Breeds Revealed Different Levels of Tolerance to Heat Stress. J. Agric. Food Chem. 2018, 66, 6926–6935. [Google Scholar] [CrossRef] [PubMed]
- Crawford, R.J.; Forwood, J.R.; Belyea, R.L.; Garner, G.B. Relationship between Level of Endophyte Infection and Cattle Gains on Tall Fescue. J. Prod. Agric. 1989, 2, 147–151. [Google Scholar] [CrossRef]
- Mote, R.S.; Hill, N.S.; Skarlupka, J.H.; Turner, Z.B.; Sanders, Z.P.; Jones, D.P.; Suen, G.; Filipov, N.M. Response of Beef Cattle Fecal Microbiota to Grazing on Toxic Tall Fescue. Appl. Environ. Microbiol. 2019, 85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saleem, F.; Bouatra, S.; Guo, A.C.; Psychogios, N.; Mandal, R.; Dunn, S.M.; Ametaj, B.N.; Wishart, D.S. The Bovine Ruminal Fluid Metabolome. Metabolomics 2013, 9, 360–378. [Google Scholar] [CrossRef]
- Artegoitia, V.M.; Foote, A.P.; Lewis, R.M.; Freetly, H.C. Rumen Fluid Metabolomics Analysis Associated with Feed Efficiency on Crossbred Steers. Sci. Rep. 2017, 7, 1–14. [Google Scholar] [CrossRef]
- Clemmons, B.A.; Martino, C.; Powers, J.B.; Campagna, S.R.; Voy, B.H.; Donohoe, D.R.; Gaffney, J.; Embree, M.M.; Myer, P.R. Rumen Bacteria and Serum Metabolites Predictive of Feed Efficiency Phenotypes in Beef Cattle. Sci. Rep. 2019, 9, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Alemneh, T.; Getahun, D.; Akeberegn, D.; Getabalew, M.; Zewdie, D. Urea Metabolism and Recycling in Ruminants. Biomed. J. Sci. Tech. Res. 2019, 20, 14790–14796. [Google Scholar] [CrossRef] [Green Version]
- Flythe, M.D.; Kagan, I. Antimicrobial Effect of Red Clover (Trifolium pratense) Phenolic Extract on the Ruminal Hyper Ammonia-Producing Bacterium, Clostridium sticklandii. Curr. Microbiol. 2010, 61, 125–131. [Google Scholar] [CrossRef]
- Leonardi, R.; Zhang, Y.-M.; Rock, C.O.; Jackowski, S. Coenzyme A: Back in action. Prog. Lipid Res. 2005, 44, 125–153. [Google Scholar] [CrossRef]
- Sibley, D.R.; Creese, I. Interactions of ergot alkaloids with anterior pituitary D-2 dopamine receptors. Mol. Pharmacol. 1983, 23, 585–593. [Google Scholar]
- Fernstrom, J.D. Dietary Precursors and Brain Neurotransmitter Formation. Annu. Rev. Med. 1981, 32, 413–425. [Google Scholar] [CrossRef]
- Lin, F.D.; Smith, T.K.; Bayley, H.S. A Role for Tryptophan in Regulation of Protein Synthesis in Porcine Muscle. J. Nutr. 1988, 118, 445–449. [Google Scholar] [CrossRef] [PubMed]
- Dukes, A.; Davis, C.; El Refaey, M.; Upadhyay, S.; Mork, S.; Arounleut, P.; Johnson, M.H.; Hill, W.D.; Isales, C.M.; Hamrick, M.W. The aromatic amino acid tryptophan stimulates skeletal muscle IGF1/p70s6k/mTor signaling in vivo and the expression of myogenic genes in vitro. Nutrition 2015, 31, 1018–1024. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Musumeci, G.; Trovato, F.; Avola, R.; Imbesi, R.; Castrogiovanni, P. Serotonin/growth hormone/insulin-like growth factors axis on pre- and post-natal development: A contemporary review. OA Anat. 2013, 1, 12. [Google Scholar] [CrossRef] [Green Version]
- Ma, H.; Cheng, J.; Zhu, X.; Jia, Z. 2011. Effects of rumen-protected tryptophan on performance, nutrient utilization and plasma tryptophan in cashmere goats. Afr. J. Biotechnol. 2011, 10, 5806–5811. [Google Scholar]
- Lee, J.S.; Priatno, W.; Ghassemi Nejad, J.; Peng, D.Q.; Park, J.S.; Moon, J.O.; Lee, H.G. Effect of dietary rumen-protected L-Tryptophan supplementation on growth performance, blood hematological and biochemical profiles, and gene expression in Korean native steers under cold environment. Animals 2019, 9, 1036. [Google Scholar] [CrossRef] [Green Version]
- Mote, R.S.; Hill, N.S.; Uppal, K.; Tran, V.T.; Jones, D.P.; Filipov, N.M. Metabolomics of fescue toxicosis in grazing beef steers. Food Chem. Toxicol. 2017, 105, 285–299. [Google Scholar] [CrossRef] [Green Version]
- Mote, R.S.; Hill, N.S.; Skarlupka, J.H.; Tran, V.T.; Walker, D.I.; Turner, Z.B.; Sanders, Z.P.; Jones, D.P.; Suen, G.; Filipov, N.M. Toxic tall fescue grazing increases susceptibility of the Angus steer fecal microbiota and plasma/urine metabolome to environmental effects. Sci. Rep. 2020, 10, 2497. [Google Scholar] [CrossRef] [Green Version]
- Kamphorst, J.J.; Fan, J.; Lu, W.; White, E.; Rabinowitz, J.D. Liquid Chromatography–High Resolution Mass Spectrometry Analysis of Fatty Acid Metabolism. Anal. Chem. 2011, 83, 9114–9122. [Google Scholar] [CrossRef] [Green Version]
- Lu, W.; Clasquin, M.F.; Melamud, E.; Amador-Noguez, D.; Caudy, A.A.; Rabinowitz, J.D. Metabolomic Analysis via Reversed-Phase Ion-Pairing Liquid Chromatography Coupled to a Stand Alone Orbitrap Mass Spectrometer. Anal. Chem. 2010, 82, 3212–3221. [Google Scholar] [CrossRef] [Green Version]
- Chambers, M.C.; MacLean, B.; Burke, R.D.; Amodei, D.; Ruderman, D.L.; Neumann, S.; Gatto, L.; Fischer, B.; Pratt, B.; Egertson, J.D.; et al. A cross-platform toolkit for mass spectrometry and proteomics. Nat. Biotechnol. 2012, 30, 918–920. [Google Scholar] [CrossRef] [PubMed]
- Clasquin, M.F.; Melamud, E.; Rabinowitz, J.D. LC-MS Data Processing with MAVEN: A Metabolomic Analysis and Visualization Engine. Curr. Protoc. Bioinform. 2012, 37. [Google Scholar] [CrossRef] [Green Version]
- Chong, J.; Soufan, O.; Li, C.; Caraus, I.; Li, S.; Bourque, G.; Wishart, D.S.; Xia, J. MetaboAnalyst 4.0: Towards more transparent and integrative metabolomics analysis. Nucleic Acids Res. 2018, 46, W486–W494. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kanehisa, M.; Goto, S.; Sato, Y.; Kawashima, M.; Furumichi, M.; Tanabe, M. Data, information, knowledge and principle: Back to metabolism in KEGG. Nucleic Acids Res. 2013, 42, D199–D205. [Google Scholar] [CrossRef] [Green Version]

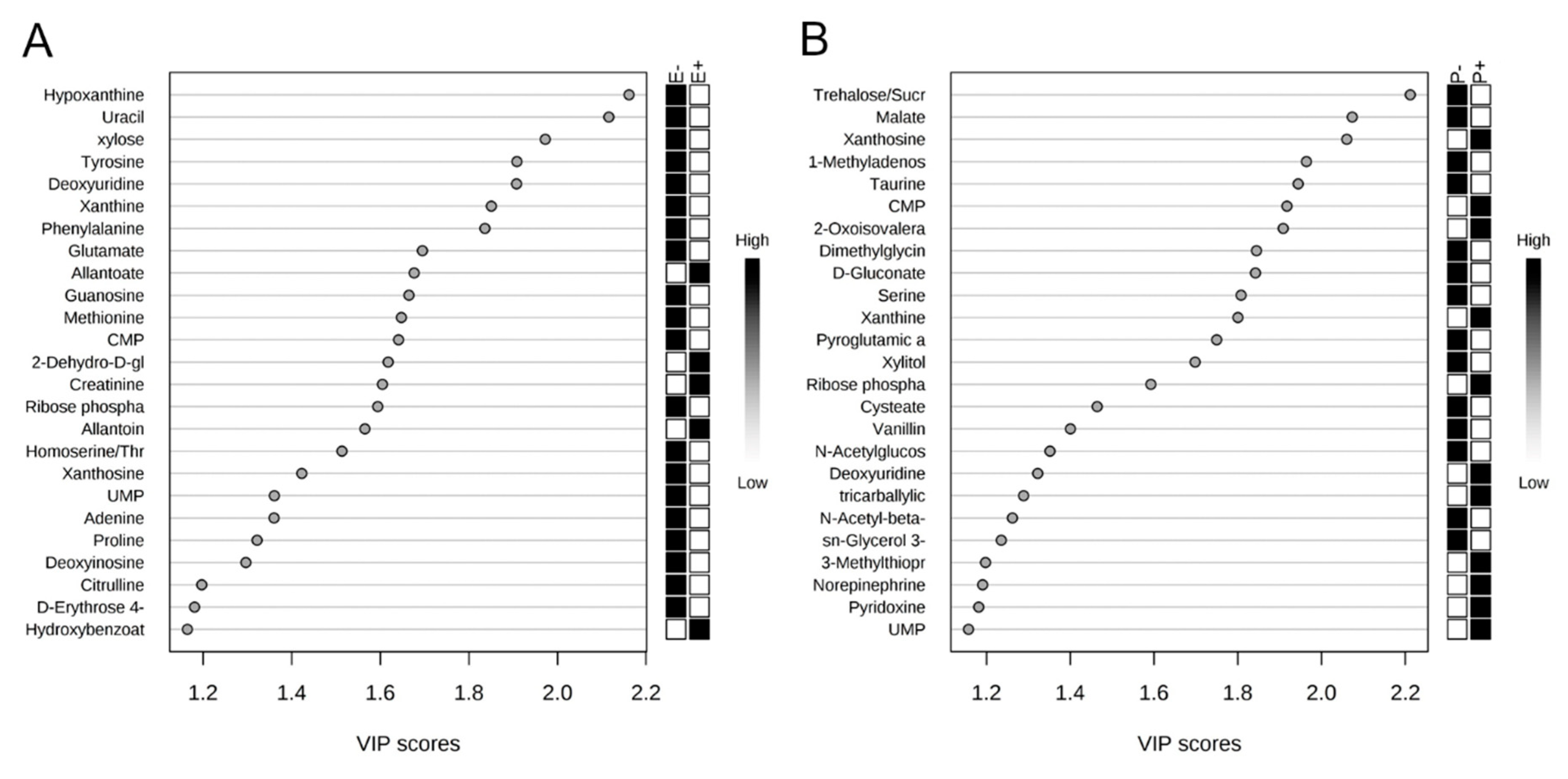
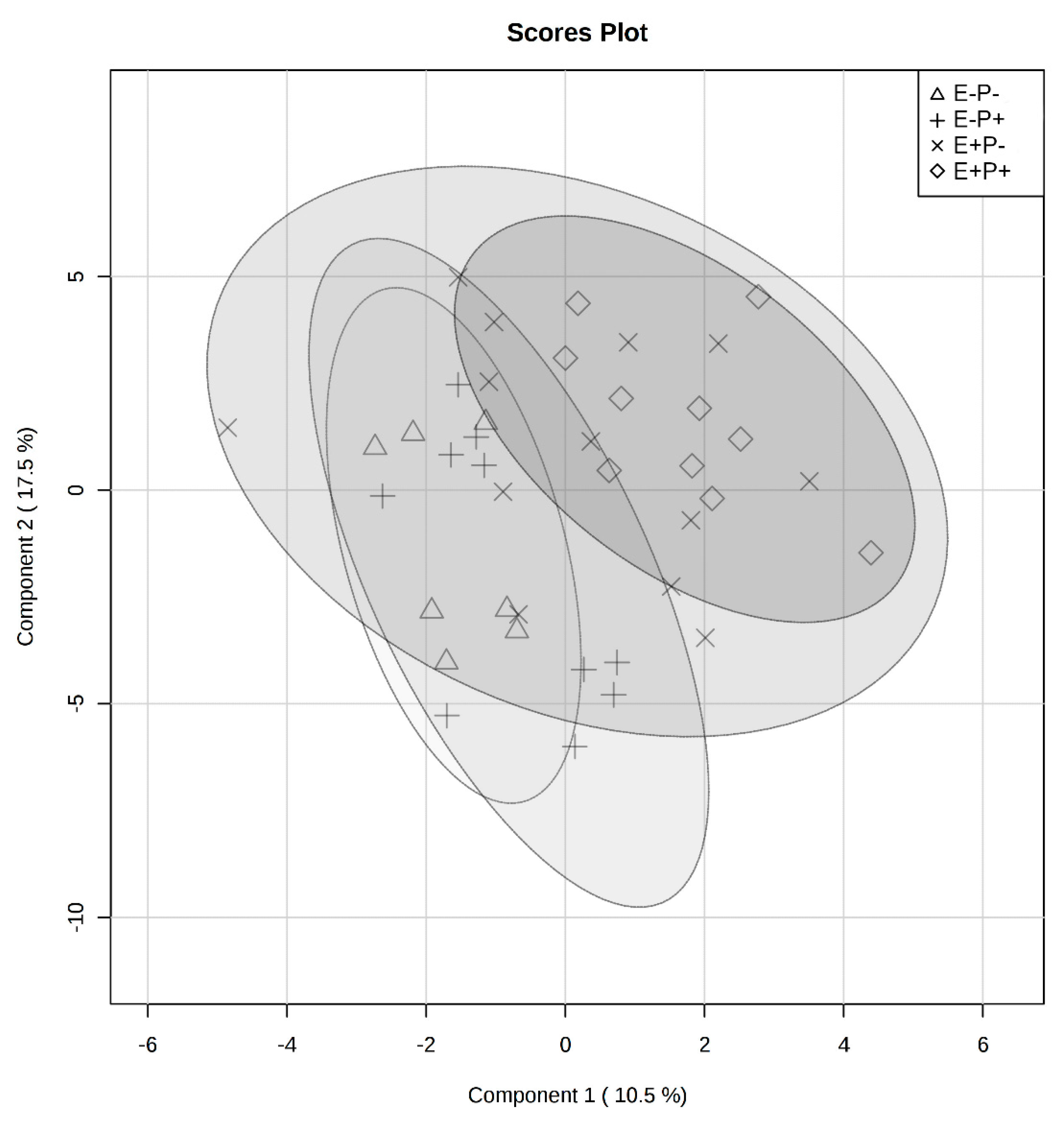
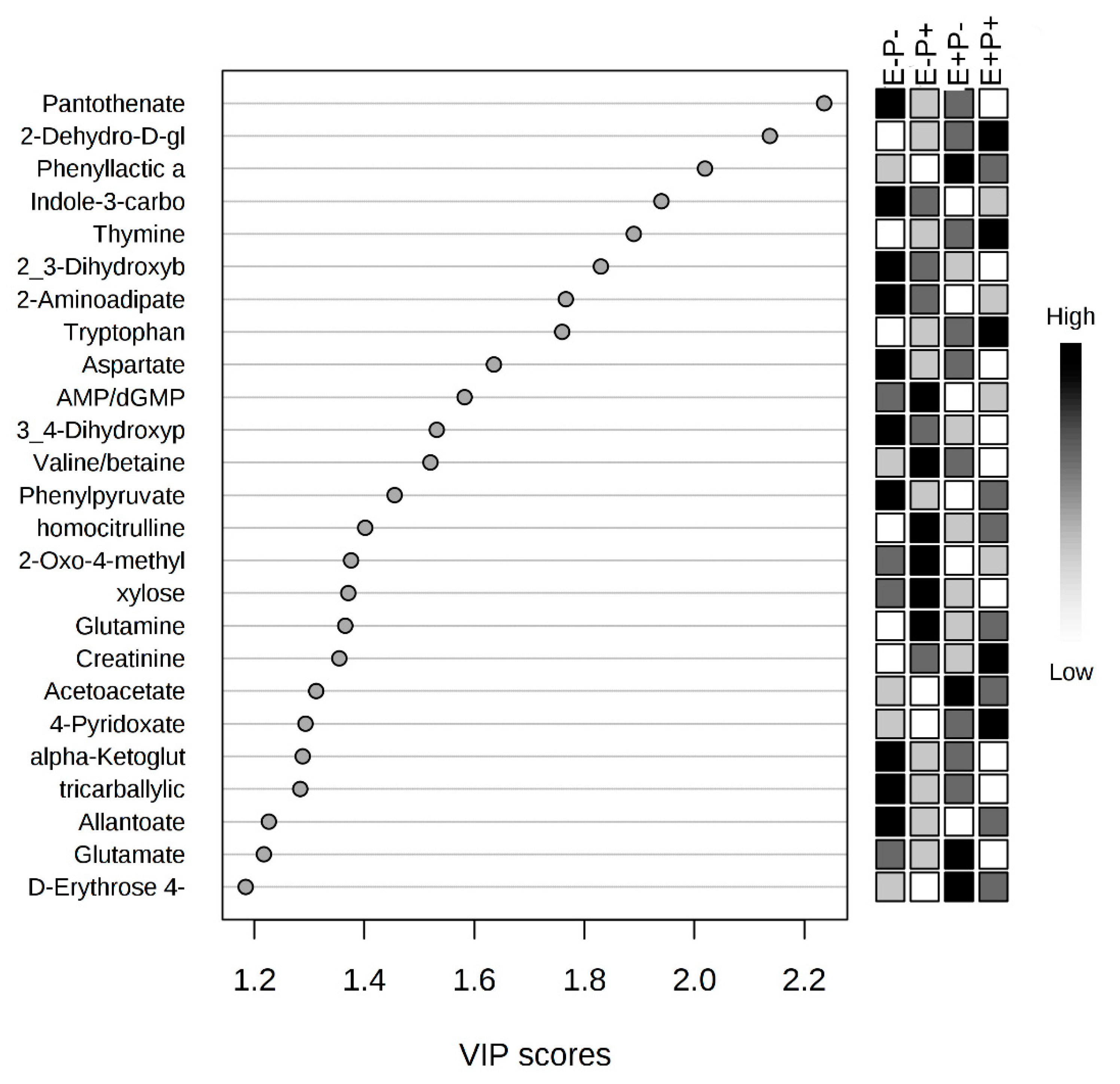
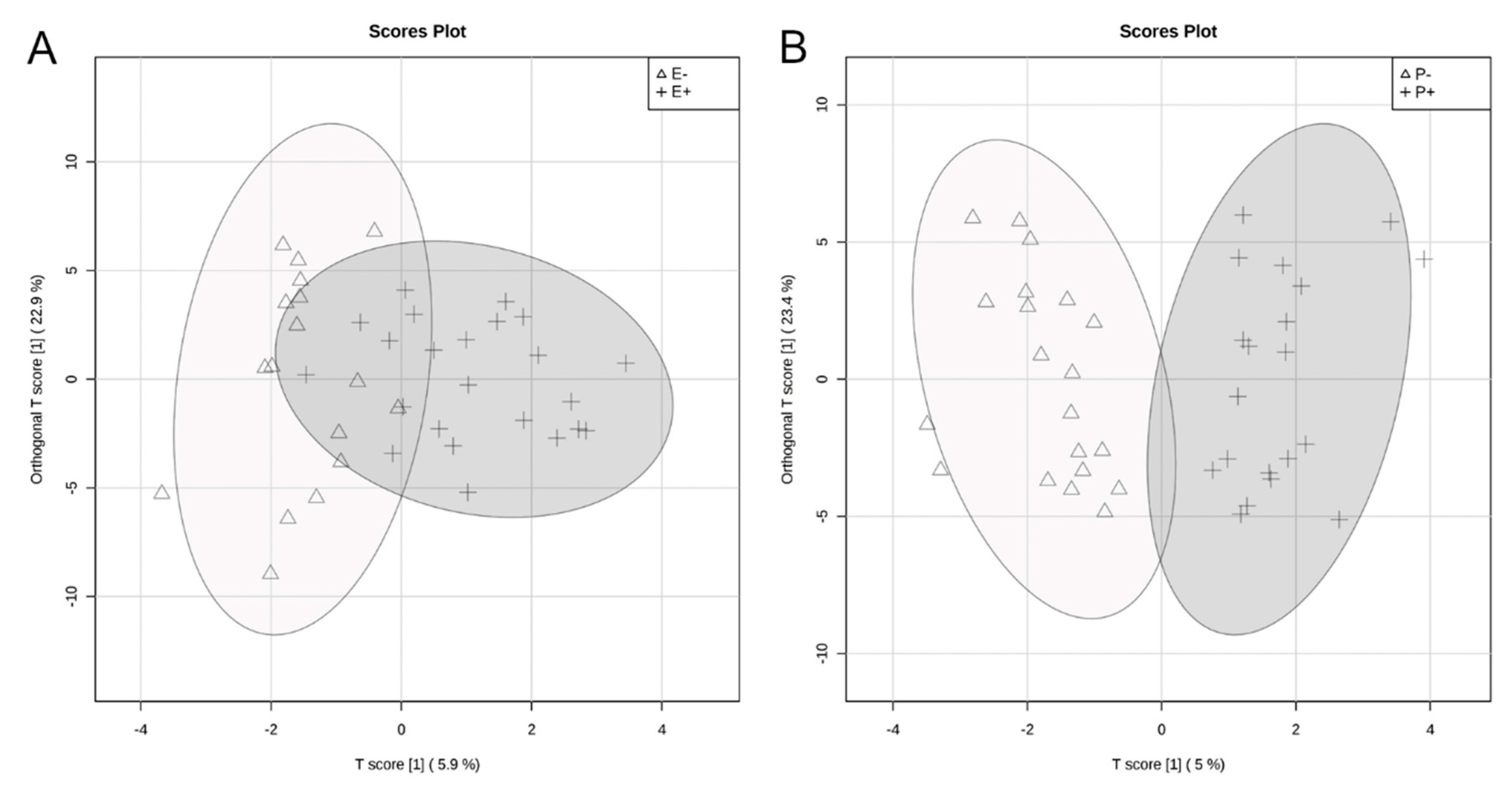
| Metabolite | Seed Type † | p Value € | |
|---|---|---|---|
| E+ | E− | ||
| Dihydroxybenzoate | 5.90 × 107 ± 5.35 × 106 B | 8.43 × 107 ± 6.26 × 106 A | 0.05 |
| Adenine * | 2.30 × 107 ± 1.13 × 107 B | 6.84 × 107 ± 1.32 × 107 A | 0.02 |
| CMP * | 9.17 × 105 ± 7.64 × 105 B | 3.17 × 106 ± 8.95 × 105 A | 0.04 |
| Deoxyuridine * | 8.04 × 105 ± 2.71 × 105 B | 1.74 × 106 ± 3.18 × 105 A | 0.02 |
| Glutamate * | 7.18 × 107 ± 2.17 × 107 B | 1.57 × 108 ± 2.54 × 107 A | 0.05 |
| Guanosine * | 3.00 × 105 ± 1.44 × 105 B | 8.63 × 105 ± 1.69 × 105 A | 0.05 |
| Homoserine/threonine | 1.02 × 107 ± 8.90 × 105 B | 6.65 × 106 ± 7.60 × 105 A | 0.05 |
| Hypoxanthine * | 4.40 × 107 ± 1.66 × 107 B | 1.17 × 108 ± 1.94 × 107 A | 0.01 |
| Uracil * | 5.76 × 107 ± 1.19 × 107 B | 1.08 × 108 ± 1.39 × 107 A | 0.02 |
| Xanthine * | 1.79 × 108 ± 4.34 × 107 B | 3.48 × 108 ± 5.09 × 107 A | 0.01 |
| Xylose * | 3.63 × 106 ± 1.05 × 106 B | 8.69 × 106 ± 1.23 × 106 A | 0.01 |
| Pathway | FDR | Impact | p Value |
|---|---|---|---|
| Seed Type | |||
| Purine metabolism | 2.73 × 10−4 | 0.338 | 6.89 × 10−6 |
| Arginine and proline metabolism | 2.73 × 10−4 | 0.075 | 1.07 × 10−5 |
| Pentose and glucuronate interconversions | 3.42 × 10−4 | 0 | 2.01 × 10−5 |
| Beta-Alanine metabolism | 3.69 × 10−4 | 0 | 2.89 × 10−5 |
| Pyrimidine metabolism | 3.99 × 10−4 | 0.494 | 5.92 × 10−5 |
| Pantothenate and CoA biosynthesis | 3.99 × 10−4 | 0.229 | 6.7 × 10−5 |
| Aminoacyl-tRNA biosynthesis | 3.99 × 10−4 | 0.2 | 7.81 × 10−5 |
| Tyrosine metabolism | 3.99 × 10−4 | 0 | 7.83 × 10−5 |
| Novobiocin biosynthesis | 3.99 × 10−4 | 0 | 7.83 × 10−5 |
| Thiamine metabolism | 3.99 × 10−4 | 0 | 7.83 × 10−5 |
| Phenylalanine metabolism | 7.94 × 10−4 | 0.001 | 1.71 × 10−4 |
| Phenylalanine, tyrosine, and tryptophan biosynthesis | 0.001 | 4.60 × 10−4 | 2.78 × 10−4 |
| Carbapenem biosynthesis | 0.001 | 0 | 3.25 × 10−4 |
| Butanoate metabolism | 0.001 | 0 | 3.25 × 10−4 |
| Porphyrin and chlorophyll metabolism | 0.001 | 0 | 3.25 × 10−4 |
| Pentose phosphate pathway | 0.003 | 0.07 | 0.001 |
| Amino sugar and nucleotide sugar metabolism | 0.004 | 0.109 | 0.001 |
| Glutathione metabolism | 0.004 | 0.014 | 0.002 |
| D-Glutamine and D-glutamate metabolism | 0.004 | 0.172 | 0.002 |
| Nitrogen metabolism | 0.004 | 0 | 0.002 |
| Isoflavone Treatment | |||
| Methane metabolism | 0.84824 | 0.154 | 0.032 |
| Sulfur metabolism | 0.84824 | 0 | 0.033 |
| Metabolite | Isoflavone Treatment † | p Value € | |
|---|---|---|---|
| P+ | P− | ||
| Histidine * | 8.50 × 106 ± 8.94 × 105 | 1.05 × 107 ± 9.37 × 105 | 0.05 |
| Cytidine * | 1.51 × 106 ± 4.89 × 105 B | 2.67 × 107 ± 5.12 × 105 A | 0.01 |
| Pantothenate | 6.64 × 106 ± 1.84 × 106 B | 1.51 × 107 ± 1.93 × 106 A | 0.01 |
| Homocysteine | 1.47 × 106 ± 1.28 × 105 B | 2.02 × 106 ± 1.35 × 105 A | 0.02 |
| Allantoin | 1.94 × 108 ± 1.14 × 107 B | 2.37 × 108 ± 1.19 × 107 A | 0.03 |
| GABA | 9.68 × 105 ± 1.40 × 105 B | 1.41 × 106 ± 1.44 × 105 A | 0.05 |
| Methylhistidine | 8.35 × 105 ± 6.25 × 104 | 1.04 × 106 ± 6.51 × 104 | 0.05 |
| Pathway | FDR | Impact | p Value |
|---|---|---|---|
| Seed Type | |||
| Glyoxylate and dicarboxylate metabolism | 0.013 | 0.11 | 0.005 |
| Arginine biosynthesis | 0.013 | 0.51 | 0.006 |
| Alanine, aspartate, and glutamate metabolism | 0.015 | 0.73 | 0.007 |
| Cysteine and methionine metabolism | 0.051 | 0.14 | 0.024 |
| Glycine, serine, and threonine metabolism | 0.054 | 0.16 | 0.029 |
| Ubiquinone and other terpenoid-quinone biosynthesis | 0.054 | 0 | 0.029 |
| Aminobenzoate degradation | 0.054 | 0 | 0.029 |
| Vitamin B6 metabolism | 0.069 | 0.05 | 0.039 |
| Monobactam biosynthesis | 0.069 | 0 | 0.041 |
| Lysine biosynthesis | 0.069 | 0 | 0.041 |
| Nicotinate and nicotinamide metabolism | 0.069 | 0.06 | 0.042 |
| Tryptophan metabolism | 0.07 | 0 | 0.044 |
| Cyanoamino acid metabolism | 0.076 | 0 | 0.05 |
| Isoflavone Treatment | |||
| Pyrimidine metabolism | 0.151 | 0.37 | 0.007 |
| Arginine and proline metabolism | 0.151 | 0.19 | 0.008 |
| D-glutamine and D-glutamate metabolism | 0.151 | 0.17 | 0.013 |
| Nitrogen metabolism | 0.151 | 0 | 0.013 |
| Arginine biosynthesis | 0.151 | 0.51 | 0.015 |
| Glutathione metabolism | 0.173 | 0.01 | 0.02 |
| Purine metabolism | 0.209 | 0.09 | 0.029 |
| Glyoxylate and dicarboxylate metabolism | 0.269 | 0.13 | 0.045 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ault-Seay, T.B.; Melchior-Tiffany, E.A.; Clemmons, B.A.; Cordero, J.F.; Bates, G.E.; Flythe, M.D.; Klotz, J.L.; Ji, H.; Goodman, J.P.; McLean, K.J.; et al. Rumen and Serum Metabolomes in Response to Endophyte-Infected Tall Fescue Seed and Isoflavone Supplementation in Beef Steers. Toxins 2020, 12, 744. https://doi.org/10.3390/toxins12120744
Ault-Seay TB, Melchior-Tiffany EA, Clemmons BA, Cordero JF, Bates GE, Flythe MD, Klotz JL, Ji H, Goodman JP, McLean KJ, et al. Rumen and Serum Metabolomes in Response to Endophyte-Infected Tall Fescue Seed and Isoflavone Supplementation in Beef Steers. Toxins. 2020; 12(12):744. https://doi.org/10.3390/toxins12120744
Chicago/Turabian StyleAult-Seay, Taylor B., Emily A. Melchior-Tiffany, Brooke A. Clemmons, Juan F. Cordero, Gary E. Bates, Michael D. Flythe, James L. Klotz, Huihua Ji, Jack P. Goodman, Kyle J. McLean, and et al. 2020. "Rumen and Serum Metabolomes in Response to Endophyte-Infected Tall Fescue Seed and Isoflavone Supplementation in Beef Steers" Toxins 12, no. 12: 744. https://doi.org/10.3390/toxins12120744






