Simulation of Food Folate Digestion and Bioavailability of an Oxidation Product of 5-Methyltetrahydrofolate
Abstract
:1. Introduction
2. Experimental
2.1. Chemicals and Reagents
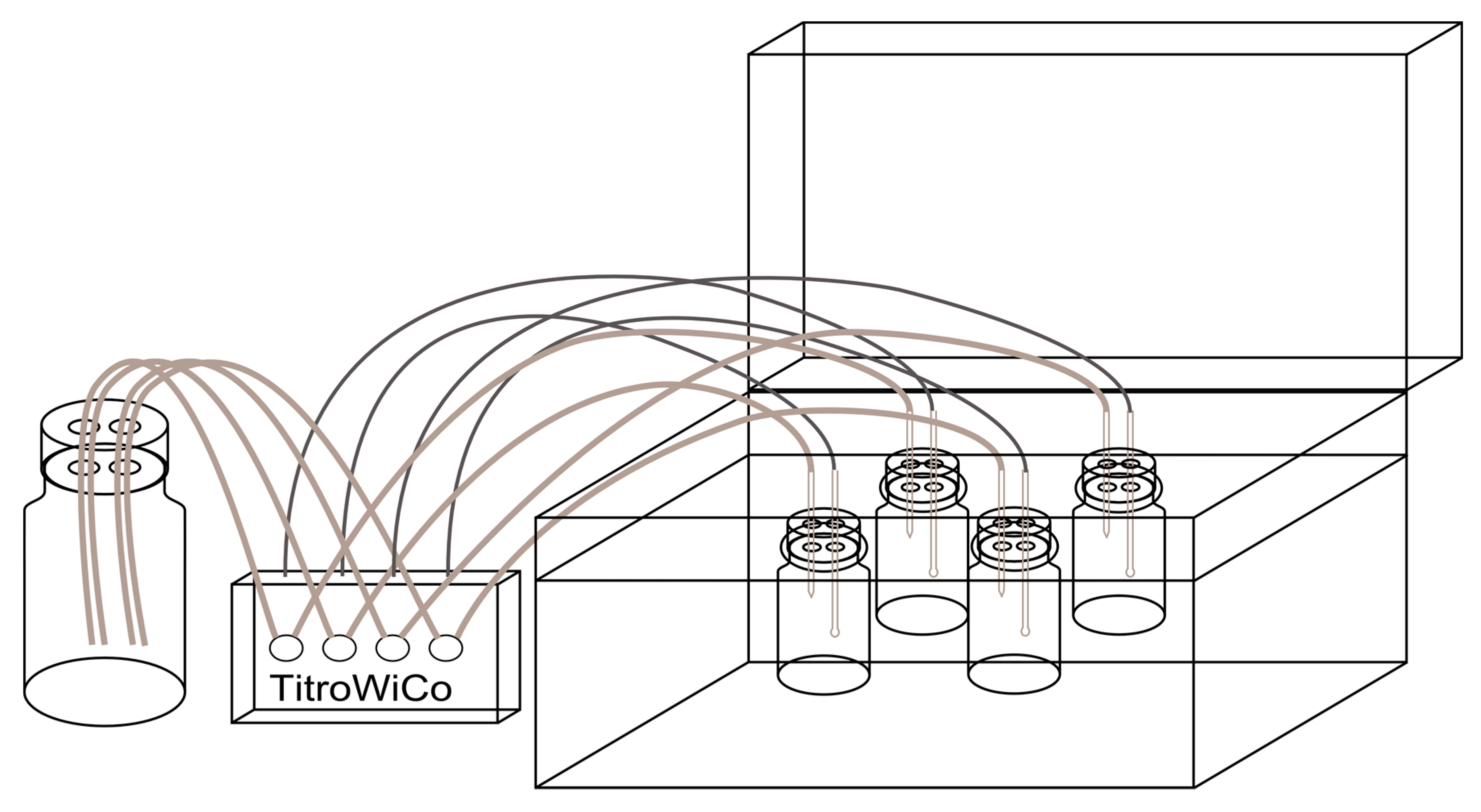
2.2. In Vitro Digestion Model
2.3. Quantification of Folates
2.4. Applicability of the Simulated Digestion
2.4.1. Deconjugation Efficiency
2.4.2. Effectiveness of the In Vitro Model in the Digestion of Starch, Proteins and Fat
2.5. Preliminary Human Study
3. Results
3.1. Effectiveness of the In Vitro Model Regarding Digestion of Starch, Proteins and Triglycerides
3.2. Variability of the In Vitro Digestion Model Using Spinach as a Model Matrix
3.3. Stability of the Single Vitamers
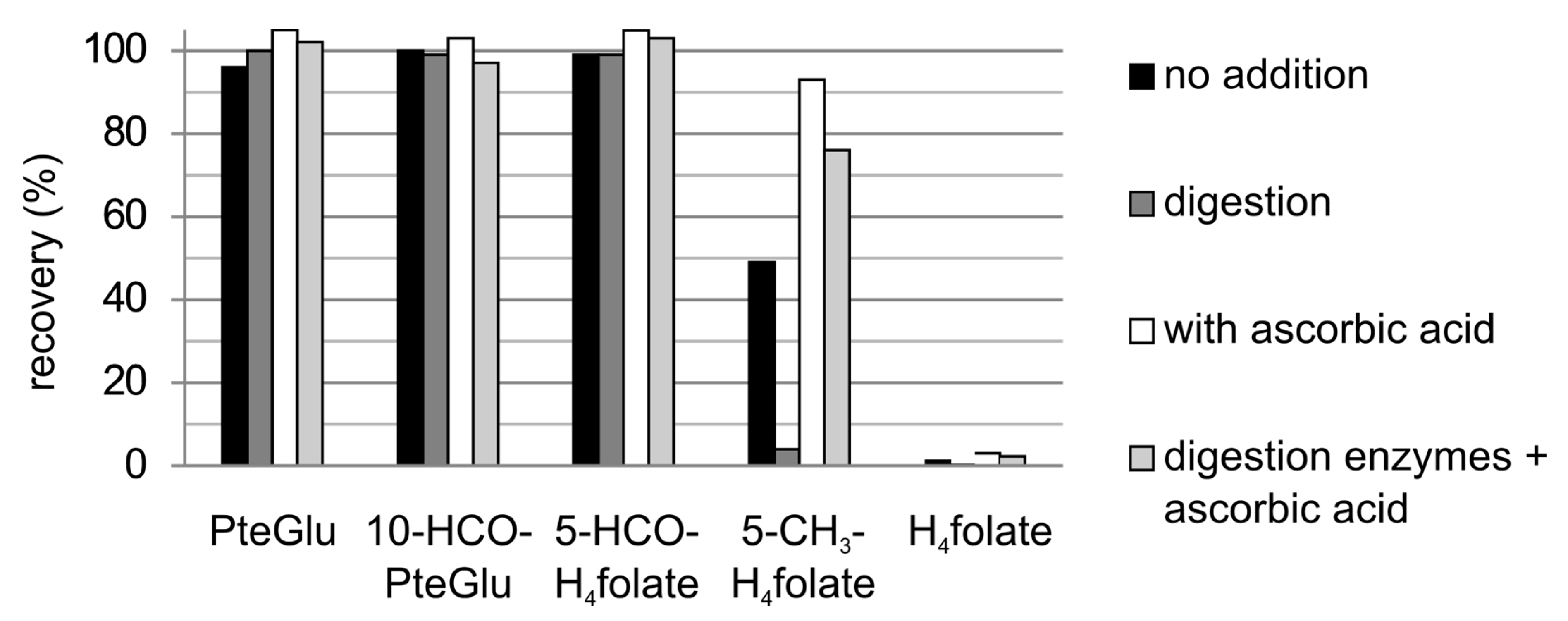
3.3.1. Recovery
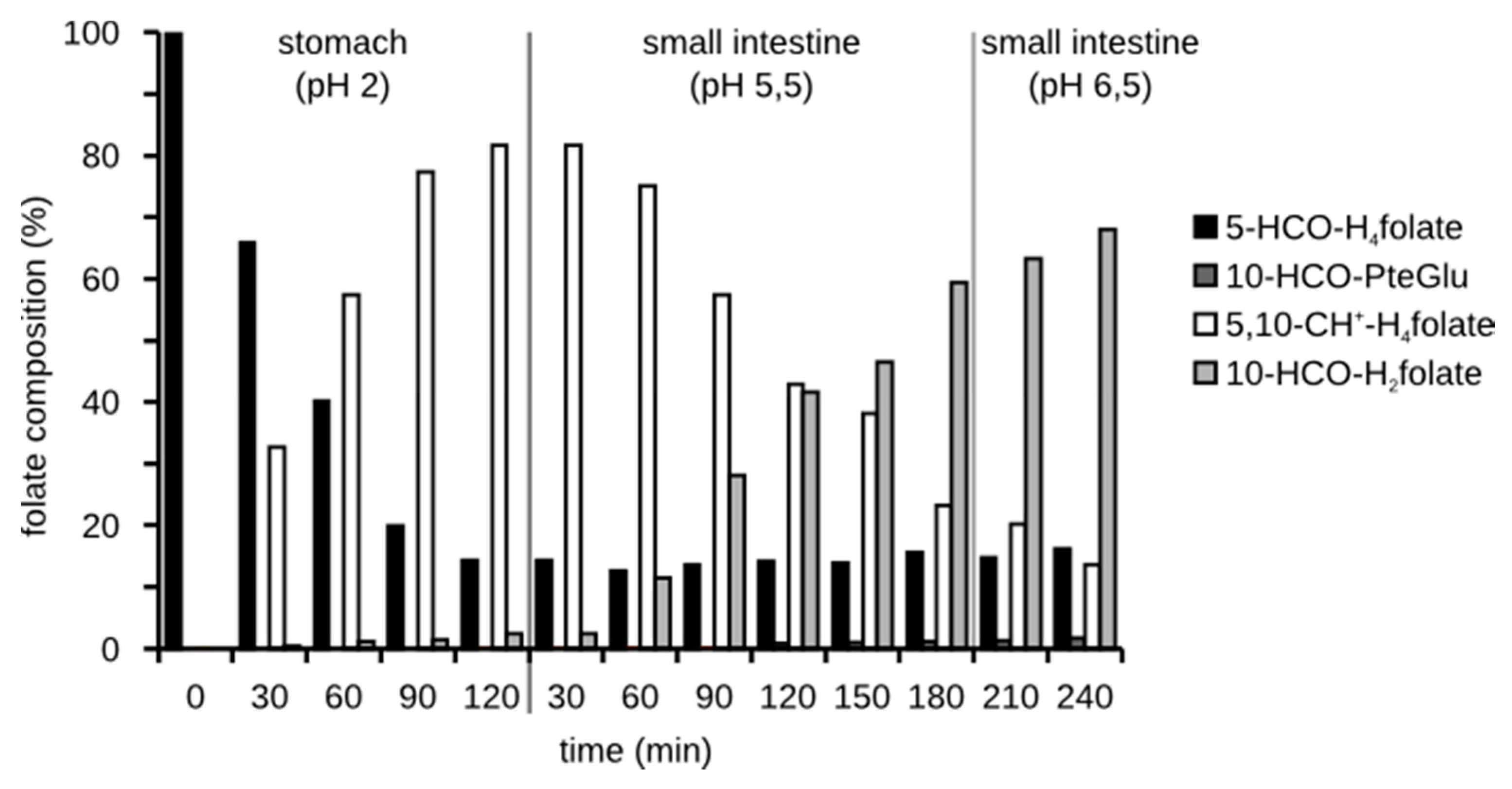
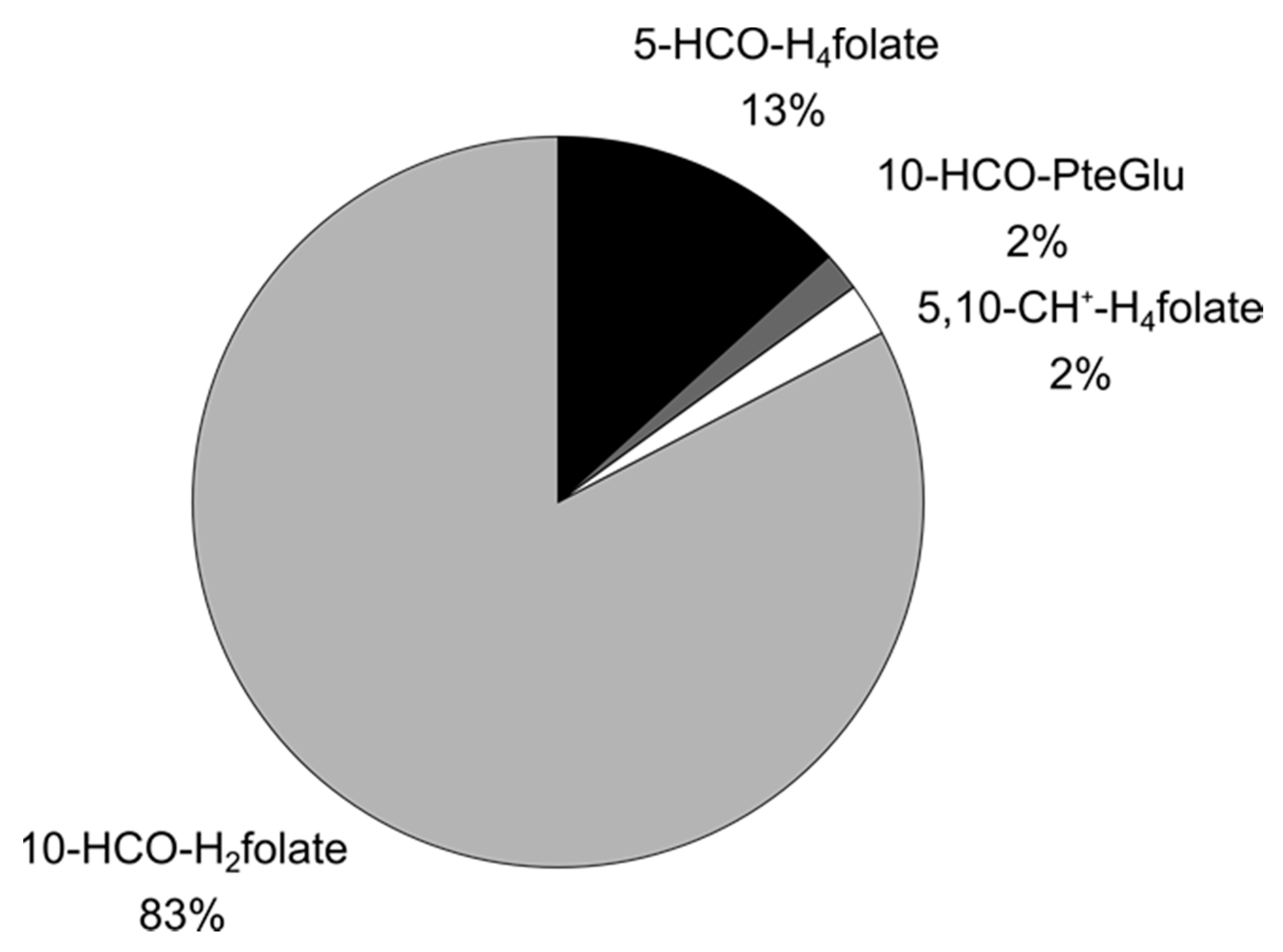
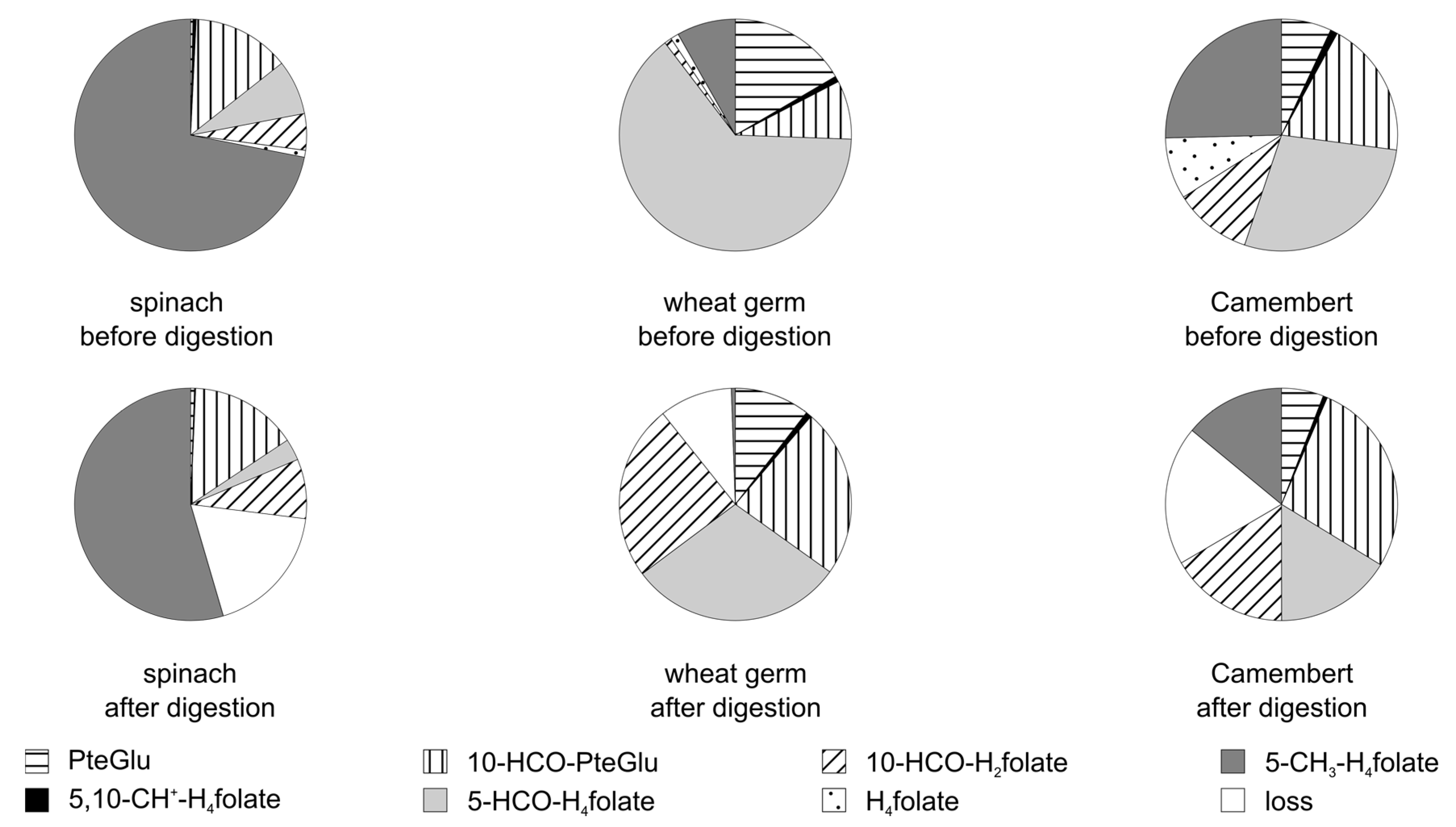
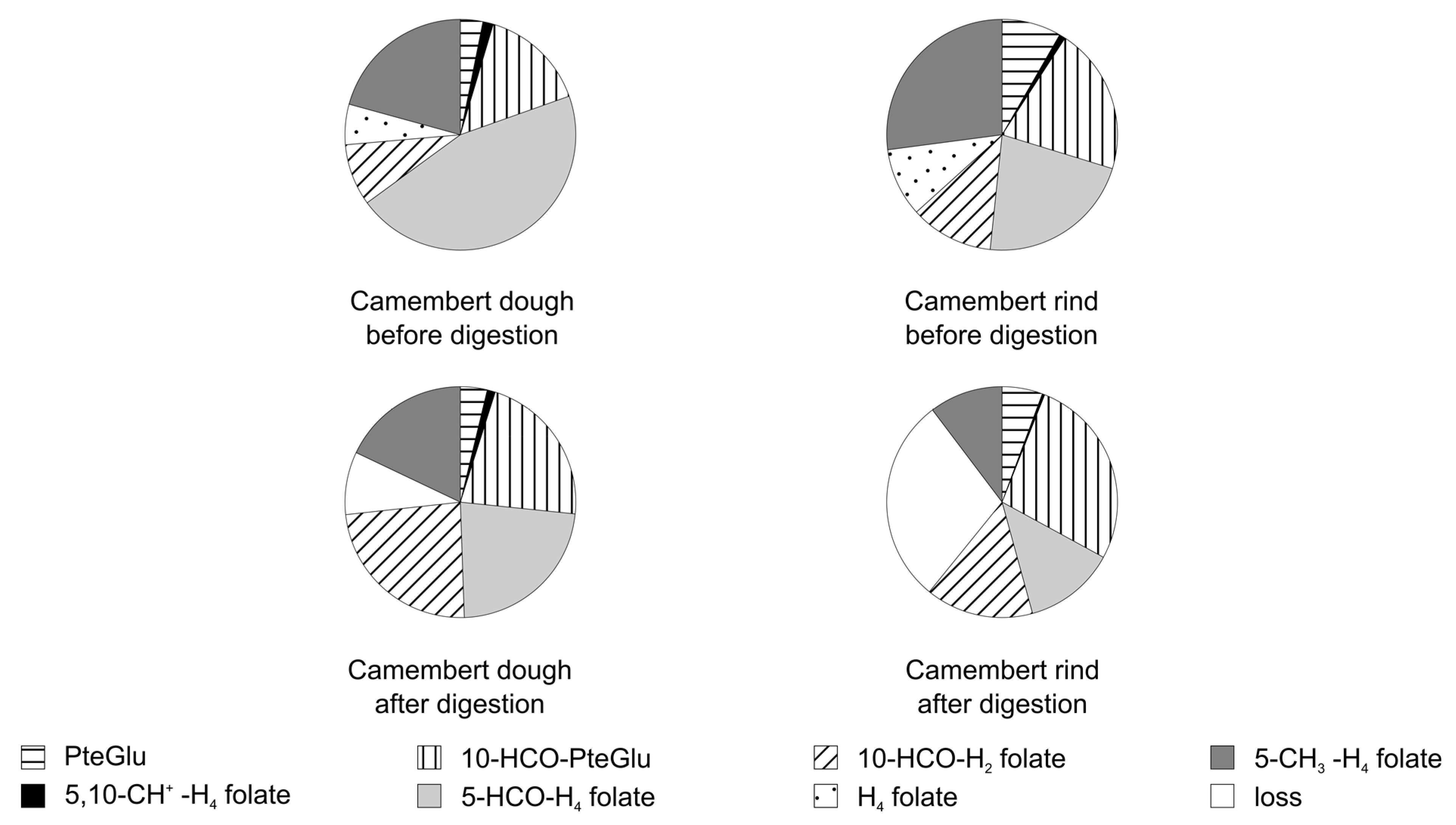
3.3.2. Folate Composition after Digestion
3.4. Stability of Food Folate
3.5. Deconjugation
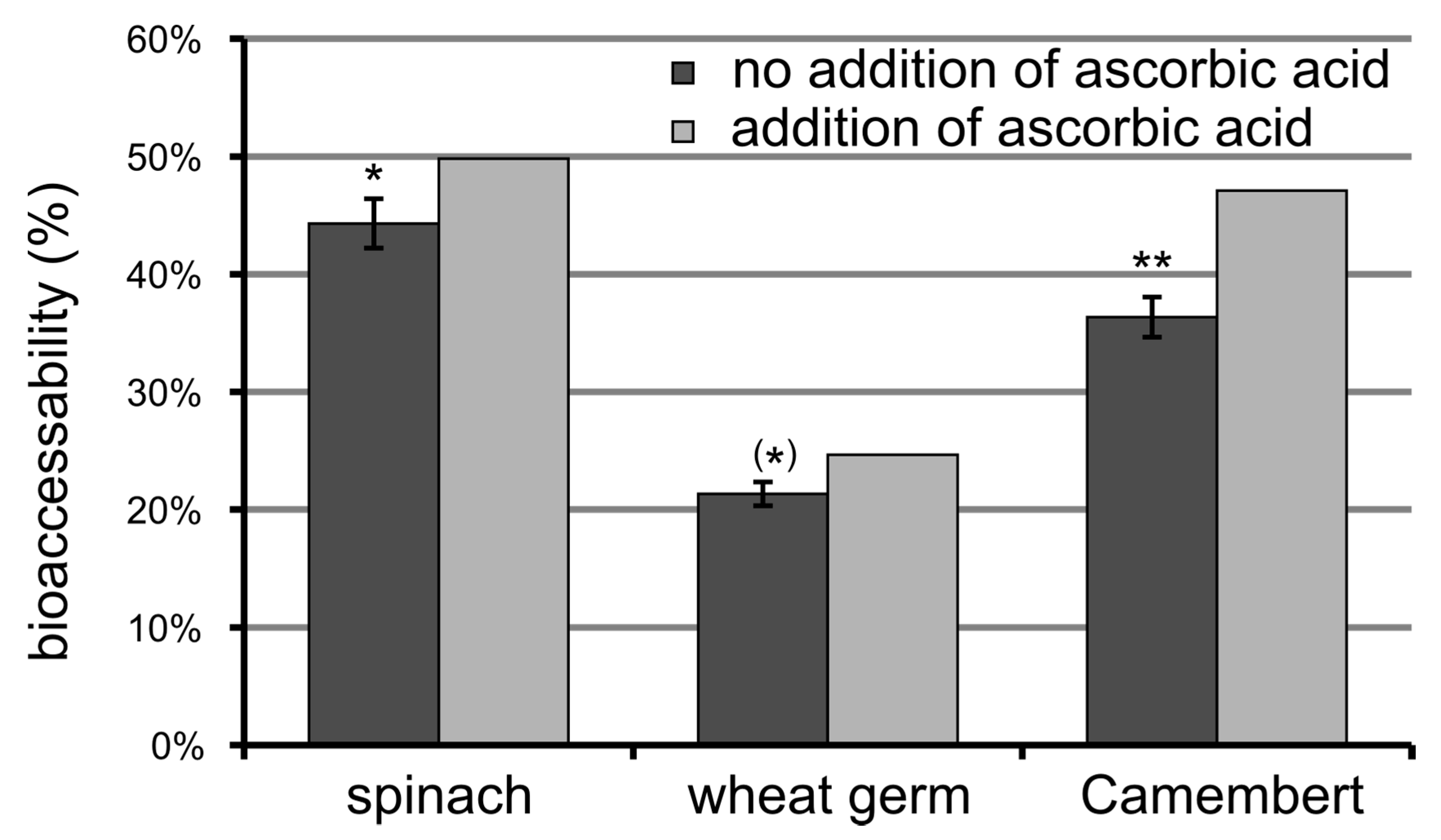
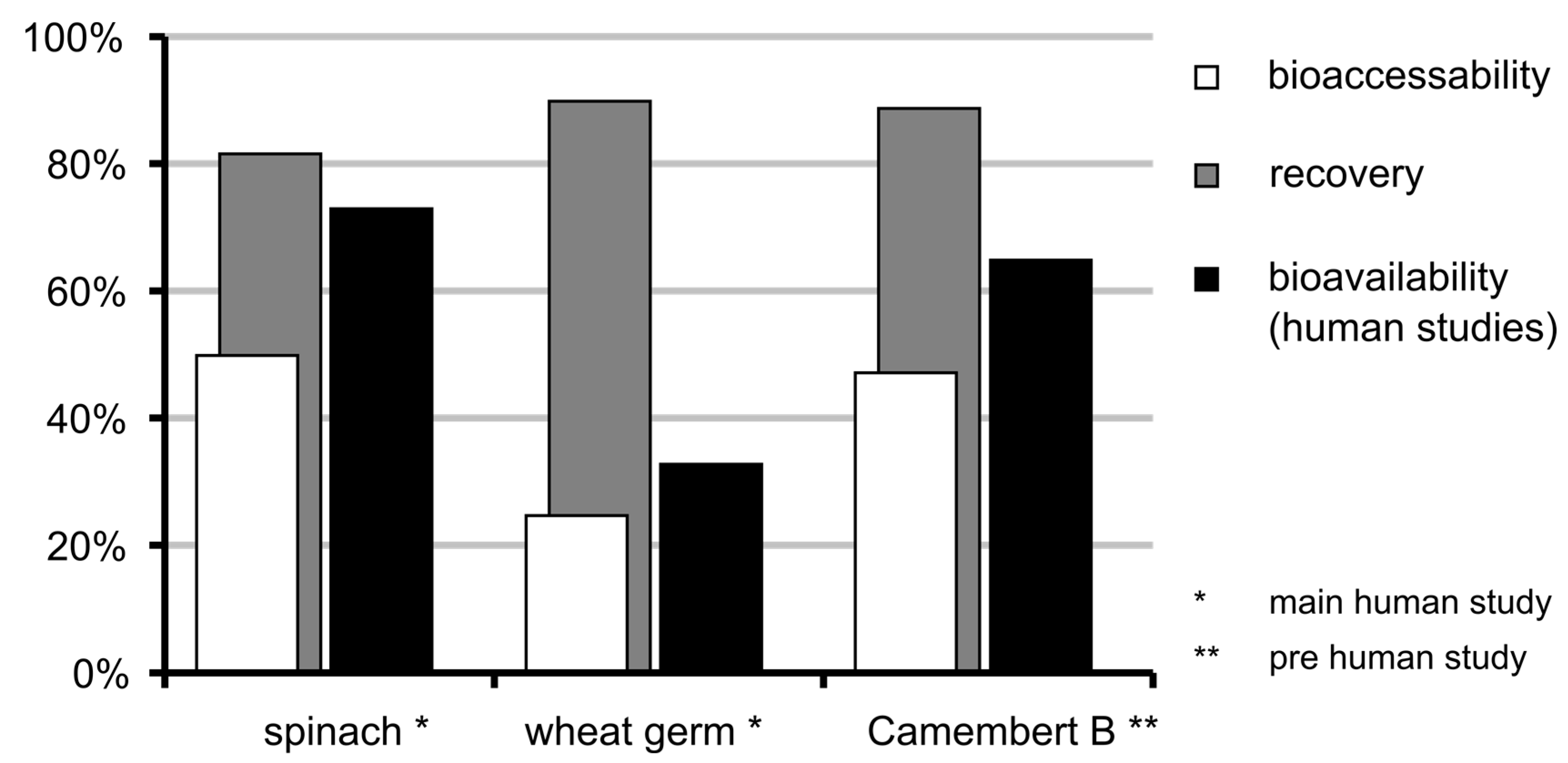
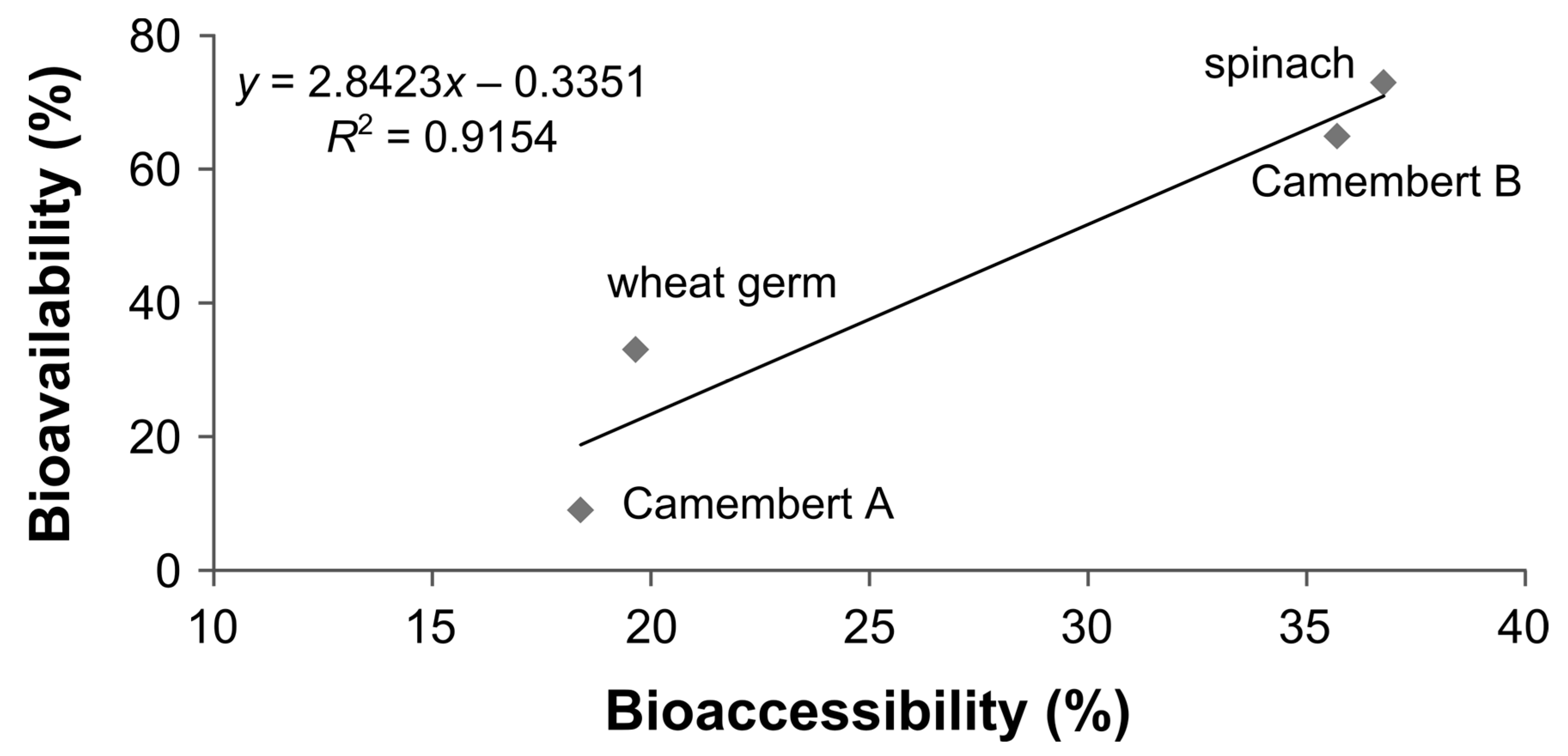
3.6. Bioaccessibility of Food Folate
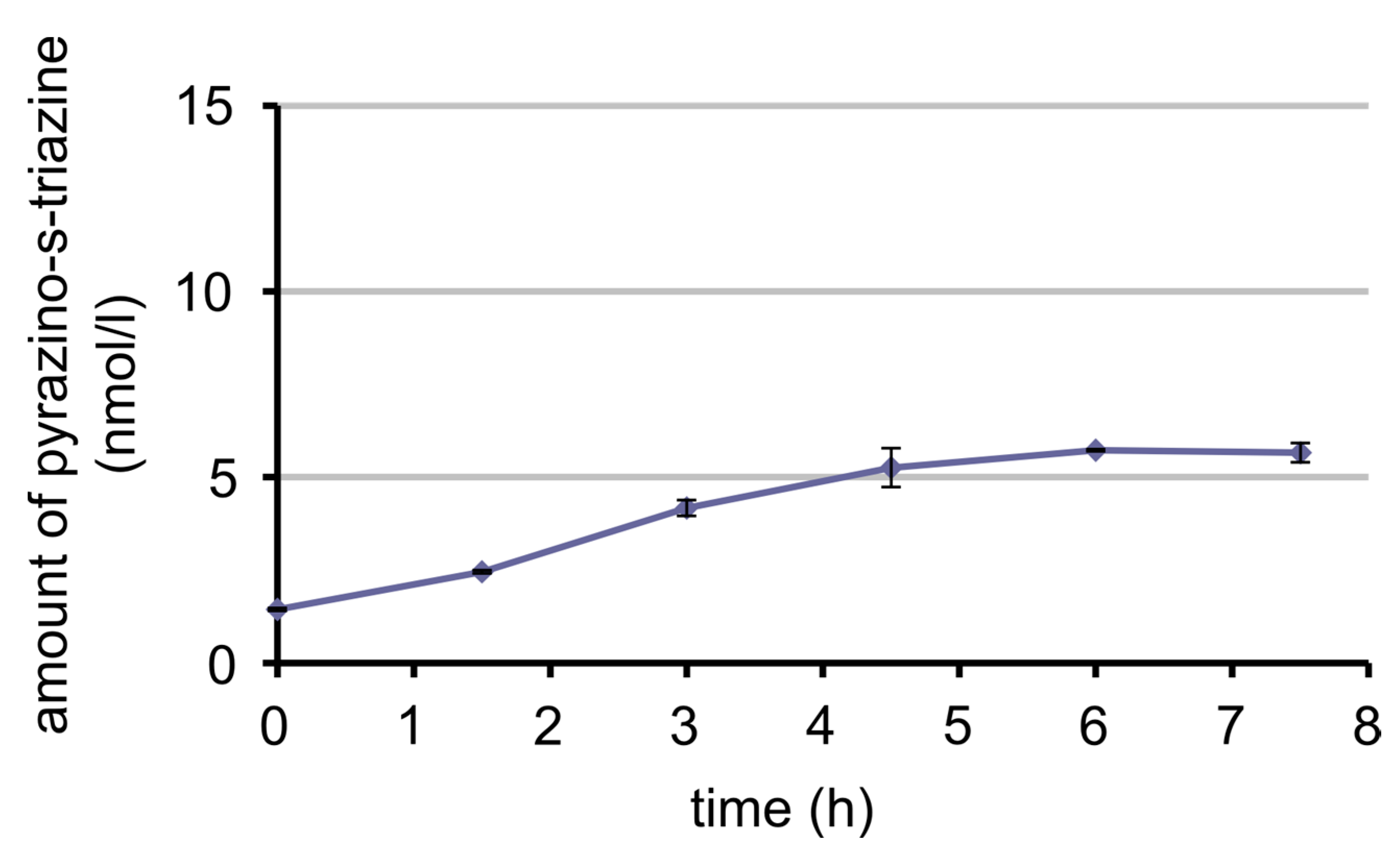
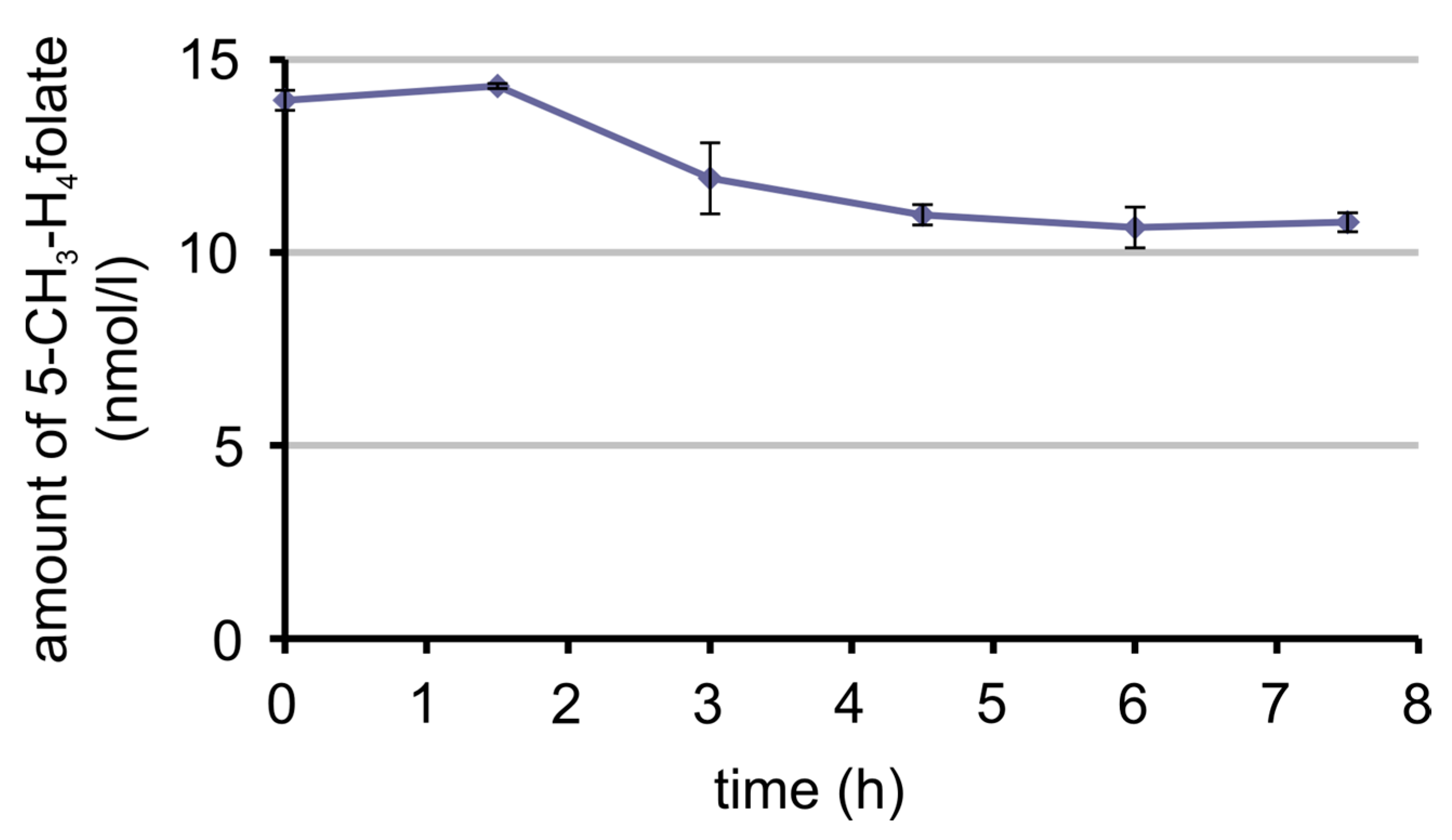
3.7. Bioavailability of MeFox
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Robinson, K. Homocysteine, B vitamins, and risk of cardiovascular disease. Heart 2000, 83, 127–130. [Google Scholar] [CrossRef] [PubMed]
- Czeizel, A.E.; Dudás, I. Prevention of the first occurrence of neural-tube defects by periconceptional vitamin supplementation. N. Engl. J. Med. 1992, 327, 1832–1835. [Google Scholar] [CrossRef] [PubMed]
- Institute of Medicine (US) Standing Committee on the Scientific Evaluation of Dietary Reference Intakes. In Dietary Reference Intakes for Thiamin, Riboflavin, Niacin, Vitamin B6, Folate, Vitamin B12, Pantothenic Acid, Biotin, and Choline; National Academy Press: Washington, DC, USA, 1998.
- Krawinkel, M.B.; Strohm, D.; Weissenborn, A.; Watzl, B.; Eichholzer, M.; Bärlocher, K.; Elmadfa, I.; Leschik-Bonnet, E.; Heseker, H. Revised D-A-CH intake recommendations for folate: How much is needed. Eur. J. Clin. Nutr. 2014, 68, 719–723. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Matherly, L.H.; Goldman, I.D. Membrane transporters and folate homeostasis: Intestinal absorption and transport into systemic compartments and tissues. Expert Rev. Mol. Med. 2009, 11, e4. [Google Scholar] [CrossRef] [PubMed]
- Ball, G.F.M. Bioavailability and Analysis of Vitamins in Foods; Chapman & Hall: London, UK, 1998. [Google Scholar]
- Ndaw, S.; Bergaentzle, M.; Aoude-Werner, D.; Lahely, S.; Hasselmann, C. Determination of folates in foods by high-performance liquid chromatography with fluorescence detection after precolumn conversion to 5-methyltetrahydrofolates. J. Chrom. A 2001, 928, 77–90. [Google Scholar] [CrossRef]
- Garratt, L.C.; Ortori, C.A.; Tucker, G.A.; Sablitzky, F.; Bennett, M.J.; Barrett, D.A. Comprehensive metabolic profiling of mono- and polyglutamated folates and their precursors in plant and animal tissue using liquid chromatography/negative ion electrospray ionisation tandem mass spectrometry. Rapid Commun. Mass Spectrom. 2005, 19, 2390–2398. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.T.; Reisenauer, A.M.; Halsted, C.H. Comparison of folate conjugase activities in human, pig, rat and monkey intestine. J. Nutr. 1985, 115, 814–819. [Google Scholar] [PubMed]
- Chanarin, I.; Perry, J. Evidence for reduction and methylation of folate in the intestine during normal absorption. Lancet 1969, 294, 776–778. [Google Scholar] [CrossRef]
- Perry, J.; Chanarin, I. Intestinal absorption of reduced folate compounds in man. Brit. J. Haematol. 1970, 18, 329–339. [Google Scholar] [CrossRef]
- Shane, B. Folate Chemistry and Metabolism. In Folate in Health and Disease, 2nd ed.; Bailey, L.B., Ed.; Taylor & Francis: Boca Raton, FL, USA, 2010. [Google Scholar]
- Caudill, M.A. Folate bioavailability: Implications for establishing dietary recommendations and optimizing status. Am. J. Clin. Nutr. 2010, 91, 1455–1460. [Google Scholar] [CrossRef] [PubMed]
- McNulty, H.; Pentieva, K. Folate bioavailability. Proc. Nutr. Soc. 2004, 63, 529–536. [Google Scholar] [CrossRef] [PubMed]
- Mönch, S.; Netzel, M.; Netzel, G.; Ott, U.; Frank, T.; Rychlik, M. Folate bioavailability from foods rich in folates assessed in a short term human study using stable isotope dilution assays. Food Function 2015, 6, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Mönch, S.; Netzel, M.; Netzel, G.; Ott, U.; Frank, T.; Rychlik, M. Pilot Study on Folate Bioavailability from a Camembert Cheese Reveals Contradictory Findings to Recent Results from a Human Short-term Study. Front. Nutr. 2016, 3, 9. [Google Scholar] [CrossRef] [PubMed]
- Sauberlich, H.E.; Kretsch, M.J.; Skala, J.H.; Johnson, H.L.; Taylor, P.C. Folate requirement and metabolism in nonpregnant women. Am. J. Clin. Nutr. 1987, 46, 1016–1028. [Google Scholar] [PubMed]
- Verwei, M.; Arkbage, K.; Havenaar, R.; van den Berg, H.; Witthoft, C.; Schaafsma, G. Folic acid and 5-methyltetrahydrofolate in fortified milk are bioaccessible as determined in a dynamic in vitro gastrointestinal model. J. Nutr. 2003, 133, 2377–2383. [Google Scholar] [PubMed]
- Wei, M.M.; Gregory, J.F. Organic acids in selected foods inhibit intestinal brush border pteroylpolyglutamate hydrolase in vitro: Potential mechanism affecting the bioavailability of dietary polyglutamyl folate. J. Agric. Food Chem. 1998, 46, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Bhandari, S.D.; Gregory, J.F. Inhibition by selected food components of human and porcine intestinal pteroylpolyglutamate hydrolase activity. Am. J. Clin. Nutr. 1990, 51, 87–94. [Google Scholar] [PubMed]
- Seyoum, E.; Selhub, J. Properties of food folates determined by stability and susceptibility to intestinal pteroylpolyglutamate hydrolase action. J. Nutr. 1998, 128, 1956–1960. [Google Scholar] [PubMed]
- Lucock, M.D.; Priestnall, M.; Daskalakis, I.; Schorah, C.J.; Wild, J.; Levene, M.I. Nonenzymatic Degradation and Salvage of Dietary Folate: Physicochemical Factors Likely to Influence Bioavailability. Biochem. Mol. Med. 1995, 55, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Öhrvik, V.E.; Witthoft, C. Orange juice is a good folate source in respect to folate content and stability during storage and simulated digestion. Eur. J. Nutr. 2008, 47, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Öhrvik, V.; Öhrvik, H.; Tallkvist, J.; Witthöft, C. Folates in bread: Retention during bread-making and in vitro bioaccessibility. Eur. J. Nutr. 2010, 49, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Verwei, M.; Freidig, A.P.; Havenaar, R.; Groten, J.P. Predicted serum folate concentrations based on in vitro studies and kinetic modeling are consistent with measured folate concentrations in humans. J. Nutr. 2006, 136, 3074–3078. [Google Scholar] [PubMed]
- Arkbåge, K.; Verwei, M.; Havenaar, R.; Witthöft, C. Bioaccessibility of folic acid and (6S)-5-methyltetrahydrofolate decreases after the addition of folate-binding protein to yogurt as studied in a dynamic in vitro gastrointestinal model. J. Nutr. 2003, 133, 3678–3683. [Google Scholar] [PubMed]
- Minekus, M.; Marteau, P.; Havenaar, R.; Huisintveld, J.H.J. A multicompartmental dynamic computer-controlled model simulating the stomach and small-Intestine. ATLA 1995, 23, 197–209. [Google Scholar]
- Deutsches Insitut für Normung. Bodenbeschaffenheit-Resporptionsverfügbarkeit von Organischen und Anorganischen Schadstoffen aus Kontaminiertem Bodenmaterial; Deutsches Institut für Normung: Berlin, Germany, 2004. [Google Scholar]
- Freisleben, A.; Schieberle, P.; Rychlik, M. Syntheses of labeled vitamers of folic acid to be used as internal standards in stable isotope dilution assays. J. Agric. Food Chem. 2002, 50, 4760–4768. [Google Scholar] [CrossRef] [PubMed]
- Ringling, C.; Rychlik, M. Analysis of seven folates in food by LC-MS/MS to improve accuracy of total folate data. Eur. Food Res. Technol. 2013, 236, 17–28. [Google Scholar] [CrossRef]
- Ringling, C.; Rychlik, M. Origins of the difference between food folate analysis by LC-MS/MS and microbiological assays. Anal. Bioanal. Chem. 2017, 409, 1815–1825. [Google Scholar] [CrossRef] [PubMed]
- Naumann, C.; Bassler, R. (Eds.) Handbuch der Landwirtschaftlichen Versuchs- und Untersuchungsmethodik. (Methodenbuch); VDLUFA Publishers: Darmstadt, Germany, 2007; Volume III. [Google Scholar]
- Firl, N.; Kienberger, H.; Hauser, T.; Rychlik, M. Determination of the fatty acid profile of neutral lipids, free fatty acids and phospholipids in human plasma. Clin. Chem. Lab. Med. 2013, 51, 799–810. [Google Scholar] [CrossRef] [PubMed]
- Gapski, G.R.; Whiteley, J.M.; Huennekens, F.M. Hydroxylated Derivatives of 5-Methy-5,6,7,8-tetrahydrofolate. Biochemistry 1971, 10, 2930–2934. [Google Scholar] [PubMed]
- Jongejan, J.A.; Mager, H.I.X.; Berends, W. Autoxidation of 5-Alkyl-Tetrahydropteridines. The Oxidation Product of 5-Methyl-THF. In Chemistry and Biology of Pteridines; Kisliuk, R.L., Brown, G.M., Eds.; Elsevier/North-Holland: New York, NY, USA, 1979; pp. 241–246. [Google Scholar]
- Fazili, Z.; Whitehead, J.R.D.; Paladugula, N.; Pfeiffer, C.M. A high-throughput LC-MS/MS method suitable for population biomonitoring measures five serum folate vitamers and one oxidation product. Anal. Bioanal. Chem. 2013, 405, 4549–4560. [Google Scholar] [CrossRef] [PubMed]
- Robinson, D.R. The nonenzymatic hydrolysis of N5,N10-methenyltetrahydrofolic acid and related reactions. In Vitamins and Coenzymes, Part B; Academic Press: New York, NY, USA, 1971; pp. 716–725. [Google Scholar]
- Savoie, L.; Gauthier, S.F.; Marin, J.; Pouliot, Y. In vitro determination of the release kinetics of peptides and free amino acids during the digestion of food proteins. J. AOAC Internat. 2005, 88, 935–948. [Google Scholar]
- Waring, A.J.; Drake, I.M.; Schorah, C.J.; White, K.L.M.; Lynch, D.A.F.; Axon, A.T.R.; Dixon, M.F. Ascorbic acid and total vitamin C concentrations in plasma, gastric juice, and gastrointestinal mucosa: Effects of gastritis and oral supplementation. Gut 1996, 38, 171–176. [Google Scholar] [CrossRef] [PubMed]
- Sobala, G.M.; Schorah, C.J.; Sanderson, M.; Dixon, M.F.; Tompkins, D.S.; Godwin, P.; Axon, A.T.R. Ascorbic acid in the human stomach. Gastroenterology 1989, 97, 357–363. [Google Scholar] [CrossRef]
- Sobala, G.M.; Pignatelli, B.; Schorah, C.J.; Bartsch, H.; Sanderson, M.; Dixon, M.F.; Shires, S.; King, R.F.G.; Axon, A.T.R. Levels of nitrite, nitrate, N-nitroso compounds, ascorbic acid and total bile acids in gastric juice of patients with and without precancerous conditions of the stomach. Carcinogenesis 1991, 12, 193–198. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.W.; Patchett, S.E.; Perrett, D.; Katelaris, P.H.; Domizio, P.; Farthing, M.J. The relation between gastric vitamin C concentrations, mucosal histology, and CagA seropositivity in the human stomach. Gut 1998, 43, 322–326. [Google Scholar] [CrossRef] [PubMed]
- Capurso, G.; Ricci, R.; Panzuto, F.; Baccini, F.; Passi, S.; Di Giulio, E.; Delle Fave, G.; Annibale, B. Intragastric ascorbic but not uric acid is depleted in relation with the increased pH in patients with atrophic body gastritis and H. pylori gastritis. Helicobacter 2003, 8, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Correa, P.; Malcom, G.; Schmidt, B.; Fontham, E.; Ruiz, B.; Bravo, J.C.; Bravo, L.E.; Zarama, G.; Realpe, J.L. Antioxidant micronutrients and gastric cancer. Aliment. Pharmacol. Ther. 1998, 12, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Fraser, A.G.; Woollard, G.A. Gastric juice ascorbic acid is related to Helicobacter pylori infection but not ethnicity. J. Gastroenterol. Hepatol. 1999, 14, 1070–1073. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, H.J.; Schorah, C.J.; Habibzedah, N.; Axon, A.T.R.; Cockel, R. Vitamin C in the human stomach: Relation to gastric pH, gastroduodenal disease, and possible sources. Gut 1989, 30, 436–442. [Google Scholar] [CrossRef] [PubMed]
- Temple, C.; Elliott, R.D.; Rose, J.D.; Montgomery, J.A. Preparation and purification of L-(.+/-.)-5-Formyl-5,6,7,8-tetrahydrofolic Acid. J. Med. Chem. 1979, 22, 731–734. [Google Scholar] [CrossRef] [PubMed]
- Benkovic, S.J.; Benkovic, P.A.; Bullard, W.P. Studies on Models for Tetrahydrofolic acid. III. Hydrolytic Interconversions of Tetrahydroquinoxaline Analogs at the Formate Level of Oxidation. J. Am. Chem. Soc. 1972, 94, 7542–7549. [Google Scholar] [CrossRef] [PubMed]
- Baggott, J.E.; Robinson, C.B.; Eto, I.; Johanning, G.L.; Cornwell, P.E. Iron compounds catalyze the oxidation of 10-formyl-5,6,7,8-tetrahydrofolic acid to 10-formyl-7,8-dihydrofolic acid. J. Inorg. Biochem. 1998, 71, 181–187. [Google Scholar] [CrossRef]
- Souci, S.W.; Fachmann, W.; Kraut, H. Food Composition and Nutrition Tables: Die Zusammensetzung der Lebensmittel, Nährwert-Tabellen La Composition des Aliments Tableaux des Valeurs Nutritives; Medpharm Scientific Publishers: Stuttgart, Germany, 2008; p. 1364. [Google Scholar]
- Reisenauer, A.M.; Halsted, C.H. Human Folate Requirements. J. Nutr. 1987, 117, 600–602. [Google Scholar] [PubMed]
- McNulty, H.; Pentieva, K. Folate Bioavailability. In Folate in Health and Disease; Bailey, L.B., Ed.; Taylor & Francis: Boca Raton, FL, USA, 2010; pp. 25–47. [Google Scholar]
- Brown, J.P.; Scott, J.M.; Foster, F.G.; Weir, D.G. Ingestion and absorption of naturally occurring pteroylmonoglutamates (folates) in man. Gastroenterology 1973, 64, 223–232. [Google Scholar] [PubMed]
- Gregory, J.F.; Bhandari, S.D.; Bailey, L.B.; Toth, J.P.; Baumgartner, T.G.; Cerda, J.J. Relative bioavailability of deuterium-labeled monoglutamyl tetrahydrofolates and folic acid in human subjects. Am. J. Clin. Nutr. 1992, 55, 1147–1153. [Google Scholar] [PubMed]
- Konings, E.J.; Goldbohm, R.A.; Brants, H.A.; Saris, W.H.; van den Brandt, P.A. Intake of dietary folate vitamers and risk of colorectal carcinoma. Cancer 2002, 95, 1421–1433. [Google Scholar] [CrossRef] [PubMed]
- McKillop, D.J.; McNulty, H.; Scott, J.M.; McPartlin, J.M.; Strain, J.J.; Bradbury, I.; Girvan, J.; Hoey, L.; McCreedy, R.; Alexander, J.; et al. The rate of intestinal absorption of natural food folates is not related to the extent of folate conjugation. Am. J. Clin. Nutr. 2006, 84, 167–173. [Google Scholar] [PubMed]
- Hannisdal, R.; Ueland, P.M.; Eussen, S.J.P.M.; Svardal, A.; Hustad, S. Analytical Recovery of Folate Degradation Products Formed in Human Serum and Plasma at Room Temperature. J. Nutr. 2009, 139, 1415–1418. [Google Scholar] [CrossRef] [PubMed]
- Kennelly, J.C.; Blair, J.A.; Pheasant, A.E. The metabolism of 5-methyltetrahydropteroyl-l-glutamic acid and its oxidationproducts in the rat. Biochem. J. 1982, 206, 373–378. [Google Scholar] [CrossRef] [PubMed]
| Intra-Day | Inter-Day | Inter-Batch | |
|---|---|---|---|
| efficiency of deconjugation | 1.1% | 4% | 14% |
| recovery | 1.5% | 6% | 3% |
| bioaccessibility | 0.6% | 5% | 15% |
| Food Stuff | Total Folate (in µg/100 g) | Relative Amount of Monoglutamates (%) | |
|---|---|---|---|
| before digestion | after digestion | ||
| Spinach, young | 98 | 30 | 56 |
| Spinach leaves | 117 | 52 | |
| Wheat germ | 397 | 5 | 23 |
| Camembert C | 39 | 14 | 46 |
| Camembert B | 107 | 19 | 48 |
| dough | 87 | 64 | |
| rind | 213 | 34 | |
| Camembert A | 88 | 44 | |
| dough | 45 | 46 | |
| rind | 133 | 37 | |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ringling, C.; Rychlik, M. Simulation of Food Folate Digestion and Bioavailability of an Oxidation Product of 5-Methyltetrahydrofolate. Nutrients 2017, 9, 969. https://doi.org/10.3390/nu9090969
Ringling C, Rychlik M. Simulation of Food Folate Digestion and Bioavailability of an Oxidation Product of 5-Methyltetrahydrofolate. Nutrients. 2017; 9(9):969. https://doi.org/10.3390/nu9090969
Chicago/Turabian StyleRingling, Christiane, and Michael Rychlik. 2017. "Simulation of Food Folate Digestion and Bioavailability of an Oxidation Product of 5-Methyltetrahydrofolate" Nutrients 9, no. 9: 969. https://doi.org/10.3390/nu9090969





