Urinary Metabolomic Approach Provides New Insights into Distinct Metabolic Profiles of Glutamine and N-Carbamylglutamate Supplementation in Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animal Experiment and Sample Collection
2.2. Sample Preparation and NMR Spectroscopy
2.3. NMR Spectroscopic Processes and Analysis
3. Results
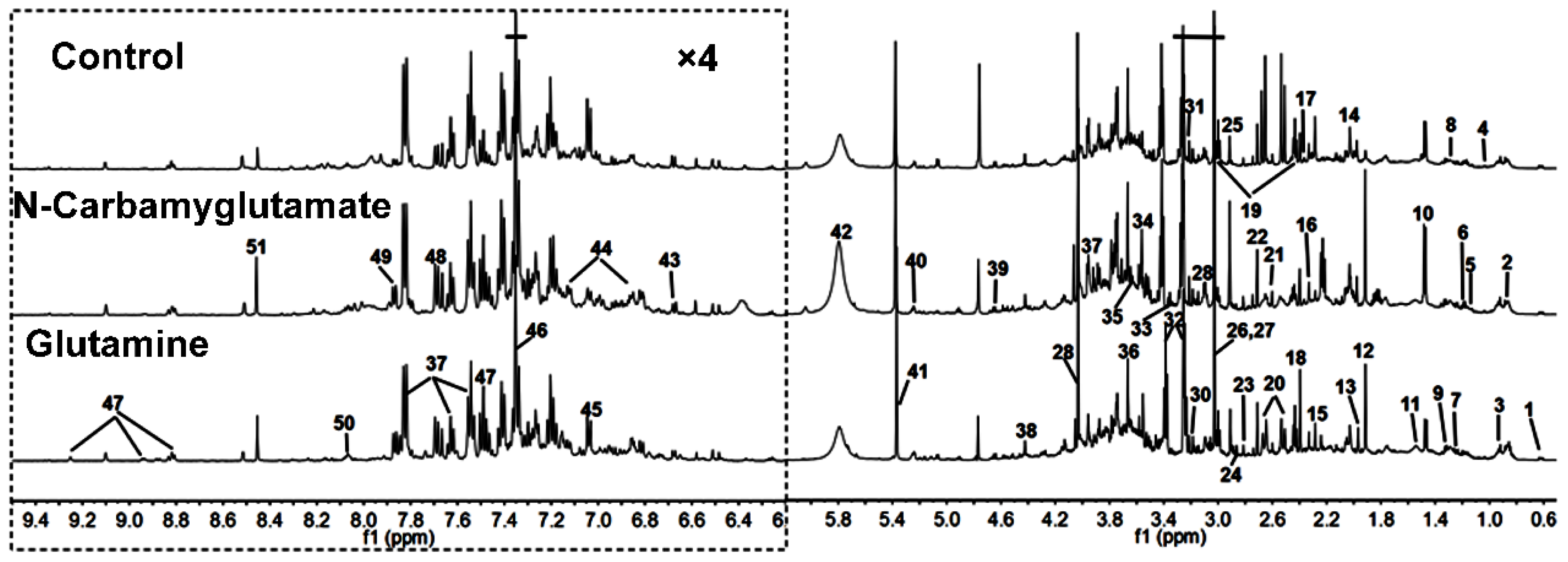
3.1. 1H NMR Spectra of Urine Samples
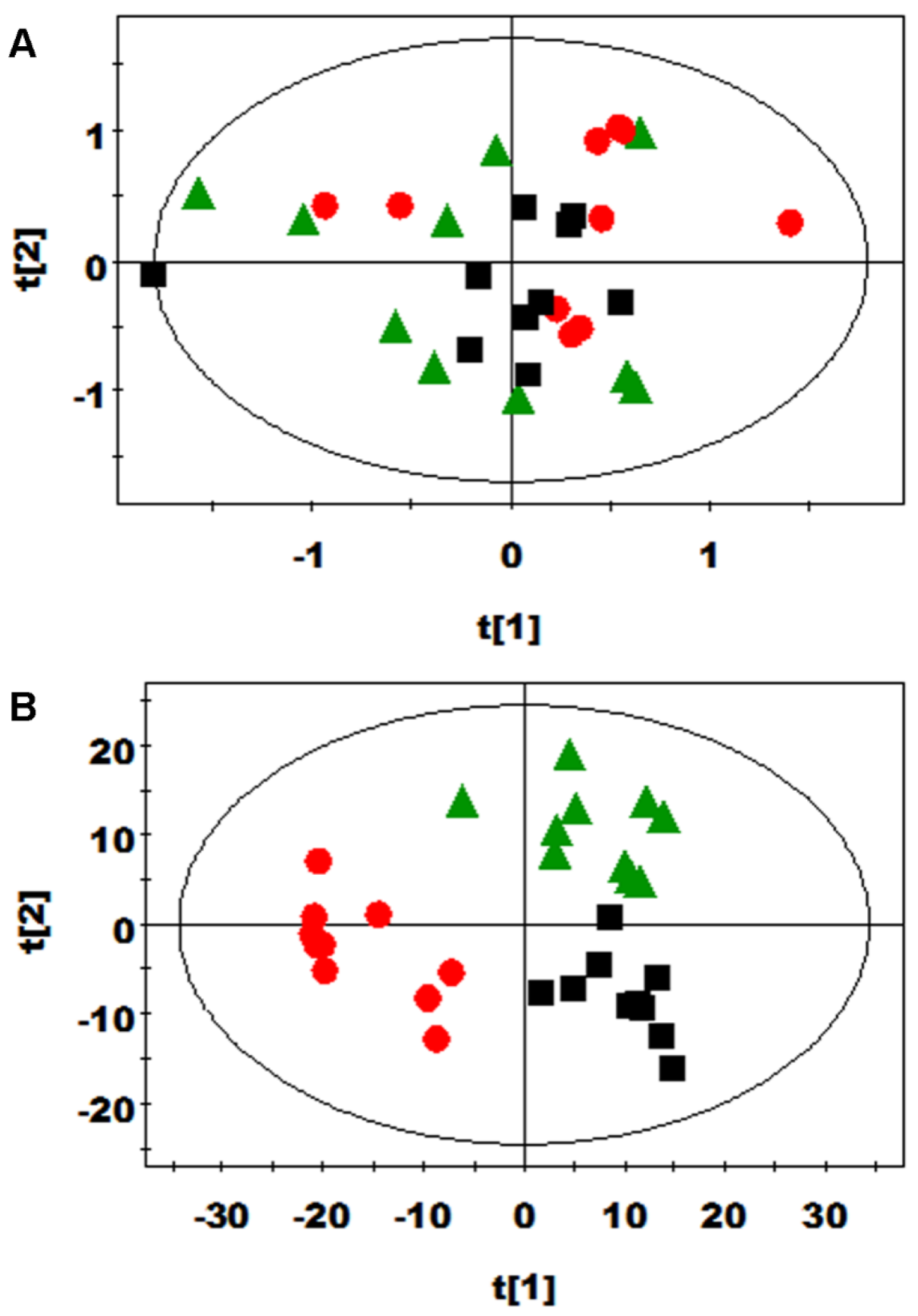
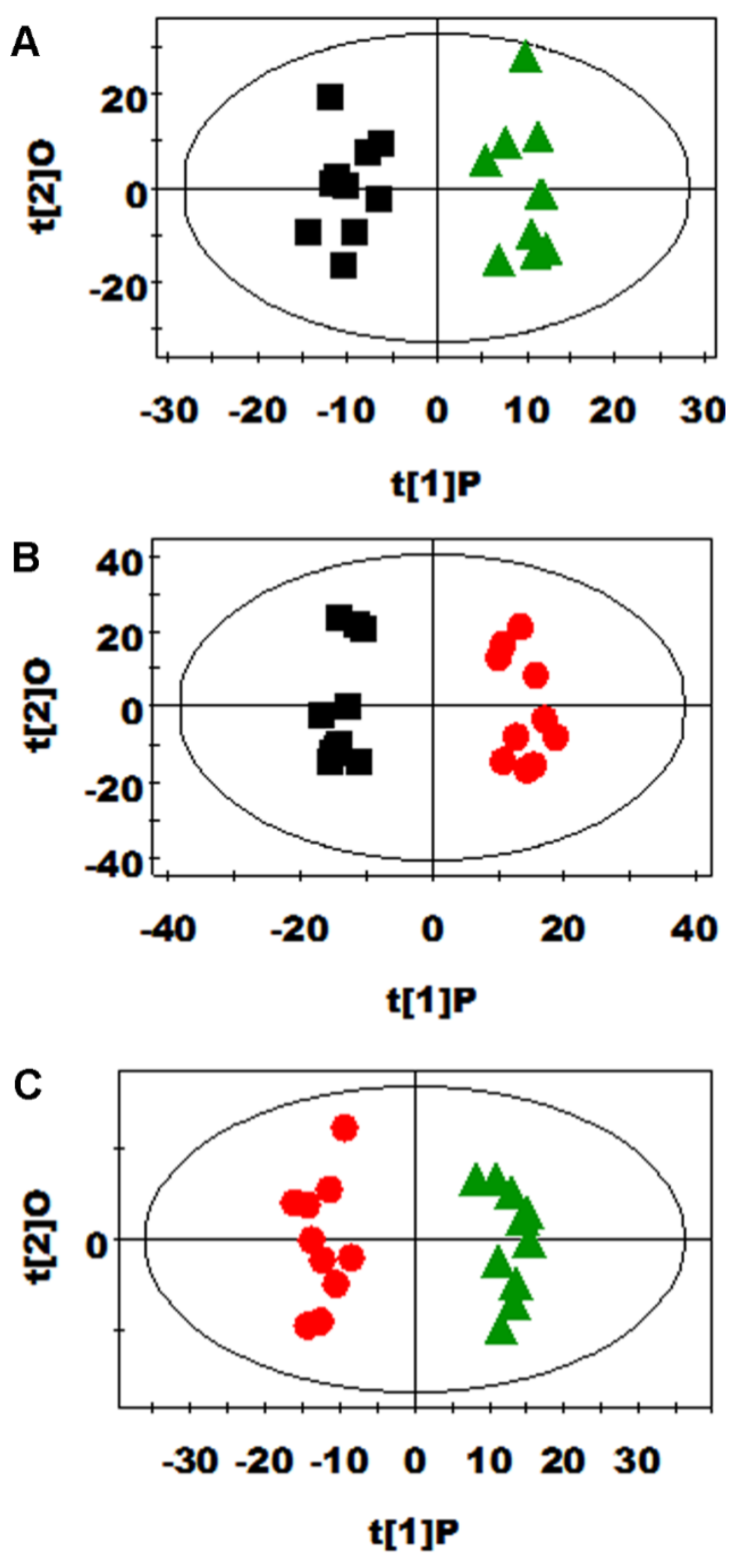
3.2. Multivariate Data Analysis of NMR Data
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wu, G.; Bazer, F.W.; Johnson, G.A.; Knabe, D.A.; Burghardt, R.C.; Spencer, T.E.; Li, X.L.; Wang, J.J. Triennial Growth Symposium: Importants roles for l-glutamine in swine nutrition and production. J. Anim. Sci. 2011, 89, 2017–2030. [Google Scholar] [CrossRef] [PubMed]
- Wu, G. Functional amino acids in nutrition and health. Amino Acids 2013, 45, 407–411. [Google Scholar] [CrossRef] [PubMed]
- Barnabé, N.; Butler, M. The effect of glucose and glutamine on the intracellular nucleotide pool and oxygen uptake rate of a murine hybridoma. Cytotechnology 2000, 34, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.; White, C.W.; Chang, L.Y.; Schneider, B.K.; Allen, C.B. Glutamine protects mitochondrial structure and function in oxygen toxicity. Am. J. Physiol. Lung Cell Mol. Physiol. 2001, 280, 779–791. [Google Scholar]
- Wu, G. Intestinal mucosal amino acid catabolism. J. Nutr. 1998, 128, 1249–1252. [Google Scholar] [PubMed]
- Reeds, P.J.; Burrin, D.G. Glutamine and the bowel. J. Nutr. 2001, 131, 2505S–2508S. [Google Scholar] [PubMed]
- Wang, J.; Chen, L.; Li, P.; Li, X.; Zhou, H.; Wang, F.; Li, D.; Yin, Y.; Wu, G. Gene expression is altered in piglet small intestine by weaning and dietary glutamine supplementation. J. Nutr. 2008, 138, 1025–1032. [Google Scholar] [PubMed]
- Ban, K.; Kozar, R.A. Glutamine protects against apoptosis via downregulation of Sp3 in intestinal epithelial cells. Am. J. Physiol. Gastrointest. Liver Physiol. 2010, 299, G1344–G1353. [Google Scholar] [CrossRef] [PubMed]
- Yoo, S.S.; Field, C.J.; McBurney, M.I. Glutamine supplementation maintains intramuscular glutamine concentrations and normalizes lymphocyte function in infected early weaned pigs. J. Nutr. 1997, 127, 2253–2259. [Google Scholar] [PubMed]
- Xi, P.; Jiang, Z.; Dai, Z.; Li, X.; Yao, K.; Zheng, C.; Lin, Y.; Wang, J.; Wu, G. Regulation of protein turnover by l-glutamine in porcine intestinal epithelial cells. J. Nutr. Biochem. 2012, 23, 1012–1017. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Lin, G.; Dai, Z.; Zhou, T.; Li, T.; Yuan, T.; Wu, Z.; Wu, G.; Wang, J. l-glutamine deprivation induces autophagy and alters the mTOR and MAPK signaling pathways in porcine intestinal epithelial cells. Amino Acids 2015, 47, 2185–2197. [Google Scholar] [CrossRef] [PubMed]
- Groening, P.; Huang, Z.; La Gamma, E.F.; Levy, R.J. Glutamine restores myocardial cytochrome C oxidase activity and improves cardiac function during experimental sepsis. JPEN J. Parenter. Enter. Nutr. 2011, 35, 249–254. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Knabe, D.A.; Kim, S.W. Arginine nutrition in neonatal pigs. J. Nutr. 2004, 134, S2783–S2790. [Google Scholar]
- Cohen, P.P.; Grisolia, S. The role of carbamyl-l-glutamic acid in the enzymatic synthesis of citrulline from ornithine. J. Biol. Chem. 1950, 182, 747–761. [Google Scholar]
- Yap, S.; Leong, H.Y.; Abdul Aziz, F.; Hassim, H.; Sthaneshwar, P.; Teh, S.H.; Abdullah, I.S.; Ngu, L.H.; Mohamed, Z. N-carbamylglutamate is an effective treatment for acute neonatal hyperammonaemia in a patient with methylmalonic aciduria. Neonatology 2016, 109, 303–307. [Google Scholar] [CrossRef] [PubMed]
- Guffon, N.; Vianey-Saban, C.; Bourgeois, J.; Rabier, D.; Colombo, J.; Guibaud, P. A new neonatal case of N-acetylglutamate synthase deficiency treated by carbamylglutamate. J. Inher. Metab. Dis. 1995, 18, 61–65. [Google Scholar] [CrossRef] [PubMed]
- Frank, J.W.; Escobar, J.; Nguyen, H.V.; Jobgen, S.C.; Jobgen, W.S.; Davis, T.A.; Wu, G. Oral N-carbamylglutamate supplementation increases protein synthesis in skeletal muscle of piglets. J. Nutr. 2007, 137, 315–319. [Google Scholar] [PubMed]
- Zeng, X.; Huang, Z.; Mao, X.; Wang, J.; Wu, G.; Qiao, S. N-carbamylglutamate enhances pregnancy outcome in rats through activation of the PI3K/PKB/mTOR signaling pathway. PLoS ONE 2012, 7, e41192. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Ruan, Z.; Gao, Y.; Yin, Y.; Zhou, X.; Wang, L.; Geng, M.; Hou, Y.; Wu, G. Dietary supplementation with l-arginine or N-carbamylglutamate enhances intestinal growth and heat shock protein-70 expression in weanling pigs fed a corn- and soybean meal-based diet. Amino Acids 2010, 39, 831–839. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.P.; Wu, T.X.; Sun, J.M.; Yang, L.; Hong, Q.H.; Chen, A.G.; Yang, C.M. Response to dietary l-glutamine supplementation in weaned piglets: A serum metabolomic comparison and hepatic metabolic regulation analysis. J. Anim. Sci. 2012, 90, 4421–4430. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.M.; Xiao, L.; Cao, W.; Fang, T.T.; Jia, G.; Chen, X.L.; Zhao, H.; Wu, C.M.; Wang, J. Changes in the metabolome of rats after exposure to arginine and N-carbamylglutamate in combination with diquat a compound that causes oxidative stress assessed by 1H NMR spectroscopy. Food Funct. 2016, 7, 964–974. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, J.K.; Foxall, P.J.D.; Spraul, M.; Farrant, R.D.; Lindon, J.C. 750 MHz 1H and 1H-13C NMR spectroscopy of human blood plasma. Anal. Chem. 1995, 67, 793–811. [Google Scholar] [CrossRef] [PubMed]
- Fan, T.W.M. Metabolite profiling by one- and two-dimensional NMR analysis of complex mixtures. Prog. Nucl. Magn. Reson. Spectrosc. 1996, 28, 161–219. [Google Scholar] [CrossRef]
- Wishart, D.S.; Jewison, T.; Guo, A.C.; Wilson, M.; Knox, C.; Liu, Y.; Djoumbou, Y.; Mandal, R.; Aziat, F.; Dong, E.; et al. HMDB 3.0-the human metabolome database in 2013. Nucleic Acids Res. 2013, 41, D801–D807. [Google Scholar] [CrossRef] [PubMed]
- Cloarec, O.; Dumas, M.E.; Trygg, J.; Craig, A.; Barton, R.H.; Lindon, J.C.; Nicholson, J.K.; Holmes, E. Evaluation of the orthogonal projection on latent structure model limitations caused by chemical shift variability and improved visualization of biomarker changes in 1H NMR spectroscopic metabonomic studies. Anal. Chem. 2005, 77, 517–526. [Google Scholar] [CrossRef] [PubMed]
- Lindgren, F.; Hansen, B.; Karcher, W.; SjÖstrÖm, M.; Eriksson, L. Model validation by permutation tests: Applications to variable selection. J. Chemom. 1996, 10, 521–532. [Google Scholar] [CrossRef]
- Liu, G.M.; Xiao, L.; Fang, T.T.; Cai, Y.M.; Jia, G.; Zhao, H.; Wang, J.; Chen, X.L.; Wu, C.M. Pea fiber and wheat bran fiber show distinct metabolic profiles in rats as investigated by a 1H NMR-based metabollomic approach. PLoS ONE 2014, 9, e115561. [Google Scholar]
- Huynh, N.N.; Chin-Dusting, J. Amino acids, arginase and nitric oxide in vascular health. Clin. Exp. Pharmacol. Physiol. 2006, 33, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Ligthart-Melis, G.C.; Deutz, N.E.P. Is glutamine still an important precursor of citrulline? Am. J. Physiol. Endocrinol. Metab. 2011, 301, E264–E266. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Zeng, X.; Yang, F.; Huang, Z.; Liu, H.; Ma, X.; Qiao, S. Dietary N-carbamylglutamate supplementation boosts intestinal mucosal immunity in Escherichia coli challenged piglets. PLoS ONE 2013, 8, e66280. [Google Scholar] [CrossRef] [PubMed]
- Meijer, A.J.; Verhoeven, A.J. N-acetylglutamate and urea synthesis. Biochem. J. 1984, 56, 559–560. [Google Scholar] [CrossRef]
- Arias-Barrau, E.; Olivera, E.R.; Luengo, J.M.; Fernández, C.; Galán, B.; García, J.L.; Díaz, E.; Miñambres, B. The homogentisate pathway: A central catabolic pathway involved in the degradation of l-phenylalanine, l-tyrosine, and 3-hydroxyphenylacetate in Pseudomonas putida. J. Bacteriol. 2004, 186, 5062–5077. [Google Scholar] [CrossRef] [PubMed]
- Tannock, G.W. A special fondness for lactobacilli. Appl. Environ. Microbiol. 2004, 70, 3189–3194. [Google Scholar] [CrossRef] [PubMed]
- Rechner, A.R.; Kuhnle, G.; Hu, H.; Roedig-Penman, A.; van den Braak, M.H.; Moore, K.P.; Rice-Evans, C.A. The metabolism of dietary polyphenols and the relevance to circulating levels of conjugated metabolites. Free Radic. Res. 2002, 36, 1229–1241. [Google Scholar] [CrossRef] [PubMed]
- Bohus, E.; Coen, M.; Keun, H.C.; Ebbels, T.M.D.; Beckonert, O.; Lindon, J.C.; Holmes, E.; Noszál, B.; Nicholson, J.K. Temporal metabonomic modeling of l-arginine-induced exocrine pancreatitis. J. Proteome Res. 2008, 7, 4435–4445. [Google Scholar] [CrossRef] [PubMed]
- Jawed, H.; Shah, S.U.A.; Jamall, S.; Simjee, S.U. N-(2-hydroxy phenyl) acetamide inhibits inflammation-related cytokines and ROS in adjuvant-induced arthritic (AIA) rats. Int. Immunopharmacol. 2010, 10, 900–905. [Google Scholar] [CrossRef] [PubMed]
- Muri, E.M.; Williamson, J.S. Anti-Helicobacter pylori agents. An update. Mini Rev. Med. Chem. 2004, 4, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Bjursell, M.K.; Himrod, J.; Deng, S.; Carmichael, L.K.; Chiang, H.C.; Hooper, L.V.; Gordon, J.I. A genomic view of the human-Bacteroides thetaiotaomicron symbiosis. Science 2003, 299, 2074–2076. [Google Scholar] [CrossRef] [PubMed]
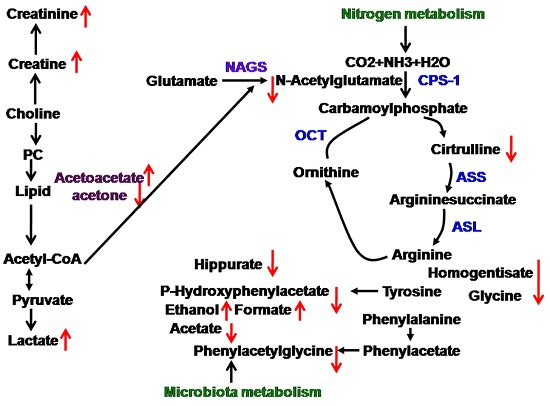
 upregulated,
upregulated,  downegulated; PC, phosphorylcholine; NAGS, N-acetylglutamate; CPS-1, carbamoylphosphate synthetase-1; ASS, argininosuccinate synthase; ASL, argininosuccinate lyase; OCT, ornithine carbamonyltransferase.
downegulated; PC, phosphorylcholine; NAGS, N-acetylglutamate; CPS-1, carbamoylphosphate synthetase-1; ASS, argininosuccinate synthase; ASL, argininosuccinate lyase; OCT, ornithine carbamonyltransferase.
 upregulated,
upregulated,  downegulated; PC, phosphorylcholine; NAGS, N-acetylglutamate; CPS-1, carbamoylphosphate synthetase-1; ASS, argininosuccinate synthase; ASL, argininosuccinate lyase; OCT, ornithine carbamonyltransferase.
downegulated; PC, phosphorylcholine; NAGS, N-acetylglutamate; CPS-1, carbamoylphosphate synthetase-1; ASS, argininosuccinate synthase; ASL, argininosuccinate lyase; OCT, ornithine carbamonyltransferase.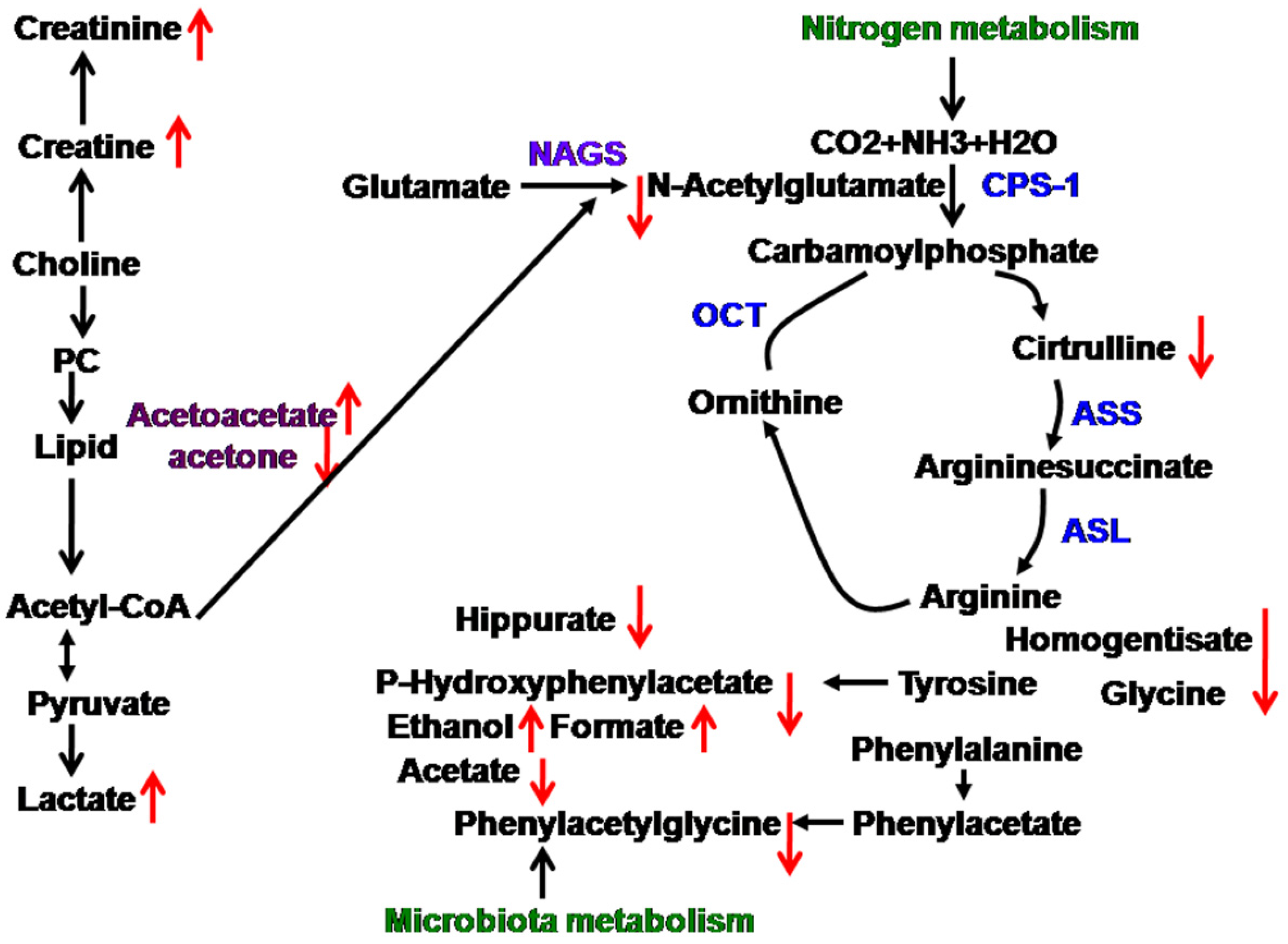
| Keys | Metabolites | Moieties | δ 1H (ppm) and Multiplicity | Samples |
|---|---|---|---|---|
| 1 | Bile acids | CH3 | 0.64(m), 0.75(m) | U |
| 2 | α-Hydroxy-iso-valerate | δCH3, CH3 | 0.83(d), 0.97(d) | U |
| 3 | α-Hydroxybutyrate | CH3 | 0.89(t) | U |
| 4 | Propionate | CH3 | 1.06(t) | U |
| 5 | Isobutyrate | CH3 | 1.13(d) | U |
| 6 | Ethanol | CH3 | 1.19(t) | U |
| 7 | Methylmalonate | CH3, CH | 1.25(d), 3.75(m) | U |
| 8 | α-Hydroxy-n-valerate | CH3, γCH2 | 0.89(t), 1.31(m) | U |
| 9 | Lactate | αCH, βCH3 | 4.14(q), 1.33(d) | U |
| 10 | Alanine | αCH, βCH3 | 3.77(q), 1.47(d) | U |
| 11 | Citrulline | γCH2, βCH2 | 1.56(m), 1.82(m) | U |
| 12 | Acetate | CH3 | 1.92(s) | U |
| 13 | Acetamide | CH3 | 1.99(s) | U |
| 14 | N-Acetylglutamate | βCH2, γCH2, CH3 | 2.06(m), 1.87(m), 2.03(s) | U |
| 15 | Acetone | CH3 | 2.24(s) | U |
| 16 | Acetoacetate | CH3 | 2.28(s) | U |
| 17 | Pyruvate | CH3 | 2.33(s) | U |
| 18 | Succinate | CH2 | 2.40(s) | U |
| 19 | α-Ketoglutarate | βCH2, γCH2 | 2.45(t), 3.01(t) | U |
| 20 | Citrate | CH2 | 2.54(d), 2.68(d) | U |
| 21 | Methylamine | CH3 | 2.61(s) | U |
| 22 | Dimethylamine | CH3 | 2.71(s) | U |
| 23 | Methylguanidine | CH3 | 2.81(s) | U |
| 24 | Trimethylamine | CH3 | 2.88(s) | U |
| 25 | Dimethylglycine | CH3 | 2.93(s) | U |
| 26 | Creatine | CH3, CH2 | 3.04(s), 3.93(s) | U |
| 27 | Creatinine | CH3, CH2 | 3.04(s), 4.05(s) | U |
| 28 | Ornithine | CH2 | 3.06(t) | U |
| 29 | Ethanolamine | CH2 | 3.11(t) | U |
| 30 | Malonate | CH2 | 3.15(s) | U |
| 31 | Choline | OCH2, NCH2, N(CH3)3 | 4.07(t), 3.53(t), 3.21(s) | U |
| 32 | Taurine | –CH2-S, –CH2–NH2 | 3.27(t), 3.43(t) | U |
| 33 | TMAO a | CH3 | 3.27(s) | U |
| 34 | Glycine | CH2 | 3.57(s) | U |
| 35 | Sarcosine | CH2 | 3.6(s) | U |
| 36 | Phenylacetyglycine | 2,6–CH, 3,5–CH, 7–CH, 10–CH | 7.30(t), 7.36(m), 7.42(m), 3.67(s) | U |
| 37 | Hippurate | CH2, 3,5–CH, 4–CH, 2,6–CH | 3.97(d), 7.55(t), 7.63(t), 7.84(d) | U |
| 38 | N-Methylnicotinamide | CH3, 5–CH, 4–CH, 6–CH, CH2 | 4.42(s), 8.21(d), 8.87(d), 8.93(d), 9.24(s) | U |
| 39 | β-Glucose | 1–CH, 2–CH, 3–CH, 4–CH, 5–CH, 6–CH | 4.47(d), 3.25(dd), 3.49(t), 3.41(dd), 3.46(m), 3.73(dd), 3.90(dd) | U |
| 40 | α-Glucose | 1–CH, 2–CH, 3–CH, 4–CH, 5–CH, 6–CH | 5.24(d), 3.54(dd), 3.71(dd), 3.42(dd), 3.84(m), 3.78(m) | U |
| 41 | Allantoin | CH | 5.39(s) | U |
| 42 | Urea | NH2 | 5.82(s) | U |
| 43 | Homogentisate | 6–CH, 5–CH | 6.67(d), 6.82(d) | U |
| 44 | p-Hydroxyphenylacetate | 6–CH, 2–CH, 3,5–CH | 3.6(s), 6.85(d), 7.15(d) | U |
| 45 | m-Hydroxyphenylacetate | 6–CH, 4–CH, 3–CH | 6.92(m), 7.04(d), 7.26(t) | U |
| 46 | Indoxyl sulfate | 4–CH, 5–CH, 6–CH, 7–CH, CH | 7.51(m), 7.22(m), 7.28(m), 7.71(m), 7.37(s) | U |
| 47 | Nicotinate | 2,6–CH, 4–CH, 5–CH | 8.60(d), 8.25(d), 7.5(dd) | U |
| 48 | 4-Aminohippurate | CH2, CH | 7.6(d), 6.8(d), 3.9(d) | U |
| 49 | Benzoate | 2,6–CH, 3,5–CH, 4–CH | 7.87(d), 7.49(dd), 7.56(t) | U |
| 50 | Trigonelline | 2–CH, 4–CH, 6–CH, 5–CH, CH3 | 9.09(s), 8.85(m), 8.81(dd), 8.07(m), 4.44(s) | U |
| 51 | Formate | CH | 8.46(s) | U |
| Metabolite | B (vs. A) a | C (vs. A) a | B (vs. C) a |
|---|---|---|---|
| Acetamide (13) | 0.608 | −0.728 | 0.906 |
| Acetate (12) | 0.713 | −0.742 | 0.768 |
| Citrulline (11) | 0.758 | −0.962 | 0.966 |
| Creatine (26) | — | −0.790 | 0.783 |
| Creatinine (27) | 0.723 | 0.717 | — |
| Ethanol (6) | −0.630 | 0.692 | −0.631 |
| Formate (51) | −0.621 | — | — |
| Glycine (34) | — | −0.616 | — |
| Hippurate (37) | — | −0.914 | 0.906 |
| Homogentisate (43) | — | −0.810 | 0.834 |
| Indoxyl sulfate (46) | — | 0.786 | −0.786 |
| Lactate (9) | — | 0.653 | — |
| Methylmalonate (7) | 0.738 | 0.608 | −0.653 |
| N-Acetylglutamate (14) | — | −0.967 | 0.978 |
| Phenylacetyglycine (36) | — | −0.634 | — |
| α-Hydroxy-n-valerate (8) | −0.684 | — | — |
| α-Ketoglutarate (19) | — | — | −0.623 |
| Acetoacetate (16) | — | 0.786 | −0.815 |
| Acetone (15) | — | −0.912 | 0.944 |
| m-Hydroxyphenylacetate (45) | — | 0.815 | −0.883 |
| p-Hydroxyphenylacetate (44) | — | −0.813 | 0.844 |
| Sarcosine (35) | — | 0.865 | −0.853 |
| α-Hydroxy-iso-valerate (2) | — | 0.607 | — |
| Pyruvate (4) | — | — | −0.608 |
| Methylamine (21) | — | — | −0.635 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, G.; Cao, W.; Fang, T.; Jia, G.; Zhao, H.; Chen, X.; Wu, C.; Wang, J. Urinary Metabolomic Approach Provides New Insights into Distinct Metabolic Profiles of Glutamine and N-Carbamylglutamate Supplementation in Rats. Nutrients 2016, 8, 478. https://doi.org/10.3390/nu8080478
Liu G, Cao W, Fang T, Jia G, Zhao H, Chen X, Wu C, Wang J. Urinary Metabolomic Approach Provides New Insights into Distinct Metabolic Profiles of Glutamine and N-Carbamylglutamate Supplementation in Rats. Nutrients. 2016; 8(8):478. https://doi.org/10.3390/nu8080478
Chicago/Turabian StyleLiu, Guangmang, Wei Cao, Tingting Fang, Gang Jia, Hua Zhao, Xiaoling Chen, Caimei Wu, and Jing Wang. 2016. "Urinary Metabolomic Approach Provides New Insights into Distinct Metabolic Profiles of Glutamine and N-Carbamylglutamate Supplementation in Rats" Nutrients 8, no. 8: 478. https://doi.org/10.3390/nu8080478