Aqueous Extract of Nypa fruticans Wurmb. Vinegar Alleviates Postprandial Hyperglycemia in Normoglycemic Rats
Abstract
:1. Introduction
2. Experimental Section
2.1. Chemicals
2.2. Plant Preparation and Extraction
2.3. Animals
2.4. Intestinal Glucose Absorption by the Everted Sac Technique
2.5. Oral Glucose Tolerance Test (OGTT)
2.6. Oral Sucrose Tolerance Test (OSucTT)
2.7. Oral Starch Tolerance Test (OSTT)
2.8. In Vitro Enzymatic Inhibition Study: α-Glucosidase
2.9. In Vitro Enzymatic Inhibition Study: α-Amylase
2.10. Statistical Analysis
3. Results
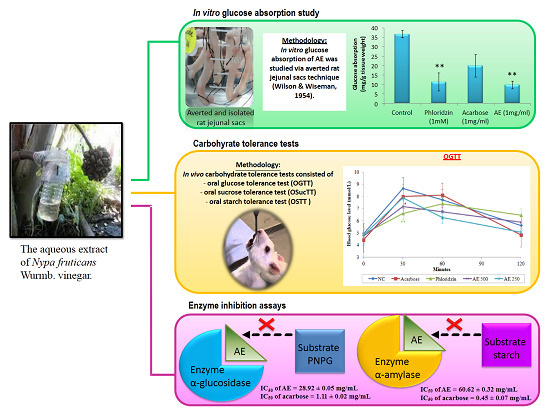
3.1. AE Attenuates Intestinal Glucose Absorption
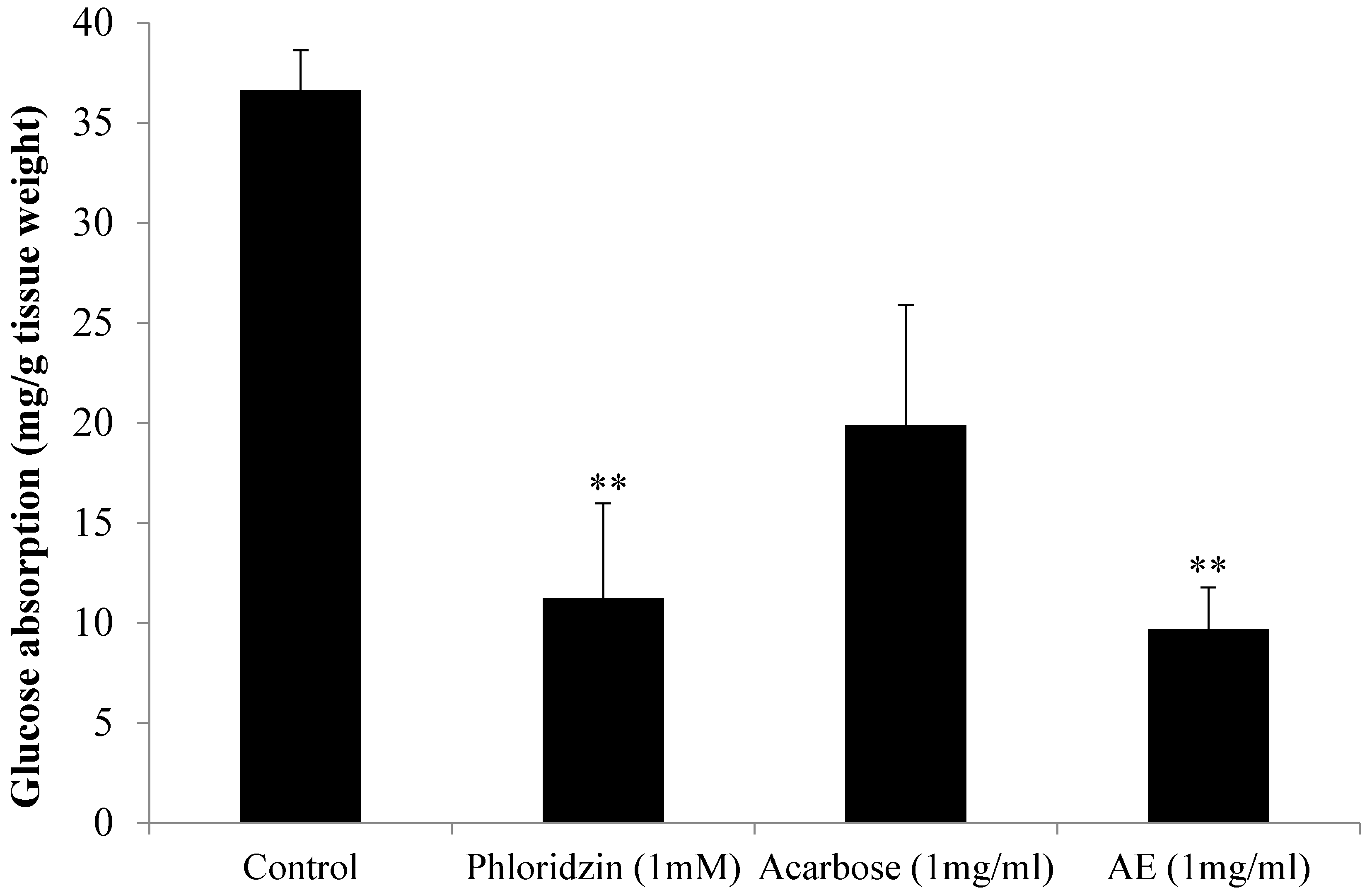
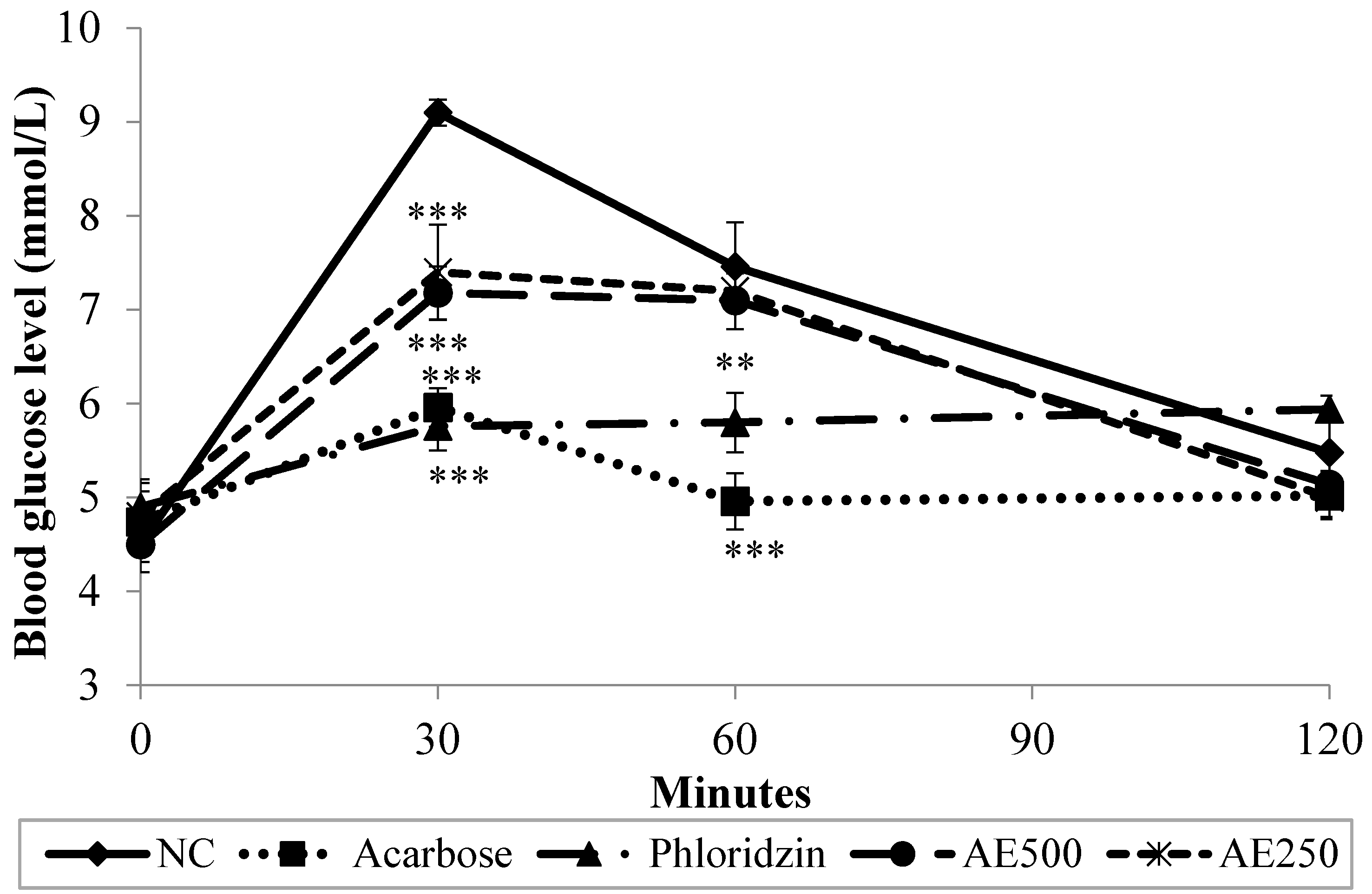
3.2. Oral Glucose Tolerance Test (OGTT) in Normal Rats
| Group 1 | AUC (mmol·min/L) | ||
|---|---|---|---|
| OGTT | OSucTT | OSTT | |
| NC | 851.7 ± 59.5 b | 855.0 ± 58.8 c | 840.6 ± 55.5 c |
| Acarbose | 816.0 ± 60.2 ab | 668.7 ± 58.9 a | 623.7 ± 45.8 a |
| Phloridzin | 798.3 ± 75.6 ab | 676.2 ± 63.9 a | 685.5 ± 62.0 ab |
| AE 500 | 756.3 ± 62.3 a | 737.4 ± 58.0 b | 756.6 ± 58.6 bc |
| AE 250 | 742.8 ± 46.4 a | 776.7 ± 62.6 b | 768.9 ± 56.1 bc |
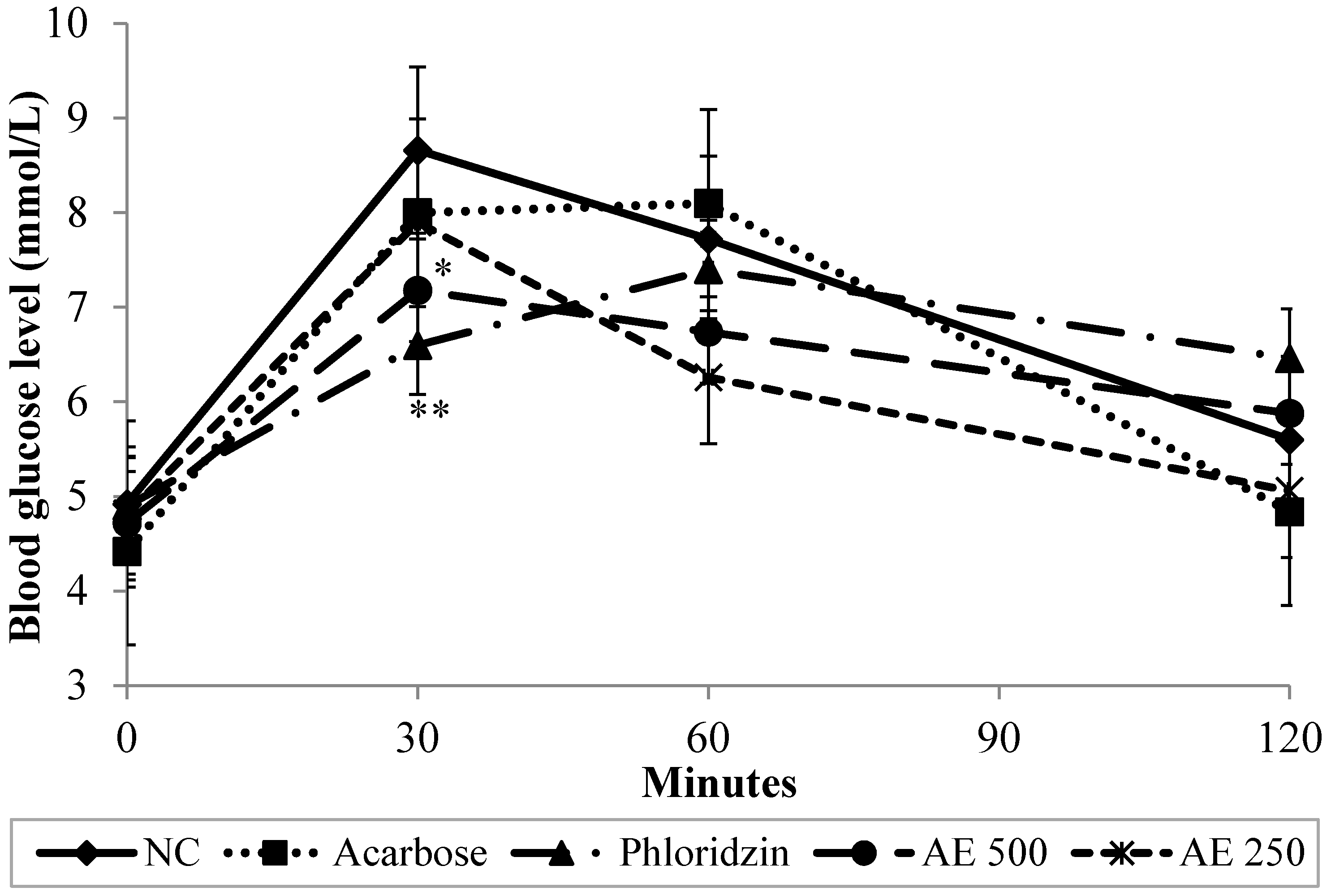
3.3. Oral Sucrose Tolerance Test (OSucTT) in Normal Rats
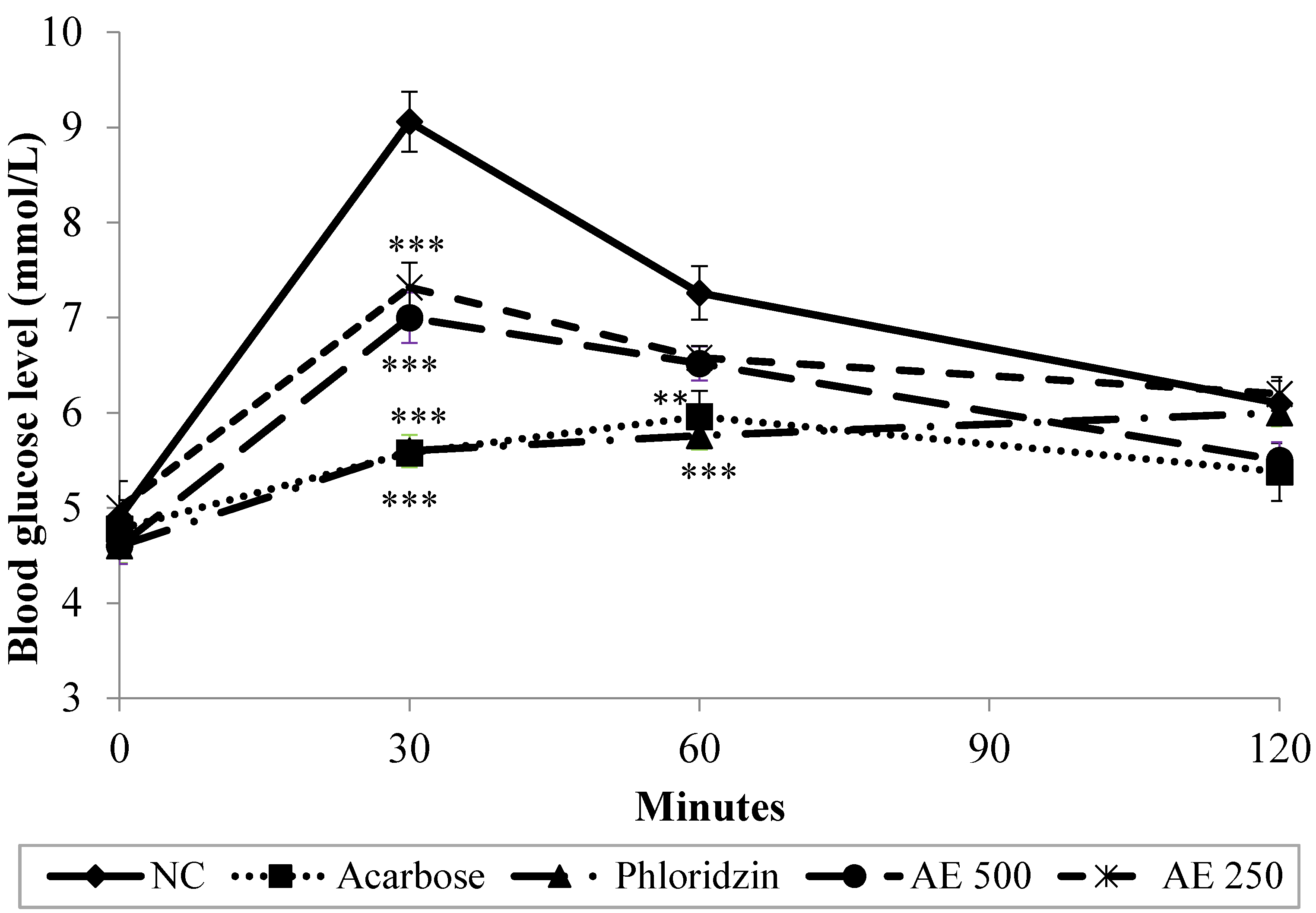
3.4. Oral Starch Tolerance Test (OSTT) in Normal Rats
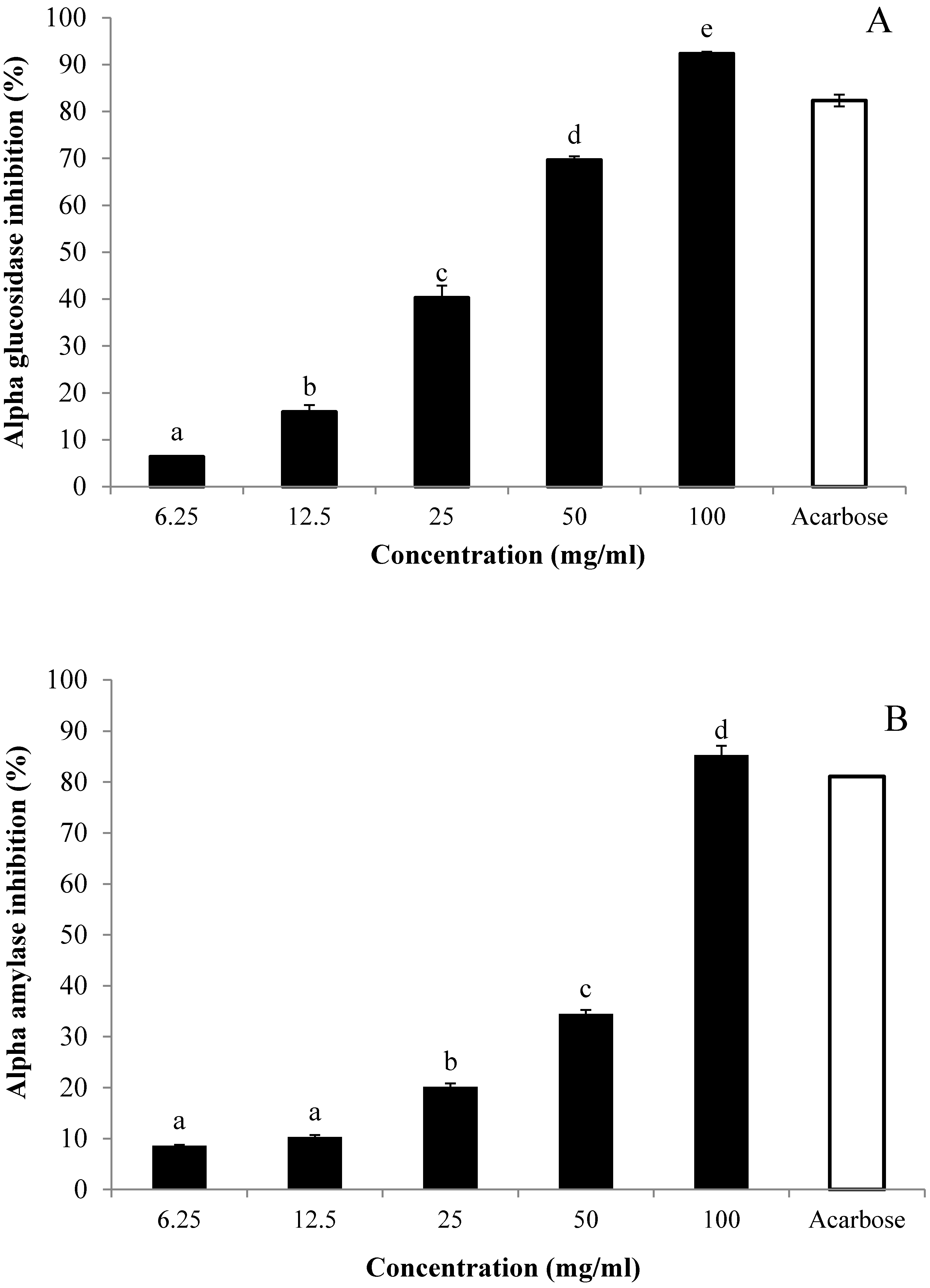
3.5. In Vitro Inhibitory Effects of AE against α-Glucosidase and α-Amylase
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care 2010, 33, S62–S69. [Google Scholar]
- Kanauchi, M.; Nakajima, M.; Saito, Y.; Kanauchi, K. Pancreatic beta-cell function and insulin sensitivity in Japanese subjects with impaired glucose tolerance and newly diagnosed type 2 diabetes mellitus. Metabolism 2003, 52, 476–481. [Google Scholar] [CrossRef] [PubMed]
- Ceriello, A. Postprandial hyperglycemia and diabetes complications is it time to treat? Diabetes 2005, 54, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.K. Post-prandial hyperglycemia. Indian J. Endocrinol. Metab. 2012, 16, S245–S247. [Google Scholar] [PubMed]
- Abid, S.; Lekchiri, A.; Mekhfi, H.; Ziyyat, A.; Legssyer, A.; Aziz, M.; Bnouham, M. Inhibition of α-glucosidase and glucose intestinal absorption by Thymelaea hirsuta fractions. J. Diabetes 2014, 6, 351–359. [Google Scholar] [CrossRef] [PubMed]
- Hollander, P.; Pi-Sunyer, X.; Coniff, R.F. Acarbose in the treatment of type 1 diabetes. Diabetes Care 1997, 20, 248–253. [Google Scholar] [CrossRef] [PubMed]
- Standl, E.; Baumgartl, H.; Fuchtenbusch, M.; Stemplinger, J. Effect of acarbose on additional insulin therapy in type 2 diabetic patients with late failure of sulphonylurea therapy. Diabetes Obes. Metab. 1999, 1, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Andrianesis, V.; Doupis, J. The role of kidney in glucose homeostasis-SGLT2 inhibitors, a new approach in diabetes treatment. Expert Rev. Clin. Pharmacol. 2013, 6, 519–539. [Google Scholar] [CrossRef] [PubMed]
- Paivoke, A.; Adams, M.R.; Twiddy, D.R. Nipa Palm Vinegar in Papua-New-Guinea. Process Biochem. 1984, 19, 84–87. [Google Scholar]
- Liatis, S.; Grammatikou, S.; Poulia, K.A.; Perrea, D.; Perrea, D.; Makrilakis, K.; Diakoumopoulou, E.; Katsilambros, N. Vinegar reduces postprandial hyperglycaemia in patients with type II diabetes when added to a high, but not to a low, glycaemic index meal. Eur. J. Clin. Nutr. 2010, 64, 727–732. [Google Scholar] [CrossRef] [PubMed]
- Yusoff, N.A.; Yam, M.F.; Beh, H.K.; Abdul Razak, K.N.; Widyawati, T.; Mahmud, R.; Ahmad, M.; Asmawi, M.Z. Antidiabetic and antioxidant activities of Nypa fruticans Wurmb. vinegar sample from Malaysia. Asian Pac. J. Trop. Med. 2015. [Google Scholar] [CrossRef]
- Qiu, J.; Ren, C.; Fan, J.; Li, Z. Antioxidant activities of aged oat vinegar in vitro and in mouse serum and liver. J. Sci. Food Agric. 2010, 90, 1951–1958. [Google Scholar] [CrossRef] [PubMed]
- Wilson, T.H.; Wiseman, G. The use of sacs of everted small intestine for the study of the transference of substances from the mucosal to the serosal surface. J. Physiol. 1954, 123, 116–125. [Google Scholar] [CrossRef] [PubMed]
- Hassan, Z.; Yam, M.F.; Ahmad, M.; Yusof, A.P.M. Antidiabetic properties and mechanism of action of Gynura procumbens water extract in streptozotocin-induced diabetic rats. Molecules 2010, 15, 9008–9023. [Google Scholar] [CrossRef] [PubMed]
- Ali, R.B.; Atangwho, I.J.; Kaur, N.; Ahmad, M.; Mahmud, R.; Asmawi, M.Z. In vitro and in vivo effects of standardized extract and fractions of Phaleria macrocarpa fruits pericarp on lead carbohydrate digesting enzymes. BMC Complement. Altern. Med. 2013, 13, 1–11. [Google Scholar]
- Kwon, Y.I.; Apostolidis, E.; Shetty, K. Inhibitory potential of wine and tea against α-amylase and α-glucosidase for management of hyperglyemia linked to type 2 diabetes. J. Food Biochem. 2008, 32, 15–31. [Google Scholar] [CrossRef]
- Gerich, J.E. Clinical significance, pathogenesis, and management of postprandial hyperglycemia. Arch. Intern. Med. 2003, 163, 1306–1316. [Google Scholar] [CrossRef] [PubMed]
- Hanhineva, K.; Torronen, R.; Bondia-Pons, I.; Pekkinen, J.; Kolehmainen, M.; Mykkanen, H.; Poutanen, K. Impact of dietary polyphenols on carbohydrate metabolism. Int. J. Mol. Sci. 2010, 11, 1365–1402. [Google Scholar] [CrossRef] [PubMed]
- Orme, C.M.; Bogan, J.S. Sorting out diabetes. Science 2009, 324, 1155–1156. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, K.; Hase, K.; Taketani, Y.; Minami, H.; Oka, T.; Nakabou, Y.; Hagihira, H. Diabetes and glucose transporter gene expression in rat small intestine. Biochem. Biophys. Res. Commun. 1991, 181, 1110–1117. [Google Scholar] [CrossRef]
- Burant, C.F.; Flink, S.; Depaoli, A.M.; Chen, J.; Lee, W.S.; Hediger, M.A.; Buse, J.B.; Chang, E.B. Small intestine hexose transport in experimental diabetes. Increased transporter mRNA and protein expression in enterocytes. J. Clin. Investig. 1994, 93, 578–585. [Google Scholar] [CrossRef] [PubMed]
- Jurysta, C.; Nicaise, C.; Giroix, M.-H.; Cetik, S.; Malaisse, W.J.; Sener, A. Comparison of GLUT1, GLUT2, GLUT 4 and SGLT1 mRNA expression in the salivary glands and six other organs of control, streptozotocin-induced, goto-kakizaki diabetic rats. Cell. Physiol. Biochem. 2013, 31, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Johnston, C.S.; Kim, C.M.; Buller, A.J. Vinegar improves insulin sensitivity to a high-carbohydrate meal in subjects with insulin resistance or type 2 diabetes. Diabetes Care 2004, 27, 281–282. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, N.; Satsu, H.; Watanabe, H.; Fukaya, M.; Tsukamoto, Y.; Miyamoto, Y.; Shimizu, M. Acetic acid suppresses the increase in disaccharidase activity that occurs during culture of caco-2 cells. J. Nutr. 2000, 130, 507–513. [Google Scholar] [PubMed]
- Ehrenkranz, J.R.; Lewis, N.G.; Ronald Kahn, C.; Roth, J. Phlorizin: A review. Diabetes Metab. Res. Rev. 2005, 21, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Hayakawa, T.; Noda, A.; Kondo, T.; Okumura, N. Effects of acarbose, an α-glucosidase inhibitor (BAY g 5421), on orally loaded glucose, maltose and sucrose and on blood glucose control in non-insulin-dependent diabetics. Nagoya J. Med. Sci. 1985, 47, 35–41. [Google Scholar] [PubMed]
- Madariaga, H.; Lee, P.; Heitlinger, L.; Lebenthal, E. Effects of graded α-glucosidase inhibition on sugar absorption in vivo. Dig. Dis. Sci. 1988, 33, 1020–1024. [Google Scholar] [CrossRef] [PubMed]
- Drozdowski, L.A.; Thomson, A.B. Intestinal sugar transport. World J. Gastroenterol. 2006, 12, 1657–1670. [Google Scholar] [PubMed]
- Hassimotto, N.M.A.; Genovese, M.I.S.; Lajolo, F.M. Absorption and metabolism of cyanidin-3-glucoside and cyanidin-3-rutinoside extracted from wild mulberry (Morus nigra L.) in rats. Nutr. Res. 2008, 28, 198–207. [Google Scholar] [CrossRef] [PubMed]
- Nair, S.S.; Kavrekar, V.; Mishra, A. In vitro studies on alpha amylase and alpha glucosidase inhibitory activities of selected plant extracts. Eur. J. Exp. Biol. 2013, 3, 128–131. [Google Scholar]
- Ye, F.; Shen, Z.; Xie, M. Alpha-glucosidase inhibition from a Chinese medical herb (Ramulus mori) in normal and diabetic rats and mice. Phytomedicine 2002, 9, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Liljeberg, H.; Bjorck, I. Delayed gastric emptying rate may explain improved glycaemia in healthy subjects to a starchy meal with added vinegar. Eur. J. Clin. Nutr. 1998, 52, 368–371. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yusoff, N.A.; Ahmad, M.; Al Hindi, B.; Widyawati, T.; Yam, M.F.; Mahmud, R.; Razak, K.N.A.; Asmawi, M.Z. Aqueous Extract of Nypa fruticans Wurmb. Vinegar Alleviates Postprandial Hyperglycemia in Normoglycemic Rats. Nutrients 2015, 7, 7012-7026. https://doi.org/10.3390/nu7085320
Yusoff NA, Ahmad M, Al Hindi B, Widyawati T, Yam MF, Mahmud R, Razak KNA, Asmawi MZ. Aqueous Extract of Nypa fruticans Wurmb. Vinegar Alleviates Postprandial Hyperglycemia in Normoglycemic Rats. Nutrients. 2015; 7(8):7012-7026. https://doi.org/10.3390/nu7085320
Chicago/Turabian StyleYusoff, Nor Adlin, Mariam Ahmad, Bassel Al Hindi, Tri Widyawati, Mun Fei Yam, Roziahanim Mahmud, Khairul Niza Abdul Razak, and Mohd Zaini Asmawi. 2015. "Aqueous Extract of Nypa fruticans Wurmb. Vinegar Alleviates Postprandial Hyperglycemia in Normoglycemic Rats" Nutrients 7, no. 8: 7012-7026. https://doi.org/10.3390/nu7085320