Modifying Choroidal Neovascularization Development with a Nutritional Supplement in Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Animals
2.2. Diode Laser-Induced CNV Model
2.3. Oral Nutritional Supplements and the Vehicle

| Per Capsule | Murine Dose Per Day | |
|---|---|---|
| Vitamin C (mg) | 120 | 1.57 |
| Vitamin E (mg) | 15 | 0.20 |
| Zinc (mg) | 7.5 | 0.10 |
| Copper (µg) | 500 | 6.54 |
| EPA (mg) | 190 | 2.48 |
| DHA (mg) | 95 | 1.24 |
| Lutein (mg) | 5 | 0.07 |
| Zeaxanthin (mg) | 1 | 0.01 |
| Resveratrol (mg) | 15 | 0.20 |
2.4. Intravitreal Treatments and the Injection Procedure
2.5. Evaluation of Leakage from Choroidal Neovascular Lesions
2.6. CD31 Immunofluorescence
2.7. Determination of Total Protein Levels
2.8. Vascular Endothelial Growth Factor and NOD-like Receptor Family Pyrin Domain Containing 3
2.9. Quantitative Real Time-Polymerase Chain Reaction
2.10. Matrix Metalloproteinase-2 and -9 Activity
2.11. Statistical Analyses
3. Results
3.1. Effect of Nutritional Supplements on Choroidal Neovascular Lesion Leakage
| V | S | V + aVEGF | S + aVEGF | |
|---|---|---|---|---|
| FA | −0.030 ± 0.003 | −0.261 ± 0.04 *** | −0.112 ± 0.025 * | −0.091 ± 0.03 * |
| CD31 (% vs. V) | 100 ± 10.03 | 63 ± 5.35 ** | 80 ± 9.62 * | 64 ± 9.69 * |
| Protein expression | ||||
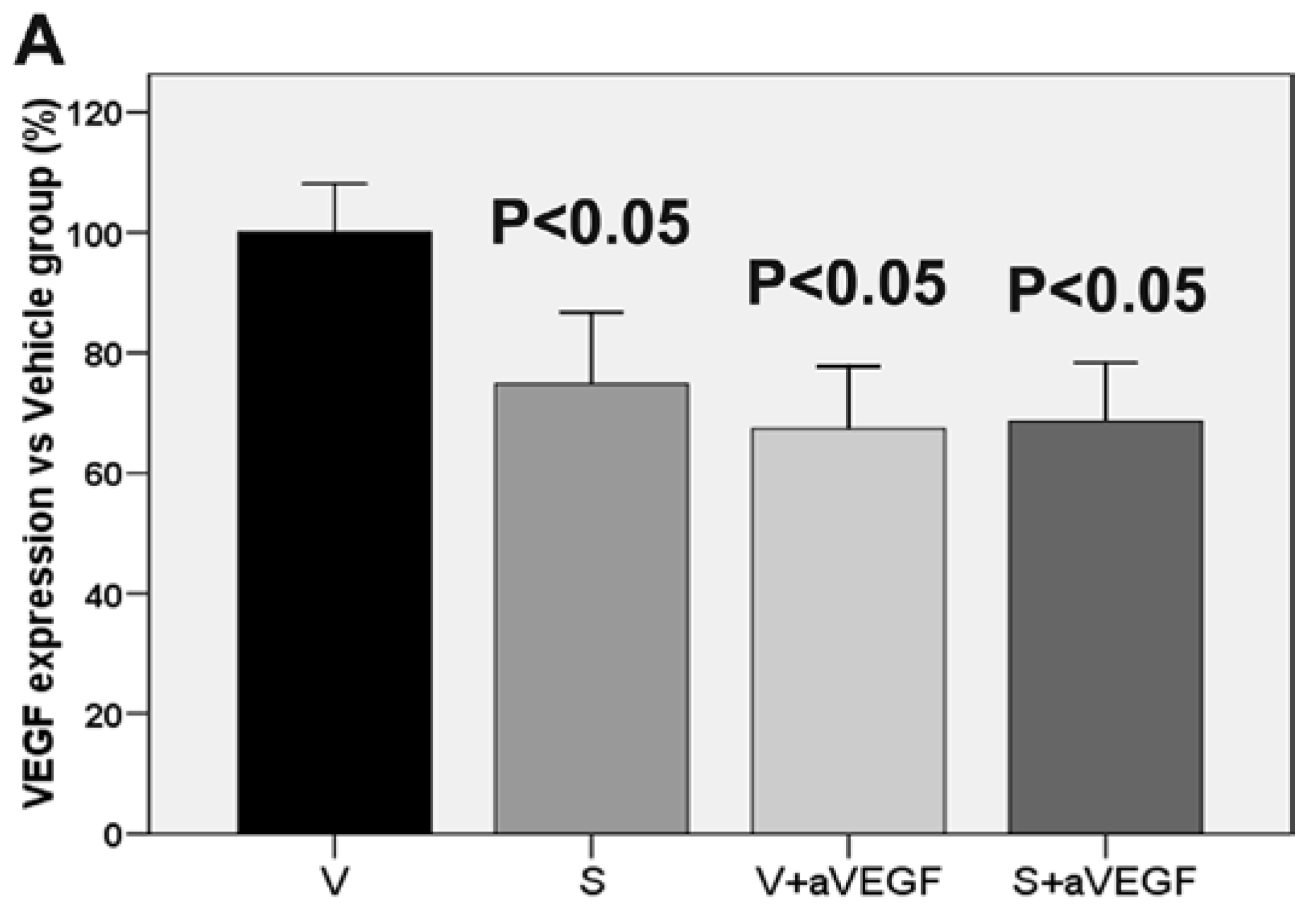
| VEGF (% vs. V) | 100 ± 8.11 | 74.78 ± 11.96 * | 67.39 ± 10.30 * | 68.59 ± 9.70 * |
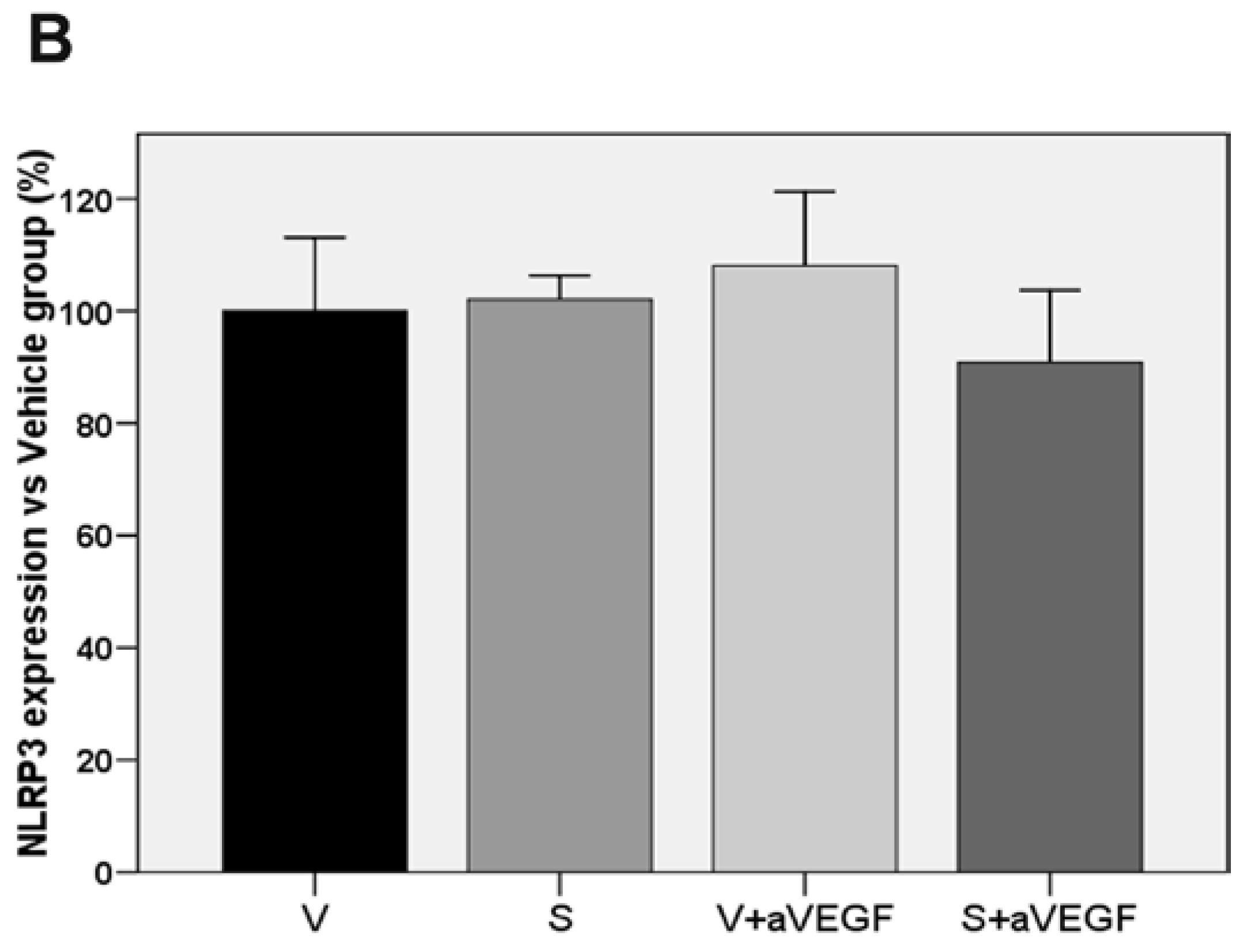
| NLRP3 (% vs. V) | 100 ± 13.12 | 102.07 ± 4.26 | 108.08 ± 13.20 | 90.8 5 ± 12.83 |
| Enzymatic activity | ||||
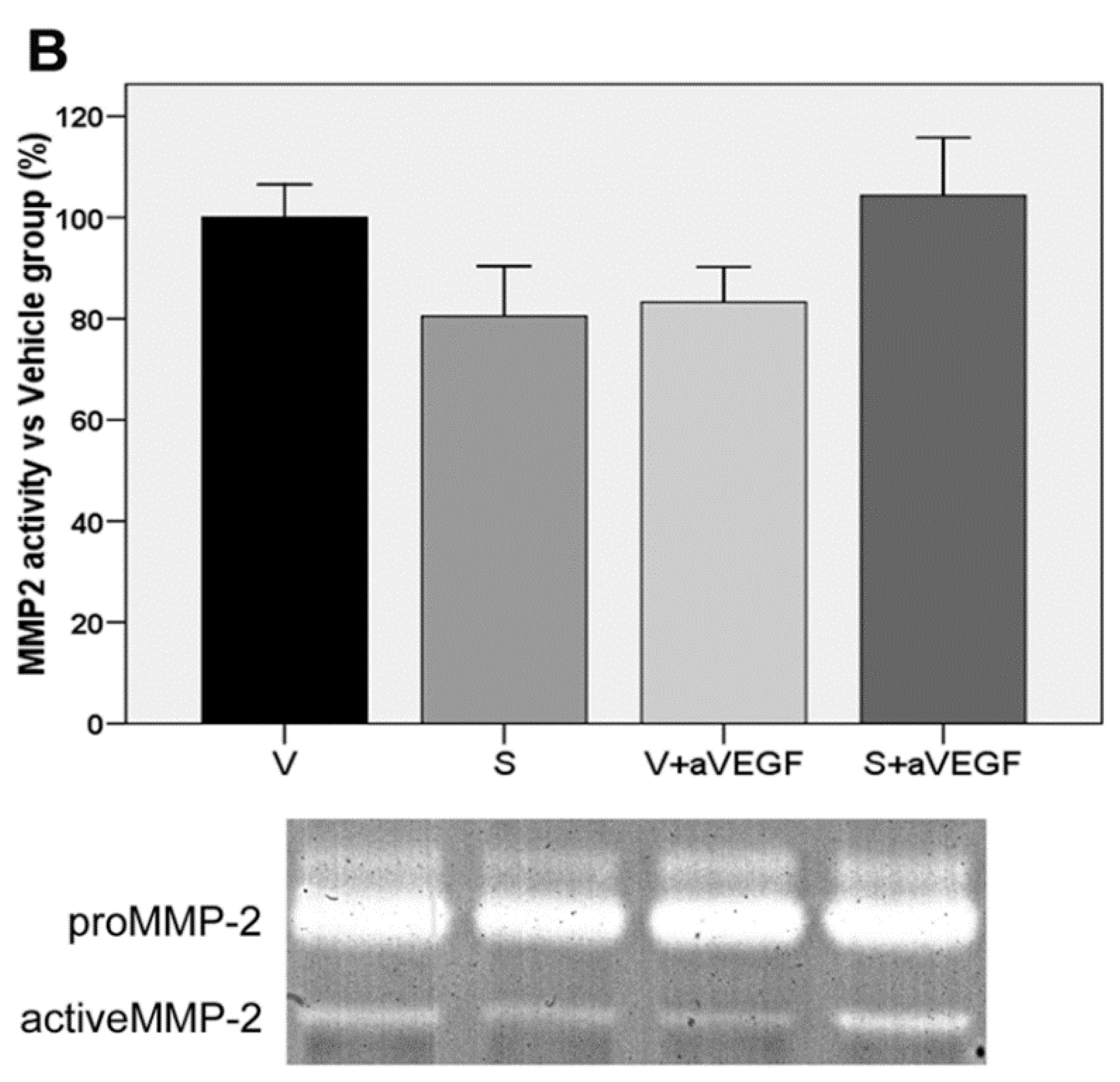
| MMP2 (% vs. V) | 100 ± 6.49 | 80.45 ± 9.93 | 83.25 ± 6.96 | 104.30 ± 11.41 |
| MMP9 (% vs. V) | 100 ± 10.08 | 105.50 ± 12.81 | 73.79 ± 10.64 | 54.75 ± 5.32 **, † |
| Gene expression (Log RQ) | ||||
| VEGF | 0.00 | −0.53 * | −0.48 * | −0.54 * |
| MMP2 | 0.00 | −0.25 | −0.11 | −0.70 |
| MMP9 | 0.00 | −0.18 | −0.09 | −0.29 *** |
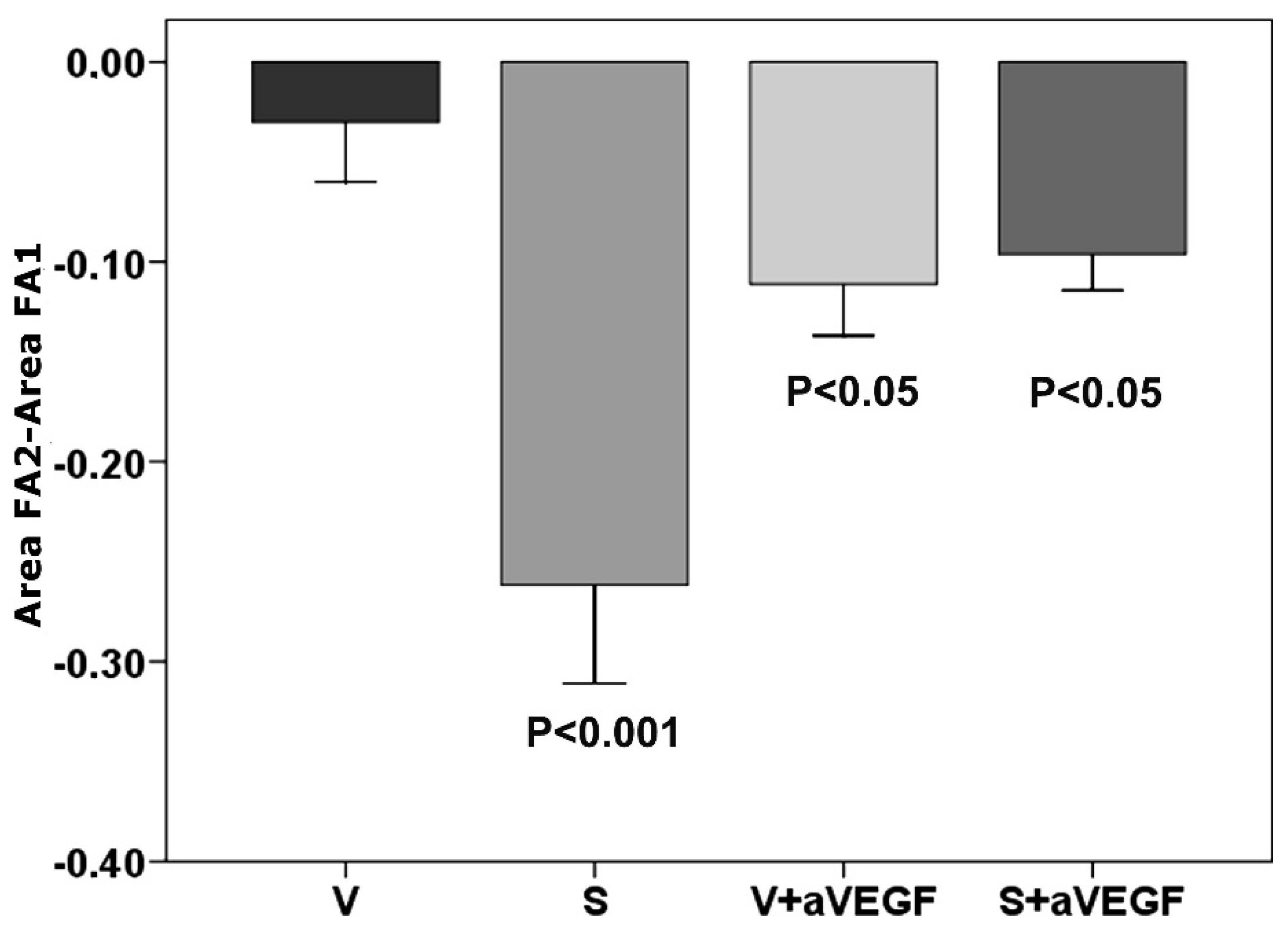
3.2. Effect of Nutritional Supplements on Choroidal Neovascular Lesion Size
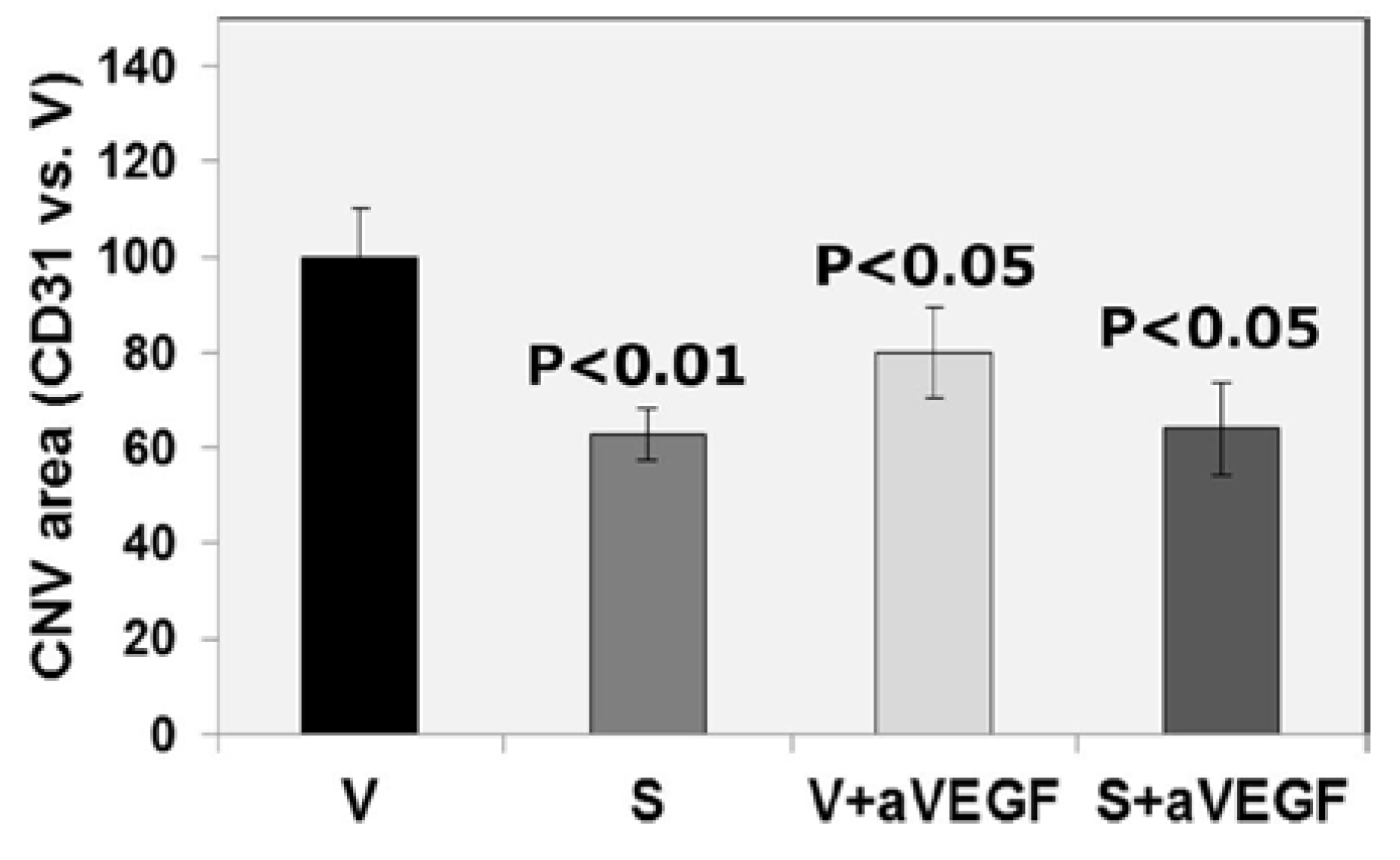
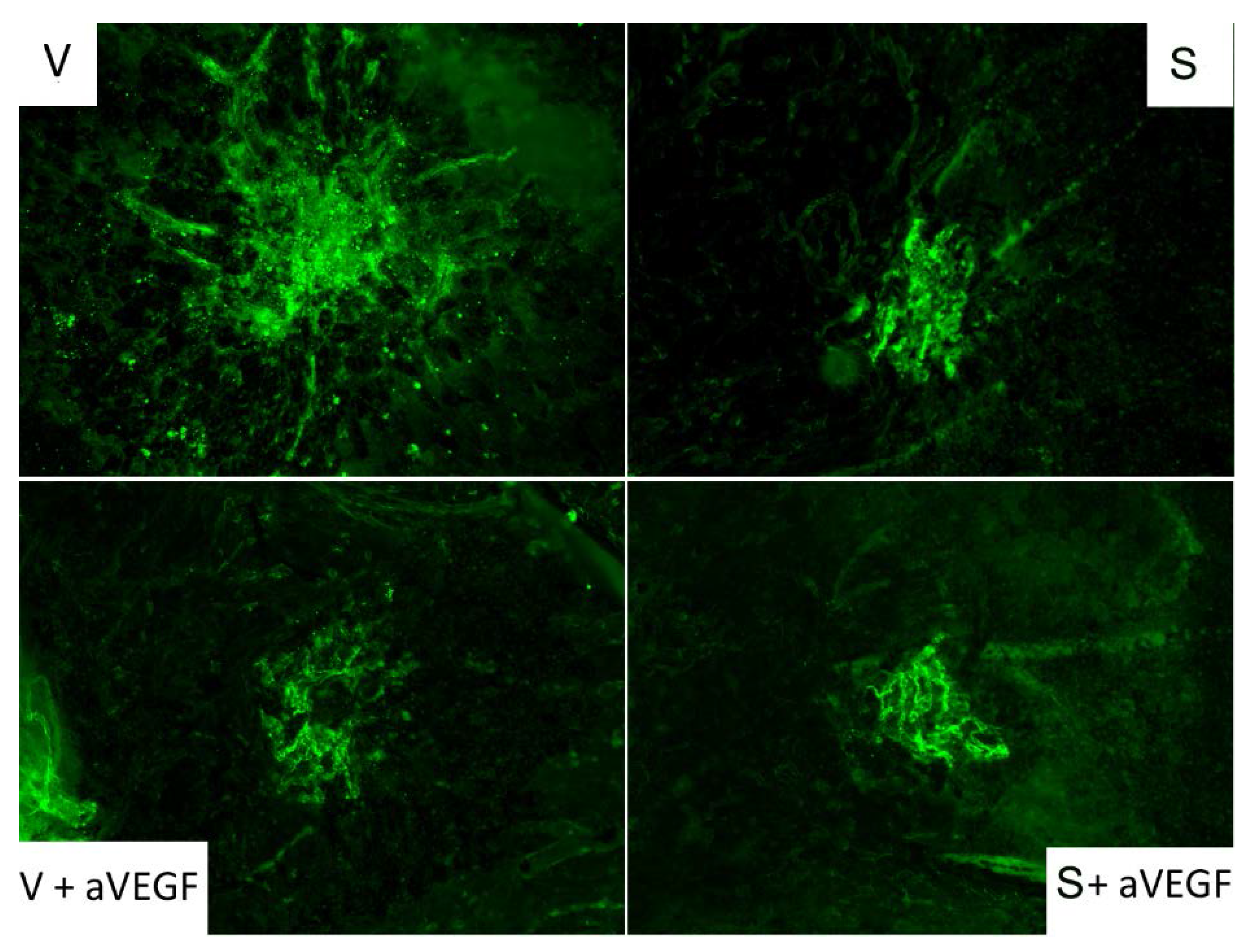
3.3. Effect of the Nutritional Supplement on Vascular Endothelial Growth Factor and NOD-Like Receptor Family Pyrin Domain Containing 3
3.4. Matrix Metalloproteinase Activity

3.5. Effect of the Nutritional Supplement on Gene Expression
4. Discussion
5. Conclusions
Supplementary Files
Supplementary File 1Acknowledgments
Author Contributions
Conflicts of Interest
References
- Grossniklaus, H.E.; Green, W.R. Choroidal neovascularisation. Am. J. Ophthalmol. 2004, 137, 496–503. [Google Scholar] [CrossRef]
- Miller, J.W. Age-related macular degeneration revisited-piecing the puzzle: The LXIX Edward Jackson Memorial Lecture. Am. J. Ophthalmol. 2013, 155, 1–35. [Google Scholar] [CrossRef]
- Augood, C.A.; Vingerling, J.R.; de Jong, P.T.; Chakravarthy, U.; Seland, J.; Soubrane, G.; Tomazzoli, L.; Topouzis, F.; Bentham, G.; Rahu, M.; et al. Prevalence of age-related maculopathy in older Europeans: The European Eye Study (EUREYE). Arch. Ophthalmol. 2006, 124, 529–535. [Google Scholar] [CrossRef]
- Lindekleiv, H.; Erke, M.G. Projected prevalence of age-related macular degeneration in Scandinavia 2012–2040. Acta Ophthalmol. 2013, 91, 307–311. [Google Scholar] [CrossRef]
- Age-Related Eye Disease Study Research Group. A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E, beta carotene, and zinc for age-related macular degeneration and vision loss: AREDS report No. 8. Arch. Ophthalmol. 2001, 119, 1417–1436. [Google Scholar]
- Aslam, T.; Delcourt, C.; Silva, R.; Holz, F.G.; Leys, A.; García Layana, A.; Souied, E. Micronutrients in age-related macular degeneration. Ophthalmologica 2013, 229, 75–79. [Google Scholar] [CrossRef]
- Benny, O.; Nakai, K.; Yoshimura, T.; Bazinet, L.; Akula, J.D.; Nakao, S.; Hafezi-Moghadam, A.; Panigrahy, D.; Pakneshan, P.; D’Amato, R.J. Broad spectrum antiangiogenic treatment for ocular neovascular disease. PLoS ONE 2010, 5, 12515. [Google Scholar] [CrossRef]
- Campochiaro, P.A. Targeted pharmacotherapy of retinal diseases with ranibizumab. Drugs Today 2007, 43, 529–537. [Google Scholar] [CrossRef]
- Garber, K. Biotech in a blink. Nat. Biotechnol. 2010, 28, 311–314. [Google Scholar] [CrossRef]
- Carmeliet, P.; de Smet, F.; Loges, S.; Mazzone, M. Branching morphogenesis and antiangiogenesis candidates: Tip cells lead the way. Nat. Rev. Clin. Oncol. 2009, 6, 315–326. [Google Scholar] [CrossRef]
- Seddon, J.M.; Ajani, U.A.; Sperduto, R.D.; Hiller, R.; Blair, N.; Burton, T.C.; Farber, M.D.; Gragoudas, E.S.; Haller, J.; Miller, D.T.; et al. Dietary carotenoids, vitamins A, C, and E, and advanced age-related macular degeneration. Eye Disease Case-Control Study Group. JAMA 1994, 272, 1413–1420. [Google Scholar] [CrossRef]
- Age-Related Eye Disease Study Research Group; SanGiovanni, J.P.; Chew, E.Y.; Clemons, T.E.; Ferris, F.L., 3rd; Gensler, G.; Lindblad, A.S.; Milton, R.C.; Seddon, J.M.; Spertudo, R.D. The relationship of dietary carotenoid and vitamin A, E, and C intake with age-related macular degeneration in a case-control study: AREDS Report No. 22. Arch. Ophthalmol. 2007, 125, 1225–1232. [Google Scholar]
- Augood, C.; Chakravarthy, U.; Young, I.; Vioque, J.; de Jong, P.T.; Bentham, G.; Rahu, M.; Seland, J.; Soubrane, G.; Tomazzoli, L.; et al. Oily fish consumption, dietary docosahexaenoic acid and eicosapentaenoic acid intakes, and associations with neovascular age-related macular degeneration. Am. J. Clin. Nutr. 2008, 88, 398–406. [Google Scholar]
- SanGiovanni, J.P.; Agron, E.; Meleth, A.D.; Reed, G.F.; Sperduto, R.D.; Clemons, T.E.; Chew, E.Y.; Age-Related Eye Disease Study Research Group. ω-3 long chain polyunsaturated fatty acid intake and 12-y incidence of neovascular age-related macular degeneration and central geographic atrophy: AREDS report 30, a prospective cohort study from the Age-Related Eye Disease Study. Am. J. Clin. Nutr. 2009, 90, 1601–1607. [Google Scholar] [CrossRef]
- Age-Related Eye Disease Study 2 Research Group. Lutein + zeaxanthin and omega-3 fatty acids for age-related macular degeneration: The Age-Related Eye Disease Study 2 (AREDS2) randomized clinical trial. JAMA 2013, 309, 2005–2015. [Google Scholar]
- Chen, P.L.; Easton, A.S. Anti-angiogenic effects of resveratrol on cerebral angiogenesis. Curr. Neurovasc. Res. 2011, 8, 14–24. [Google Scholar] [CrossRef]
- Li, C.; Wang, L.; Huang, K.; Zheng, L. Endoplasmic reticulum stress in retinal vascular degeneration: Protective role of resveratrol. Invest. Ophthalmol. Vis. Sci. 2012, 53, 3241–3249. [Google Scholar] [CrossRef]
- Yu, C.; Hhao-Di, F.; Fang, W.; Hong-Yan, L.; Rui, H. Anti-angiogenic activity of resveratrol, a natural compound from medicinal plants. J. Asian Nat. Prod. Res. 2005, 3, 205–213. [Google Scholar]
- Yun, C.; Sheng-Hong, T. Pro- and anti-angiogenesis effects of resveratrol. Vivo 2007, 21, 365–370. [Google Scholar]
- Pintea, A.; Rugină, D.; Pop, R.; Bunea, A.; Socaciu, C.; Diehl, H.A. Antioxidant effect of trans-resveratrol in cultured human retinal pigment epithelial cells. J. Ocul. Pharmacol. Ther. 2011, 27, 315–321. [Google Scholar] [CrossRef]
- Cottart, C.H.; Nivet-Antoine, V.; Laguillier-Morizot, C.; Beaudeux, J.L. Resveratrol bioavailability and toxicity in humans. Mol. Nutr. Food Res. 2010, 54, 7–16. [Google Scholar] [CrossRef]
- Kubota, S.; Kurihara, T.; Ebinuma, M.; Kubota, M.; Yuki, K.; Sasaki, M.; Noda, K.; Ozawa, Y.; Oike, Y.; Ishida, S.; et al. Resveratrol prevents light-induced retinal degeneration via suppressing activator protein-1 activation. Am. J. Pathol. 2010, 177, 1725–1731. [Google Scholar]
- Zheng, Y.; Liu, Y.; Ge, J.; Wang, X.; Liu, L.; Bu, Z.; Liu, P. Resveratrol protects human lens epithelial cells against H2O2-induced oxidative stress by increasing catalase, SOD-1, and HO-1 expression. Mol. Vis. 2010, 16, 1467–1474. [Google Scholar]
- King, R.E.; Kent, K.D.; Bomser, J.A. Resveratrol reduces oxidation and proliferation of human retinal pigment epithelial cells via extracellular signal-regulated kinase inhibition. Chem. Biol. Interact. 2005, 151, 143–149. [Google Scholar] [CrossRef]
- García-Layana, A.; Vásquez, G.; Salinas-Alamán, A.; Moreno-Montañés, J.; Recalde, S.; Fernández-Robredo, P. Development of laser-induced choroidal neovascularization in rats after retinal damage by sodium iodate injection. Ophthalmic. Res. 2009, 42, 205–212. [Google Scholar] [CrossRef]
- Wuest, T.R.; Carr, D.J. VEGF-A expression by HSV-1-infected cells drives corneal lymphangiogenesis. J. Exp. Med. 2010, 207, 101–115. [Google Scholar] [CrossRef]
- Basu, A.; Contreras, A.G.; Datta, D.; Flynn, E.; Zeng, L.; Cohen, H.T.; Briscoe, D.M.; Pal, S. Overexpression of vascular endothelial growth factor and the development of post-transplantation cancer. Cancer Res. 2008, 68, 5689–5698. [Google Scholar] [CrossRef]
- Recalde, S.; Zarranz-Ventura, J.; Fernández-Robredo, P.; García-Gómez, P.J.; Salinas-Alamán, A.; Borrás-Cuesta, F.; Dotor, J.; García-Layana, A. Transforming growth factor-β inhibition decreases diode laser-induced choroidal neovascularization development in rats: P17 and P144 peptides. Invest. Ophthalmol. Vis. Sci. 2011, 52, 7090–7097. [Google Scholar] [CrossRef]
- Richer, S.; Patel, S.; Sockanathan, S.; Ulanski, L.J., 2nd; Miller, L.; Podella, C. Resveratrol based oral nutritional supplement produces long-term beneficial effects on structure and visual function in human patients. Nutrients 2014, 6, 4404–4420. [Google Scholar] [CrossRef]
- Richer, S.; Stiles, W.; Ulanski, L.; Carroll, D.; Podella, C. Observation of human retinal remodeling in octogenarians with a resveratrol based nutritional supplement. Nutrients 2013, 5, 1989–2005. [Google Scholar] [CrossRef]
- Klein, R.; Klein, B.E.; Linton, K.L. Prevalence of age-related maculopathy. The Beaver Dam Eye Study. Ophthalmology 1992, 99, 933–943. [Google Scholar] [CrossRef]
- Seddon, J.M. Multivitamin-multimineral supplements and eye disease: Age-related macular degeneration and cataract. Am. J. Clin. Nutr. 2007, 85, 304–307. [Google Scholar]
- Charkoudian, L.D.; Gower, E.W.; Solomon, S.D.; Schachat, A.P.; Bressler, N.M.; Bressler, S.B. Vitamin usage patterns in the prevention of advanced age-related macular degeneration. Ophthalmology 2008, 115, 1032–1038. [Google Scholar] [CrossRef]
- Hernandez-Pastor, L.J.; Ortega, A.; Garcia-Layana, A.; Giraldez, J. Ranibizumab for neovascular age-related macular degeneration. Am. J. Health Syst. Pharm. 2008, 65, 1805–1814. [Google Scholar] [CrossRef]
- Sádaba, L.M.; Fernández-Robredo, P.; Rodríguez, J.A.; García-Layana, A. Antioxidant effects of vitamins C and E, multivitamin-mineral complex and flavonoids in a model of retinal oxidative stress: The ApoE-deficient mouse. Exp. Eye Res. 2008, 86, 470–479. [Google Scholar] [CrossRef]
- Fernández-Robredo, P.; Recalde, S.; Arnáiz, G.; Salinas-Alamán, A.; Sádaba, L.M.; Moreno-Orduña, M.; García-Layana, A. Effect of zeaxanthin and antioxidant supplementation on vascular endothelial growth factor (VEGF) expression in apolipoprotein-E deficient mice. Curr. Eye Res. 2009, 34, 543–552. [Google Scholar] [CrossRef]
- Koto, T.; Nagai, N.; Mochimaru, H.; Kurihara, T.; Izumi-Nagai, K.; Satofuka, S.; Shinoda, H.; Noda, K.; Ozawa, Y.; Inoue, M.; et al. Eicosapentaenoic acid is anti-inflammatory in preventing choroidal neovascularization in mice. Invest. Ophthalmol. Vis. Sci. 2007, 48, 4328–4334. [Google Scholar] [CrossRef]
- Chao, C.Y.; Lii, C.K.; Ye, S.Y.; Li, C.C.; Lu, C.Y.; Lin, A.H.; Liu, K.L.; Chen, H.W. Docosahexaenoic acid inhibits vascular endothelial growth factor (VEGF)-induced cell migration via the GPR120/PP2A/ERK1/2/eNOS signaling pathway in human umbilical vein endothelial cells. J. Agric. Food Chem. 2014, 62, 4152–4158. [Google Scholar] [CrossRef]
- Ramkumar, H.L.; Tuo, J.; Shen de, F.; Zhang, J.; Cao, X.; Chew, E.Y.; Chan, C.C. Nutrient supplementation with n3 polyunsaturated fatty acids, lutein, and zeaxanthin decrease A2E accumulation and VEGF expression in the retinas of Ccl2/Cx3cr1-deficient mice on Crb1rd8 background. J. Nutr. 2013, 143, 1129–1135. [Google Scholar] [CrossRef]
- Shen, J.; Shen, S.; Das, U.N.; Xu, G. Effect of essential fatty acids on glucose-induced cytotoxicity to retinal vascular endothelial cells. Lipids Health Dis. 2012, 11, 90. [Google Scholar] [CrossRef]
- Hua, J.; Guerin, K.I.; Chen, J.; Michán, S.; Stahl, A.; Krah, N.M.; Seaward, M.R.; Dennison, R.J.; Juan, A.M.; Hatton, C.J.; et al. Resveratrol inhibits pathologic retinal neovascularization in Vldlr(−/−) mice. J. Invest. Ophthalmol. Vis. Sci. 2011, 52, 2809–2816. [Google Scholar] [CrossRef]
- Khan, A.A.; Dace, D.S.; Ryazanov, A.G.; Kelly, J.; Apte, R.S. Resveratrol regulates pathologic angiogenesis by a eukaryotic elongation factor-2 kinase-regulated pathway. Am. J. Pathol. 2010, 177, 481–492. [Google Scholar] [CrossRef]
- Sheu, S.J.; Liu, N.C.; Ou, C.C.; Bee, Y.S.; Chen, S.C.; Lin, H.C.; Chan, J.Y. Resveratrol stimulates mitochondrial bioenergetics to protect retinal pigment epithelial cells from oxidative damage. Invest. Ophthalmol. Vis. Sci. 2013, 54, 6426–6438. [Google Scholar] [CrossRef]
- Nagai, N.; Kubota, S.; Tsubota, K.; Ozawa, Y. Resveratrol prevents the development of choroidal neovascularization by modulating AMP-activated protein kinase in macrophages and other cell types. J. Nutr. Biochem. 2014, 25, 1218–1225. [Google Scholar] [CrossRef]
- Kanavi, M.R.; Darjatmoko, S.; Wang, S.; Azari, A.A.; Farnoodian, M.; Kenealey, J.D.; van Ginkel, P.R.; Albert, D.M.; Sheibani, N.; Polans, A.S. The sustained delivery of resveratrol or a defined grape powder inhibits new blood vessel formation in a mouse model of choroidal neovascularization. Molecules 2014, 19, 17578–17603. [Google Scholar] [CrossRef]
- Ferrara, N.; Davis-Smyth, T. The biology of vascular endothelial growth factor. Endocr. Rev. 1997, 18, 4–25. [Google Scholar] [CrossRef]
- Fernández-Robredo, P.; Sádaba, L.M.; Salinas-Alamán, A.; Recalde, S.; Rodríguez, J.A.; García-Layana, A. Effect of lutein and antioxidant supplementation on VEGF expression, MMP-2 activity, and ultrastructural alterations in apolipoprotein E-deficient mouse. Oxid. Med. Cell. Longev. 2013, 2013. [Google Scholar] [CrossRef]
- Kim, Y.H.; Kim, Y.S.; Roh, G.S.; Choi, W.S.; Cho, G.J. Resveratrol blocks diabetes-induced early vascular lesions and vascular endothelial growth factor induction in mouse retinas. Acta Ophthalmol. 2012, 90, 31–37. [Google Scholar] [CrossRef]
- Li, W.; Jiang, D. Effect of resveratrol on bcl-2 and VEGF expression in oxygen-induced retinopathy of prematurity. J. Pediatr. Ophthalmol. Strabismus 2012, 49, 230–235. [Google Scholar] [CrossRef]
- Yar, A.S.; Menevse, S.; Dogan, I.; Alp, E.; Ergin, V.; Cumaoglu, A.; Aricioglu, A.; Ekmekci, A.; Menevse, A. Investigation of ocular neovascularization-related genes and oxidative stress in diabetic rat eye tissues after resveratrol treatment. J. Med. Food 2012, 15, 391–398. [Google Scholar] [CrossRef]
- Rezende, F.A.; Lapalme, E.; Qian, C.X.; Smith, L.E.; SanGiovanni, J.P.; Sapieha, P. Omega-3 supplementation combined with anti-vascular endothelial growth factor lowers vitreal levels of vascular endothelial growth factor in wet age-related macular degeneration. Am. J. Ophthalmol. 2014, 158, 1071–1078. [Google Scholar] [CrossRef]
- Chau, K.Y.; Sivaprasad, S.; Patel, N.; Donaldson, T.A.; Luthert, P.J.; Chong, N.V. Plasma levels of matrix metalloproteinase-2 and -9 (MMP-2 and MMP-9) in age-related macular degeneration. Eye 2008, 22, 855–859. [Google Scholar] [CrossRef]
- Belotti, D.; Calcagno, C.; Garofalo, A.; Caronia, D.; Riccardi, E.; Giavazzi, R.; Taraboletti, G. Vascular endothelial growth factor stimulates organ-specific host matrix metalloproteinase-9 expression and ovarian cancer invasion. Mol. Cancer Res. 2008, 6, 525–534. [Google Scholar] [CrossRef]
- Sun, C.Y.; Hu, Y.; Guo, T.; Wang, H.F.; Zhang, X.P.; He, W.J.; Tan, H. Resveratrol as a novel agent for treatment of multiple myeloma with matrix metalloproteinase inhibitory activity. Acta Pharmacol. Sin. 2006, 27, 1447–1452. [Google Scholar] [CrossRef]
- Shinto, L.; Marracci, G.; Bumgarner, L.; Yadav, V. The effects of omega-3 fatty acids on matrix metalloproteinase-9 production and cell migration in human immune cells: Implications for multiple sclerosis. Autoimmune Dis. 2011, 2011. [Google Scholar] [CrossRef]
- Liuzzi, G.M.; Latronico, T.; Rossano, R.; Viggiani, S.; Fasano, A.; Riccio, P. Inhibitory effect of polyunsaturated fatty acids on MMP-9 release from microglial cells--implications for complementary multiple sclerosis treatment. Neurochem. Res. 2007, 32, 2184–2193. [Google Scholar] [CrossRef]
- Tsuzuki, T.; Shibata, A.; Kawakami, Y.; Nakagawa, K.; Miyazawa, T. Conjugated eicosapentaenoic acid inhibits vascular endothelial growth factor-induced angiogenesis by suppressing the migration of human umbilical vein endothelial cells. J. Nutr. 2007, 137, 641–646. [Google Scholar]
- Zarranz-Ventura, J.; Fernández-Robredo, P.; Recalde, S.; Salinas-Alamán, A.; Borrás-Cuesta, F.; Dotor, J.; García-Layana, A. Transforming growth factor-beta inhibition reduces progression of early choroidal neovascularization lesions in rats: P17 and P144 peptides. PLoS ONE 2013, 8, 65434. [Google Scholar] [CrossRef]
- Dong, W.; Li, N.; Gao, D.; Zhen, H.; Zhang, X.; Li, F. Resveratrol attenuates ischemic brain damage in the delayed phase after stroke and induces messenger RNA and protein express for angiogenic factors. J. Vasc. Surg. 2008, 48, 709–714. [Google Scholar] [CrossRef]
- Lambert, V.; Munaut, C.; Jost, M.; Noël, A.; Werb, Z.; Foidart, J.M.; Rakic, J.M. Matrix metalloproteinase-9 contributes to choroidal neovascularization. Am. J. Pathol. 2002, 161, 1247–1253. [Google Scholar] [CrossRef]
- Lambert, V.; Wielockx, B.; Munaut, C.; Galopin, C.; Jost, M.; Itoh, T.; Werb, Z.; Baker, A.; Libert, C.; Krell, H.W.; et al. MMP-2 and MMP-9 synergize in promoting choroidal neovascularization. FASEB J. 2003, 17, 2290–2292. [Google Scholar] [CrossRef]
- Tseng, W.A.; Thein, T.; Kinnunen, K.; Lashkari, K.; Gregory, M.S.; D’Amore, P.A.; Ksander, B.R. NLRP3 inflammasome activation in retinal pigment epithelial cells by lysosomal destabilization: Implications for age-related macular degeneration. Invest. Ophthalmol. Vis. Sci. 2013, 7, 110–120. [Google Scholar] [CrossRef]
- Doyle, S.L.; Campbell, M.; Ozaki, E.; Salomon, R.G.; Mori, A.; Kenna, P.F.; Farrar, G.J.; Kiang, A.S.; Humphries, M.M.; Lavelle, E.C.; et al. NLRP3 has a protective role in age-related macular degeneration through the induction of IL-18 by drusen components. Nat. Med. 2012, 18, 791–798. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ivanescu, A.A.; Fernández-Robredo, P.; Heras-Mulero, H.; Sádaba-Echarri, L.M.; García-García, L.; Fernández-García, V.; Moreno-Orduna, M.; Redondo-Exposito, A.; Recalde, S.; García-Layana, A. Modifying Choroidal Neovascularization Development with a Nutritional Supplement in Mice. Nutrients 2015, 7, 5423-5442. https://doi.org/10.3390/nu7075229
Ivanescu AA, Fernández-Robredo P, Heras-Mulero H, Sádaba-Echarri LM, García-García L, Fernández-García V, Moreno-Orduna M, Redondo-Exposito A, Recalde S, García-Layana A. Modifying Choroidal Neovascularization Development with a Nutritional Supplement in Mice. Nutrients. 2015; 7(7):5423-5442. https://doi.org/10.3390/nu7075229
Chicago/Turabian StyleIvanescu, Alina Adriana, Patricia Fernández-Robredo, Henar Heras-Mulero, Luis Manuel Sádaba-Echarri, Laura García-García, Vanessa Fernández-García, Maite Moreno-Orduna, Aitor Redondo-Exposito, Sergio Recalde, and Alfredo García-Layana. 2015. "Modifying Choroidal Neovascularization Development with a Nutritional Supplement in Mice" Nutrients 7, no. 7: 5423-5442. https://doi.org/10.3390/nu7075229




