Self-Reported Prevalence of Symptomatic Adverse Reactions to Gluten and Adherence to Gluten-Free Diet in an Adult Mexican Population
Abstract
:1. Introduction
2. Experimental Section
2.1. Questionnaire Design
2.2. Questionnaire Clarity/Comprehension and Wording of Questions Evaluation
2.3. Questionnaire Test-Retest Consistency
2.4. Population Survey

2.5. Statistical and Ethical Issues
3. Results
3.1. Questionnaire Evaluation
| Assessment | Age Mean (Range) | n (Female/Male) | Adverse Reactions | Score |
|---|---|---|---|---|
| Clarity/comprehension | 34.9 (20–66) | 22 (5/17) | Gluten | Clear and comprehensible (0) * |
| 29.5 (18–50) | 20 (12/8) | Other foods | Clear and comprehensible (0) | |
| Consistency | 33.2 (20–63) | 20 (12/8) | Gluten | 0.918 # (0.88–0.96) ## |
| 22.1 (20–43) | 24 (19/5) | Other foods | 0.767 (0.71–0.82) |
3.2. Population Survey
| Gluten-Related Disorder | Mean Age in Years (Range) | Number of Cases (Female/Male) | Self-Reported | Self-Reported Physician-Diagnosed | Gluten-Free Diet (Yes/No) |
|---|---|---|---|---|---|
| Celiac disease * | 45 (−) | 1 (1/0) | 0 | 1 | 1/0 |
| Wheat allergy | 30.5 (18–45) | 9 (6/3) | 9 # | 0 | 3/6 |
| NCGS ** | 37.2 (21–56) | 12 (9/3) | 2 | 10 | 2/10 |
| Sample Size * | Assessment | (+) Cases ** | Age # (Range) | Prevalence by Gender (95% CI) | p Value | General Prevalence (95% CI) |
|---|---|---|---|---|---|---|
| 1238 M ## = 559 F ## = 679 | Adverse reactions to foods | Total = 398 M = 141 F = 257 | 31.3 (18–85) | M 25.2 (21.8–29.0) F 37.8 (34.3–41.6) | <0.001 | 32.1 (29.6–34.8) |
| 1230 M = 554 F = 676 | Recurrent adverse reactions to foods | Total = 256 M = 81 F = 175 | 32.0 (18–84) | M 14.6 (11.9–17.8) F 22.7 (22.6–29.3) | <0.001 | 20.8 (18.6–23.2) |
| 1221 M = 555 F = 666 | Food allergy | Total = 67 M = 13 F = 54 | 27.4 (18–57) | M 2.3 (1.2–3.9) F 8.1 (6.3–10.4) | <0.001 | 5.5 (4.3–6.9) |
| 1221 M = 555 F = 666 | Dairy intolerance | Total = 43 M = 18 F = 25 | 30.8 (18–58) | M 3.2 (2.1–5.1) F 3.7 (2.6–5.5) | NS | 3.5 (2.6–4.7) |
| 1238 M = 559 F = 679 | Adverse reactions to wheat/gluten | Total = 144 M = 45 F = 99 | 33.5 (18–71) | M 8.0 (6.0–10.6) F14.6 (12.1–17.4) | 0.001 | 11.9 (9.9–13.5) |
| 1237 M = 559 F = 678 | Recurrent adverse reactions to wheat/gluten | Total = 96 M = 25 F = 71 | 34.2 (18–63) | M 4.5 (3.0–6.5) F 10.5 (8.4–13.0) | <0.001 | 7.8 (6.4–9.4) |
| 1228 M = 556 F = 672 | Adherence to gluten-free diet | Total = 45 M = 13 F = 32 | 32.0 (19–57) | M 2.3 (1.4–4.0) F 4.8 (3.4–6.6) | 0.033 | 3.7 (2.7–4.8) |
| Variable | Adverse Reactions to | Odds Ratio (95% CI) | |||
|---|---|---|---|---|---|
| Wheat/Gluten (N = 90) | Other Foods (N = 140) | ||||
| --- | n | --- | n | ||
| Mean age in years (range) # | 34.1 (18–63) | --- | 30.5 (18-84) | --- | --- |
| Gender (female/male) (%) | 73.3/26.7 | 66/24 | 63.6/36.4 | 89/51 | 1.57 (0.88–2.9) |
| Bloating (%) | 83.3 | 75 | 59.3 | 83 | 3.4 (1.8–6.6) |
| Abdominal pain (%) | 44.4 | 40 | 48.6 | 68 | 0.85 (0.49–1.4) |
| Abdominal discomfort (%) | 37.8 | 34 | 30.7 | 43 | 1.4 (0.78–2.4) |
| Constipation (%) | 34.3 | 31 | 10.0 | 14 | 4.7 (2.3–9.5) |
| Flatulence (%) | 30.0 | 27 | 24.3 | 34 | 1.3 (0.74–2.4) |
| Reflux (%) | 24.4 | 22 | 22.1 | 31 | 1.1 (0.61–2.1) |
| Acidity (%) | 22.2 | 20 | 23.6 | 33 | 0.93 (0.49–1.7) |
| Nausea (%) | 16.7 | 15 | 14.3 | 20 | 1.2 (0.58–2.5) |
| Diarrhea (%) | 10.0 | 9 | 19.3 | 27 | 0.46 (0.21–1.0) |
| Vomit (%) | 5.5 | 5 | 8.6 | 12 | 0.63 (0.21–1.8) |
| Dairy intolerance (%) | 17.7 | 16 | 14.8 | 21 | 1.4 (0.72–2.9) |
| IBS (%) | 12.5 | 12 | 8.6 | 12 | 1.5 (0.67–3.4) |
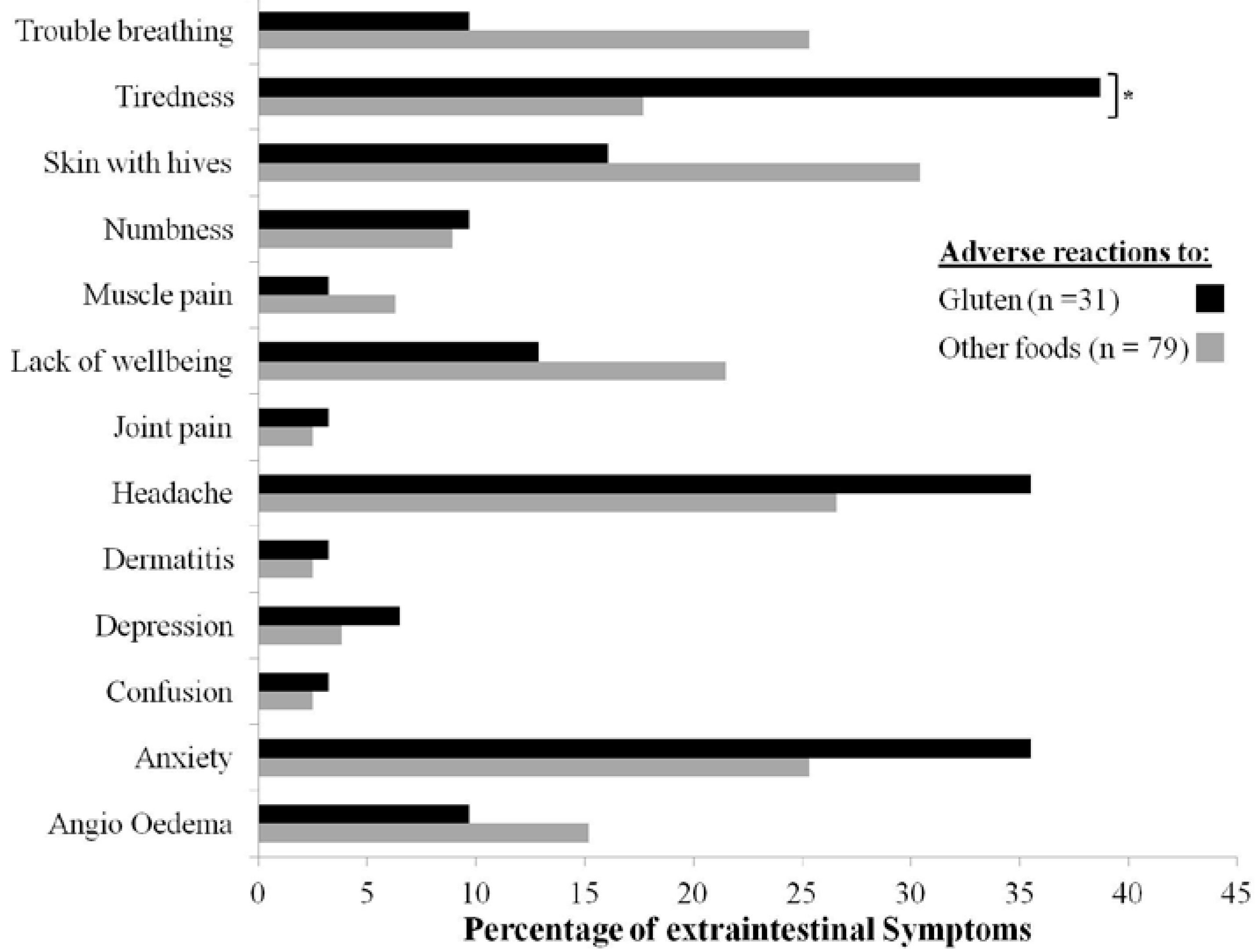
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Sapone, A.; Bai, J.C.; Ciacci, C.; Dolinsek, J.; Green, P.H.; Hadjivassiliou, M.; Kaukinen, K.; Rostami, K.; Sanders, D.S.; Schumann, M.; et al. Spectrum of gluten-related disorders: Consensus on new nomenclature and classification. BMC Med. 2012, 10, 13. [Google Scholar] [CrossRef] [PubMed]
- Ludvigsson, J.F.; Leffler, D.A.; Bai, J.C.; Biaqi, F.; Fasano, A.; Green, P.H.; Hadjivassiliou, M.; Kaukiken, K.; Kelly, C.P.; Leonard, J.N.; et al. The Oslo definitions for coeliac disease and related terms. Gut 2012, 62, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Sapone, A.; Lammers, K.M.; Casolaro, V.; Cammarota, M.; Giuliano, M.T.; de Rosa, M.; Stefanile, R.; Mazzarella, G.; Tolone, C.; Russo, M.I.; et al. Divergence of gut permeability and mucosal immune gene expression in two gluten-associated conditions: Celiac disease and gluten sensitivity. BMC Med. 2011, 9, 23. [Google Scholar] [CrossRef] [PubMed]
- Pietzak, M. Celiac disease, wheat allergy, and gluten sensivity: When gluten free is not a fad. JPEN J. Parenter. Enteral Nutr. 2012, 36, 68S–75S. [Google Scholar] [CrossRef] [PubMed]
- Biesiekierski, J.R.; Newnham, E.D.; Irving, P.M.; Barrett, J.S.; Haines, M.; Doecke, J.D.; Shepherd, S.J.; Muir, J.G.; Gibson, P.R. Gluten causes gastrointestinal symptoms in subjects without celiac disease: A double-blind randomized placebo-controlled trial. Am. J Gastroenterol. 2011, 106, 508–514. [Google Scholar] [CrossRef] [PubMed]
- Biesiekierski, J.R.; Peters, S.L.; Newnham, E.D.; Rosella, O.; Muir, J.G.; Gibson, P.R. No effects of gluten in patients with self-reported non-celiac gluten sensitivity after dietary reduction of fermentable, poorly absorbed, short-chain carbohydrates. Gastroenterology 2013, 145, 320–328. [Google Scholar] [CrossRef] [PubMed]
- Catassi, C.; Elli, L.; Bonaz, B.; Bouma, G.; Carroccio, A.; Castillejo, G.; Cellier, C.; Cristofori, F.; de Magistris, L.; Dolinsek, J.; et al. Diagnosis of Non-Celiac Gluten Sensitivity (NCGS): The Salerno Experts’ Criteria. Nutrients 2015, 7, 4966–4977. [Google Scholar] [CrossRef] [PubMed]
- Ontiveros, N.; Hardy, M.Y.; Cabrera-Chavez, F. Assessing of Celiac Disease and Nonceliac Gluten Sensivity. Gastroenterol. Res. Pract. 2015. [Google Scholar] [CrossRef]
- Rubio-Tapia, A.; Ludvingsson, J.F.; Brantner, T.L.; Murray, J.A.; Everhart, J.E. The prevalence of celiac disease in the United States. Am. J. Gastroenterol. 2012, 107, 1538–1544. [Google Scholar] [CrossRef] [PubMed]
- Tanpowpong, P.; Ingham, T.R.; Lampshire, P.K.; Kirchberg, F.F.; Epton, M.J.; Crane, J.; Camargo, C.A., Jr.; New Zealand Asthma and Allergy Cohort Study Group. Coeliac disease and gluten avoidance in New Zealand children. Arch. Dis. Child. 2012, 97, 2–6. [Google Scholar] [CrossRef] [PubMed]
- Hallert, C.; Svensson, M.; Tholstrup, J.; Hultberg, B. Clinical trial: B vitamins improve health in patients with coeliac disease living on a gluten-free diet. Aliment. Pharmacol. Ther. 2009, 29, 811–816. [Google Scholar] [CrossRef] [PubMed]
- Wild, D.; Robins, G.G.; Burly, V.J.; Howdle, P.D. Evidence of high sugar intake, and low fibre and mineral intake, in the gluten-free diet. Aliment. Pharmacol. Ther. 2010, 32, 573–581. [Google Scholar] [CrossRef] [PubMed]
- Aziz, I.; Lewis, N.R.; Hadjivassiliou, M.; Winfiled, S.N.; Rugg, N.; Kelsall, A.; Newrick, L.; Sanders, D.S. A UK study assessing the population prevalence of self-reported gluten sensivity and referral characteristics to secondary care. Eur. J. Gastroenterol. Hepatol. 2014, 26, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Volta, U.; Bardella, M.T.; Calabró, A.; Tranconne, R.; Corazza, G.R. An Italian prospective multicenter survey on patients suspected of having non-celiac-gluten sensitivity. BMC Med. 2014. [Google Scholar] [CrossRef] [PubMed]
- DiGiacomo, D.V.; Terryson, C.A.; Green, P.H.; Demmert, R.T. Prevalence of gluten-free diet adherence among individuals without celiac disease in the USA: Results from the Continuous National Health and Nutrition Examination Survey 2009–2010. Scand. J. Gastroenterol. 2013, 48, 921–925. [Google Scholar] [CrossRef] [PubMed]
- Carroccio, A.; Mansueto, P.; Iacono, G.; Soresi, M.; D’Alcamo, A.; Cavataio, F.; Brusca, I.; Florena, A.M.; Ambrosiano, G.; Seidita, A.; et al. Non-celiac wheat sensitivity diagnosed by double-blind placebo-controlled challenge: Exploring a new clinical entity. Am. J. Gastroenterol. 2012, 107, 1898–1906. [Google Scholar] [CrossRef] [PubMed]
- Peters, S.L.; Biesiekierski, J.R.; Yelland, G.W.; Muir, G.J.; Gibson, P.R. Randomised clinical trial: Gluten may cause depression in subjects with non-coeliac gluten sensitivity—An exploratory clinical study. Aliment. Pharmacol. Ther. 2014, 39, 1104–1112. [Google Scholar] [CrossRef] [PubMed]
- Ontiveros, N.; Tye-Din, J.A.; Hardy, M.Y.; Anderson, R.P. Ex-vivo whole blood secretion of interferon (IFN)-γ and IFN-γ-inducible protein-10 measured by enzyme-linked immunosorbent assay are as sensitive as IFN-γ enzyme-linked immunospot for the detection of gluten-reactive T cells in human leucocyte antigen (HLA)-DQ2–5(+) -associated coeliac disease. Clin. Exp. Immunol. 2014, 175, 305–315. [Google Scholar] [PubMed]
- Hoyos-Bachiloglu, R.; Ivanovic-Zuvic, D.; Alvarez, J.; Linn, K.; Thöne, K.; de los Ángeles, P.M.; Borzutzky, A. Prevalence of parent-reported immediate hypersensitivity food allergy in Chilean school-aged children. Allergol. Immnunopathol. 2014, 42, 527–532. [Google Scholar] [CrossRef] [PubMed]
- Sicherer, S.H.; Burks, A.W.; Sampson, H.A. Clinical features of acute allergic reactions to peanut and tree nuts in children. Pediatrics 1998, 102, e6. [Google Scholar] [CrossRef] [PubMed]
- Sicherer, S.H.; Muñoz-Furlong, A.; Godbold, J.H.; Sampson, H.A. US prevalence of selft-reported peanut, tree nut, and sesame allergy: 11-year follow-up. J. Allergy Clin. Immunol. 2010, 125, 1322–1326. [Google Scholar] [CrossRef] [PubMed]
- Gusi, N.; Badia, X.; Herdman, M.; Olivares, P. Translation and cultural adaptation of the Spanish version of EQ-5D-Y questionnaire for children and adolescents. Aten Primaria 2009, 41, 19–23. [Google Scholar] [CrossRef] [PubMed]
- Kazi, A.M.; Khalid, W. Questionnaire designing and validation. J. Pak. Med. Assoc. 2012, 62, 514–516. [Google Scholar] [PubMed]
- OpenEpi Open Source Epidemiologic Statistics for Public Health. Available online: http://www.openepi.com/ (accessed on 25 April 2015).
- Landis, J.R.; Koch, G.G. An application of hierarchical kappa-type statistics in the assessment of majority agreement among multiple observers. Biometrics 1977, 33, 363–374. [Google Scholar] [CrossRef] [PubMed]
- Beaton, D.E.; Bombardier, C.; Guillemin, F.; Ferraz, M.B. Guidelines for the process of cross-cultural adaptation of self-report measures. SPINE 2000, 25, 3186–3191. [Google Scholar] [CrossRef] [PubMed]
- Bedolla-Barajas, M.; Bedolla-Pulido, T.R.; Camacho-Peña, A.S.; González-García, E.; Morales-Romero, J. Food hypersensitivity in Mexican adults at 18 to 50 years of age: A questionnaire survey. Allergy Asthma Immunol. Res. 2014, 6, 511–516. [Google Scholar] [CrossRef] [PubMed]
- Johansson, S.G.; Hourihane, J.O.; Bousquet, J.; Bruijnzeel-Koomen, C.; Dreborg, S.; Haahtela, T.; Kowalski, M.L.; Mygind, N.; Ring, J.; van Cauwenberge, P.; et al. A revised nomenclature for allergy. An EAACI position statement from the EAACI nomenclature task force. Allergy 2011, 56, 813–824. [Google Scholar]
- Vierk, K.A.; Koehler, K.M.; Fein, S.B.; Street, D.A. Prevalence of self-reported food allergy in American adults and use of food labels. J. Allergy Clin. Immunol. 2007, 119, 1504–1510. [Google Scholar] [CrossRef] [PubMed]
- Zuidmeer, L.; Goldhahn, K.; Rona, R.J.; Gislason, D.; Madsen, C.; Summers, C.; Sodergren, E.; Dahistrom, J.; Lindner, T.; Siqurdardottir, S.T.; et al. The prevalence of plant food allergies: A systematic review. J. Allery Clin. Immunol. 2008, 121, 1210–1218. [Google Scholar] [CrossRef] [PubMed]
- Morita, E.; Chinuki, Y.; Takahashi, H.; Nabika, T.; Yamasaki, M.; Shiwaku, K. Prevalence of wheat allergy in Japanese adults. Allergol Int. 2012, 61, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Remes-Troche, J.M.; Nuñez-Alvarez, C.; Uscanga-Dominguez, L.F. Celiac Disease in Mexican population: An update. Am. J. Gastroenterol. 2013, 108, 283–284. [Google Scholar] [CrossRef] [PubMed]
- Balamtekin, N.; Uslu, N.; Baysoy, G.; Usta, Y.; Demir, H.; Saltik-Temize, I.N.; Ozen, H.; Gürakan, F.; Yüce, A. The presentation of celiac disease in 220 Turkish children. Turk. J. Pediatr. 2010, 52, 239–244. [Google Scholar] [PubMed]
- Admou, B.; Essaadouni, L.; Krati, K.; Zaher, K.; Sbihi, M.; Chabaa, L.; Belaabidia, B.; Alaoui-Yazidi, A. Atypical celiac disease: From recognizing to managing. Gastroenterol. Res. Pract. 2012. [Google Scholar] [CrossRef] [PubMed]
- Capannolo, A.; Viscido, A.; Barkad, M.A.; Valerii, G.; Ciccone, F.; Melideo, D.; Frieri, G.; Latella, G. Non-Celiac gluten sensitivity among patients perceiving gluten-related symptoms. Digestion 2015, 92, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Rubio-Tapia, A.; Hill, I.D.; Kelly, C.P.; Calderwood, A.H.; Murray, J.A. ACG clinical guidelines: Diagnosis and management of celiac disease. Am. J. Gastroenterol. 2013, 108, 656–676. [Google Scholar] [CrossRef] [PubMed]
- Rostom, A.; Murray, J.A.; Kagnoff, M.F. American Gastroenterological Association (AGA) Institute technical review on the diagnosis and management of celiac disease. Gastroenterology. 2006, 131, 1981–2002. [Google Scholar] [CrossRef] [PubMed]
- Rona, R.J.; Keil, T.; Summers, C.; Gislason, D.; Zuidmeer, L.; Sodergren, E.; Siqurdardottir, S.T.; Lindner, T.; Goldhahn, K.; Dahistrom, J.; et al. The prevalence of food allergy: A meta-analysis. Allergy Clin. Immunol. 2007, 120, 638–646. [Google Scholar] [CrossRef] [PubMed]
- Ontiveros, N.; Flores-Mendoza, L.K.; Canizalez-Román, V.A.; Cabrera-Chavez, F. Food allergy: Prevalence and food technology approaches for the Control of IgE-mediated food allergy. Austin J Nutr. Food Sci. 2014, 2, 1029. [Google Scholar]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ontiveros, N.; López-Gallardo, J.A.; Vergara-Jiménez, M.J.; Cabrera-Chávez, F. Self-Reported Prevalence of Symptomatic Adverse Reactions to Gluten and Adherence to Gluten-Free Diet in an Adult Mexican Population. Nutrients 2015, 7, 6000-6015. https://doi.org/10.3390/nu7075267
Ontiveros N, López-Gallardo JA, Vergara-Jiménez MJ, Cabrera-Chávez F. Self-Reported Prevalence of Symptomatic Adverse Reactions to Gluten and Adherence to Gluten-Free Diet in an Adult Mexican Population. Nutrients. 2015; 7(7):6000-6015. https://doi.org/10.3390/nu7075267
Chicago/Turabian StyleOntiveros, Noe, Jesús A. López-Gallardo, Marcela J. Vergara-Jiménez, and Francisco Cabrera-Chávez. 2015. "Self-Reported Prevalence of Symptomatic Adverse Reactions to Gluten and Adherence to Gluten-Free Diet in an Adult Mexican Population" Nutrients 7, no. 7: 6000-6015. https://doi.org/10.3390/nu7075267





