Menaquinone-7 Supplementation to Reduce Vascular Calcification in Patients with Coronary Artery Disease: Rationale and Study Protocol (VitaK-CAC Trial)
Abstract
:1. Introduction and Rationale
2. The Vitamin K—Coronary Artery Calcification (VitaK-CAC) Study
2.1. Trial Objectives
| Changes in plaque morphology of existing atherosclerotic lesions |
| Incidence of new calcified atherosclerotic lesions |
| Changes in arterial structure and function |
| Carotid-femoral pulse wave velocity (cfPWV) |
| Pulse-waveform and central aortic blood pressure (CABP) |
| Common carotid artery intima media thickness (cIMT) |
| Common carotid artery distensibility coefficient (DC) |
| Biochemical associations between CAC progression |
| Circulating matrix Gla protein (MGP) species with different phosphorylation and carboxylation forms |
| Osteocalcin (OC) |
| Lipid profile (Total cholesterol, Low-Density Lipoprotein-cholesterol (LDL), High-Density Lipoprotein-cholesterol (HDL) and Triglycerides) |
| Glucose status (Fasting glucose) |
| Calcium metabolism (Calcium, Albumine, Phosphate and Parathyroid Hormone) |
| Kidney function (Creatinine) |
| Prothrombin time-International normalized ratio (PT-INR) |
2.2. Patient Recruitment
2.3. Inclusion and Exclusion Criteria
| Baseline-scan of insufficient quality (due to the presence of motion artefacts, breathing artefacts or high noise-levels) |
| Heart rate greater than 70 beats per min during first scan because of impaired scan quality |
| Chronic or paroxysmal atrial fibrillation |
| Presence or scheduled bypass-grafting in more than one coronary artery |
| Presence or scheduled coronary revascularization procedure (stent-placement > 1 coronary artery) |
| History of myocardial infarction or stroke < 6 months before coronary Coronary Tomography (CT) |
| Presence of diabetes mellitus type 1 |
| Known kidney disease or an estimated Glomerular Filtration Rate (eGFR) < 60 mL/min/1.73 m2, calculated by the MDRD-formula |
| Malignant disease (exception: treated basal-cell or squamous cell carcinoma) |
| Use of Vitamin K antagonists |
| A life-expectancy < 2 years |
| Pregnancy or wish to become pregnant in the near future |
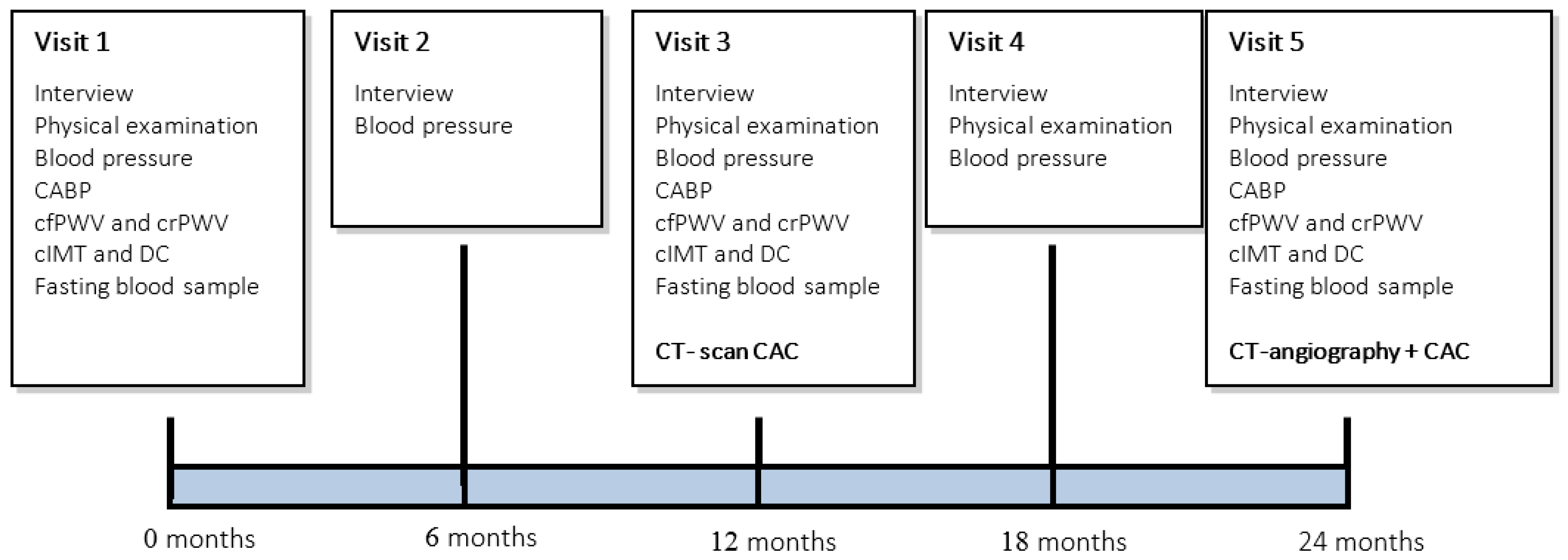
2.4. Measurements
2.4.1. Interviewing, Physical Examination and Blood-Pressure Measurement
2.4.2. Multi-Slice Computed Tomography
2.4.3. Measurement of the Central Aortic Blood Pressure (CABP)
2.4.4. Measurement of Pulse Wave Velocity (PWV)
2.4.5. Measurement of the Carotid Intima Media Thickness (cIMT)
2.4.6. Measurement of the Common Carotid Artery Distensibility Coefficient (DC)
2.4.7. Laboratory Assessment
| Routine Laboratory Variables | Specific Laboratory Variables |
|---|---|
| Total cholesterol | MGP OCN |
| LDL-cholesterol | |
| HDL-cholesterol | |
| Triglycerides | |
| Creatinine | |
| Glucose | |
| Albumin | |
| Calcium | |
| Phosphate | |
| Coagulation function (PT-INR) |
2.5. Treatment Schedule
2.6. Vitamin K Product
2.7. Randomization Procedure
2.8. Statistical Analysis
2.9. Sample Size
2.10. Organization
2.10.1. Data Safety Monitoring Board (DSMB)
2.10.2. Adverse and Serious Adverse Events (AE and SAE)
2.10.3. Suspected Unexpected Serious Adverse Reactions (SUSAR)
2.10.4. Premature Termination of the Study
2.10.5. Withdrawal of Individual Subjects
2.10.6. Participating Centers
3. Concluding Remarks
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Henein, M.Y.; Koulaouzidis, G.; Granåsen, G.; Wiklund, U.; Guerci, A.; Schmermund, A. The natural history of coronary calcification: A meta-analysis from St Francis and EBEAT trials. Int. J. Cardiol. 2013, 168, 3944–3948. [Google Scholar] [PubMed]
- Budoff, M.J.; Shaw, L.J.; Liu, S.T.; Weinstein, S.R.; Mosler, T.P.; Tseng, P.H.; Flores, F.R.; Callister, T.Q.; Raggi, P.; Berman, D.S. Long-term prognosis associated with coronary calcification: Observations from a registry of 25,253 patients. J. Am. Coll. Cardiol. 2007, 49, 1860–1870. [Google Scholar] [PubMed]
- Madhavan, M.V.; Tarigopula, M.; Mintz, G.S.; Maehara, A.; Stone, G.W.; Généreux, P. Coronary artery calcification: Pathogenesis and prognostic implications. J. Am. Coll. Cardiol. 2014, 63, 1703–1714. [Google Scholar] [PubMed]
- Luo, G.; Ducy, P.; McKee, M.D.; Pinero, G.J.; Loyer, E.; Behringer, R.R.; Karsenty, G. Spontaneous calcification of arteries and cartilage in mice lacking matrix GLA protein. Nature 1997, 386, 78–81. [Google Scholar] [PubMed]
- Schurgers, L.J.; Cranenburg, E.C.M.; Vermeer, C. Matrix Gla-protein: The calcification inhibitor in need of vitamin K. Thromb. Haemost. 2008, 100, 593–603. [Google Scholar] [PubMed]
- Schurgers, L.J.; Uitto, J.; Reutelingsperger, C.P. Vitamin K-dependent carboxylation of matrix Gla-protein: A crucial switch to control ectopic mineralization. Trends Mol. Med. 2013, 19, 217–226. [Google Scholar] [PubMed]
- Rennenberg, R.J.; Schurgers, L.J.; Kroon, A.A.; Stehouwer, C.D. Arterial calcifications. J. Cell Mol. Med. 2010, 14, 2203–2210. [Google Scholar] [PubMed]
- Price, P.A.; Faus, S.A.; Williamson, M.K. Warfarin causes rapid calcification of the elastic lamellae in rat arteries and heart valves. Arterioscler. Thromb. Vasc. Biol. 1998, 18, 1400–1407. [Google Scholar] [PubMed]
- Chatrou, M.L.L.; Winckers, K.; Hackeng, T.M.; Reutelingsperger, C.P.; Schurgers, L.J. Vascular calcification: The price to pay for anticoagulation therapy with vitamin K-antagonists. Blood Rev. 2012, 26, 155–166. [Google Scholar] [PubMed]
- Rennenberg, R.J.; van Varik, B.J.; Schurgers, L.J.; Hamulyak, K.; ten Cate, H.; Leiner, T.; Vermeer, C.; de Leeuw, P.W.; Kroon, A.A. Chronic coumarin treatment is associated with increased extracoronary arterial calcification in humans. Blood 2010, 115, 5121–5123. [Google Scholar] [PubMed]
- Weijs, B.; Blaauw, Y.; Rennenberg, R.J.M.W.; Schurgers, L.J.; Timmermans, C.C.M.M.; Pison, L.; Nieuwlaat, R.; Hofstra, L.; Kroon, A.A.; Wildberger, J.; et al. Patients using vitamin K antagonists show increased levels of coronary calcification: An observational study in low-risk atrial fibrillation patients. Eur. Heart J. 2011, 32, 2555–2562. [Google Scholar] [PubMed]
- Schurgers, L.J.; Teunissen, K.J.F.; Hamulyák, K.; Knapen, M.H.J.; Vik, H.; Vermeer, C. Vitamin K-containing dietary supplements: Comparison of synthetic vitamin K1 and natto-derived menaquinone-7. Blood 2007, 109, 3279–3283. [Google Scholar] [PubMed]
- Brandenburg, V.M.; Schurgers, L.J.; Kaesler, N.; Püsche, K.; van Gorp, R.H.; Leftheriotis, G.; Reinartz, S.; Koos, R.; Krüger, T. Prevention of vasculopathy by vitamin K supplementation: Can we turn fiction into fact? Atherosclerosis 2015, 240, 10–16. [Google Scholar] [PubMed]
- McCabe, K.M.; Booth, S.L.; Fu, X.; Shobeiri, N.; Pang, J.J.; Adams, M.A.; Holden, R.M. Dietary vitamin K and therapeutic warfarin alter the susceptibility to vascular calcification in experimental chronic kidney disease. Kidney Int. 2013, 83, 835–844. [Google Scholar] [CrossRef] [PubMed]
- Schurgers, L.J.; Spronk, H.M.H.; Soute, B.A.M.; Schiffers, P.M.; DeMey, J.G.R.; Vermeer, C. Regression of warfarin-induced medial elastocalcinosis by high intake of vitamin K in rats. Blood 2007, 109, 2823–2831. [Google Scholar] [CrossRef] [PubMed]
- Krüger, T.; Oelenberg, S.; Kaesler, N.; Schurgers, L.J.; van de Sandt, A.M.; Boor, P.; Schlieper, G.; Brandenburg, V.M.; Fekete, B.C.; Veulemans, V.; et al. Warfarin induces cardiovascular damage in mice. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 2618–2624. [Google Scholar] [CrossRef] [PubMed]
- Beulens, J.W.J.; Bots, M.L.; Atsma, F.; Bartelink, M.-L.E.L.; Prokop, M.; Geleijnse, J.M.; Witteman, J.C.M.; Grobbee, D.E.; van der Schouw, Y.T. High dietary menaquinone intake is associated with reduced coronary calcification. Atherosclerosis 2009, 203, 489–493. [Google Scholar] [PubMed]
- Geleijnse, J.M.; Vermeer, C.; Grobbee, D.E.; Schurgers, L.J.; Knapen, M.H.J.; van der Meer, I.M.; Hofman, A.; Witteman, J.C.M. Dietary intake of menaquinone is associated with a reduced risk of coronary heart disease: The Rotterdam study. J. Nutr. 2004, 134, 3100–3105. [Google Scholar] [PubMed]
- Shea, M.K.; O’Donnell, C.J.; Hoffmann, U.; Dallal, G.E.; Dawson-Hughes, B.; Ordovas, J.M.; Price, P.A.; Williamson, M.K.; Booth, S.L. Vitamin K supplementation and progression of coronary artery calcium in older men and women. Am. J. Clin. Nutr. 2009, 89, 1799–1807. [Google Scholar] [CrossRef] [PubMed]
- Braam, L.A.J.L.M.; Hoeks, A.P.G.; Brouns, F.; Hamulyák, K.; Gerichhausen, M.J.W.; Vermeer, C. Beneficial effects of vitamins D and K on the elastic properties of the vessel wall in postmenopausal women: A follow-up study. Thromb. Haemost. 2004, 91, 373–380. [Google Scholar] [CrossRef] [PubMed]
- Shea, M.K.; Holden, R.M. Vitamin K status and vascular calcification: Evidence from observational and clinical studies. Adv. Nutr. 2012, 3, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Dalmeijer, G.W.; van der Schouw, Y.T.; Magdeleyns, E.; Ahmed, N.; Vermeer, C.; Beulens, J.W.J. The effect of menaquinone-7 supplementation on circulating species of matrix Gla protein. Atherosclerosis 2012, 225, 397–402. [Google Scholar] [CrossRef] [PubMed]
- Knapen, M.H.J.; Braam, L.A.J.L.M.; Drummen, N.E.; Bekers, O.; Hoeks, A.P.G.; Vermeer, C. Menaquinone-7 supplementation improves arterial stiffness in healthy postmenopausal women. A double-blind randomised clinical trial. Thromb. Haemost. 2015, 113, 1135–1144. [Google Scholar] [CrossRef] [PubMed]
- Gast, G.C.M.; de Roos, N.M.; Sluijs, I.; Bots, M.L.; Beulens, J.W.J.; Geleijnse, J.M.; Witteman, J.C.; Grobbee, D.E.; Peeters, P.H.M.; van der Schouw, Y.T. A high menaquinone intake reduces the incidence of coronary heart disease. Nutr. Metab. Cardiovasc. Dis. 2009, 19, 504–510. [Google Scholar] [CrossRef] [PubMed]
- Schurgers, L.J.; Shearer, M.J.; Hamulyák, K.; Stöcklin, E.; Vermeer, C. Effect of vitamin K intake on the stability of oral anticoagulant treatment: Dose-response relationships in healthy subjects. Blood 2004, 104, 2682–2689. [Google Scholar] [CrossRef] [PubMed]
- Schurgers, L.J. Vitamin K: Key vitamin in controlling vascular calcification in chronic kidney disease. Kidney Int. 2013, 83, 782–784. [Google Scholar] [CrossRef] [PubMed]
- Agatston, A.S.; Janowitz, W.R.; Hildner, F.J.; Zusmer, N.R.; Viamonte, M.; Detrano, R. Quantification of coronary artery calcium using ultrafast computed tomography. J. Am. Coll. Cardiol. 1990, 15, 827–832. [Google Scholar] [CrossRef]
- Austen, W.G.; Edwards, J.E.; Frye, R.L.; Gensini, G.G.; Gott, V.L.; Griffith, L.S.; McGoon, D.C.; Murphy, M.L.; Roe, B.B. A reporting system on patients evaluated for coronary artery disease. Report of the Ad Hoc Committee for Grading of Coronary Artery Disease, Council on Cardiovascular Surgery, American Heart Association. Circulation 1975, 51, 5–40. [Google Scholar] [CrossRef] [PubMed]
- Versteylen, M.O.; Kietselaer, B.L.; Dagnelie, P.C.; Joosen, I.A.; Dedic, A.; Raaijmakers, R.H.; Wildberger, J.E.; Nieman, K.; Crijns, H.J.; Niessen, W.J.; et al. Additive value of semiautomated quantification of coronary artery disease using cardiac computed tomographic angiography to predict future acute coronary syndrome. J. Am. Coll. Cardiol. 2013, 61, 2296–2305. [Google Scholar] [CrossRef] [PubMed]
- Van Bortel, L.M.; Laurent, S.; Boutouyrie, P.; Chowienczyk, P.; Cruickshank, J.K.; de Backer, T.; Filipovsky, J.; Huybrechts, S.; Mattace-Raso, F.U.S.; Protogerou, A.D.; et al. Expert consensus document on the measurement of aortic stiffness in daily practice using carotid-femoral pulse wave velocity. J. Hypertens. 2012, 30, 445–448. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Willekes, C.; Brands, P.J.; Willigers, J.M.; Hoeks, A.P.G.; Reneman, R.S. Assessment of local differences in intima-media thickness in the human common carotid artery. J. Vasc. Res. 1999, 36, 222–228. [Google Scholar] [CrossRef] [PubMed]
- Westenfeld, R.; Krueger, T.; Schlieper, G.; Cranenburg, E.C.M.; Magdeleyns, E.J.; Heidenreich, S.; Holzmann, S.; Vermeer, C.; Jahnen-Dechent, W.; Ketteler, M.; et al. Effect of vitamin K2 supplementation on functional vitamin K deficiency in hemodialysis patients: A randomized trial. Am. J. Kidney Dis. 2012, 59, 186–195. [Google Scholar] [CrossRef] [PubMed]
- Theuwissen, E.; Cranenburg, E.C.; Knapen, M.H.; Magdeleyns, E.J.; Teunissen, K.J.; Schurgers, L.J.; Smit, E.; Vermeer, C. Low-dose menaquinone-7 supplementation improved extra-hepatic vitamin K status, but had no effect on thrombin generation in healthy subjects. Br. J. Nutr. 2012, 108, 1652–1657. [Google Scholar] [CrossRef] [PubMed]
- Scott, N.W.; McPherson, G.C.; Ramsay, C.R.; Campbell, M.K. The method of minimization for allocation to clinical trials: A review. Control. Clin. Trials 2002, 23, 662–674. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vossen, L.M.; Schurgers, L.J.; Van Varik, B.J.; Kietselaer, B.L.J.H.; Vermeer, C.; Meeder, J.G.; Rahel, B.M.; Van Cauteren, Y.J.M.; Hoffland, G.A.; Rennenberg, R.J.M.W.; et al. Menaquinone-7 Supplementation to Reduce Vascular Calcification in Patients with Coronary Artery Disease: Rationale and Study Protocol (VitaK-CAC Trial). Nutrients 2015, 7, 8905-8915. https://doi.org/10.3390/nu7115443
Vossen LM, Schurgers LJ, Van Varik BJ, Kietselaer BLJH, Vermeer C, Meeder JG, Rahel BM, Van Cauteren YJM, Hoffland GA, Rennenberg RJMW, et al. Menaquinone-7 Supplementation to Reduce Vascular Calcification in Patients with Coronary Artery Disease: Rationale and Study Protocol (VitaK-CAC Trial). Nutrients. 2015; 7(11):8905-8915. https://doi.org/10.3390/nu7115443
Chicago/Turabian StyleVossen, Liv M., Leon J. Schurgers, Bernard J. Van Varik, Bas L. J. H. Kietselaer, Cees Vermeer, Johannes G. Meeder, Braim M. Rahel, Yvonne J. M. Van Cauteren, Ge A. Hoffland, Roger J. M. W. Rennenberg, and et al. 2015. "Menaquinone-7 Supplementation to Reduce Vascular Calcification in Patients with Coronary Artery Disease: Rationale and Study Protocol (VitaK-CAC Trial)" Nutrients 7, no. 11: 8905-8915. https://doi.org/10.3390/nu7115443






