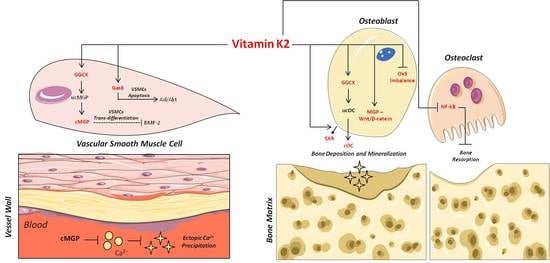
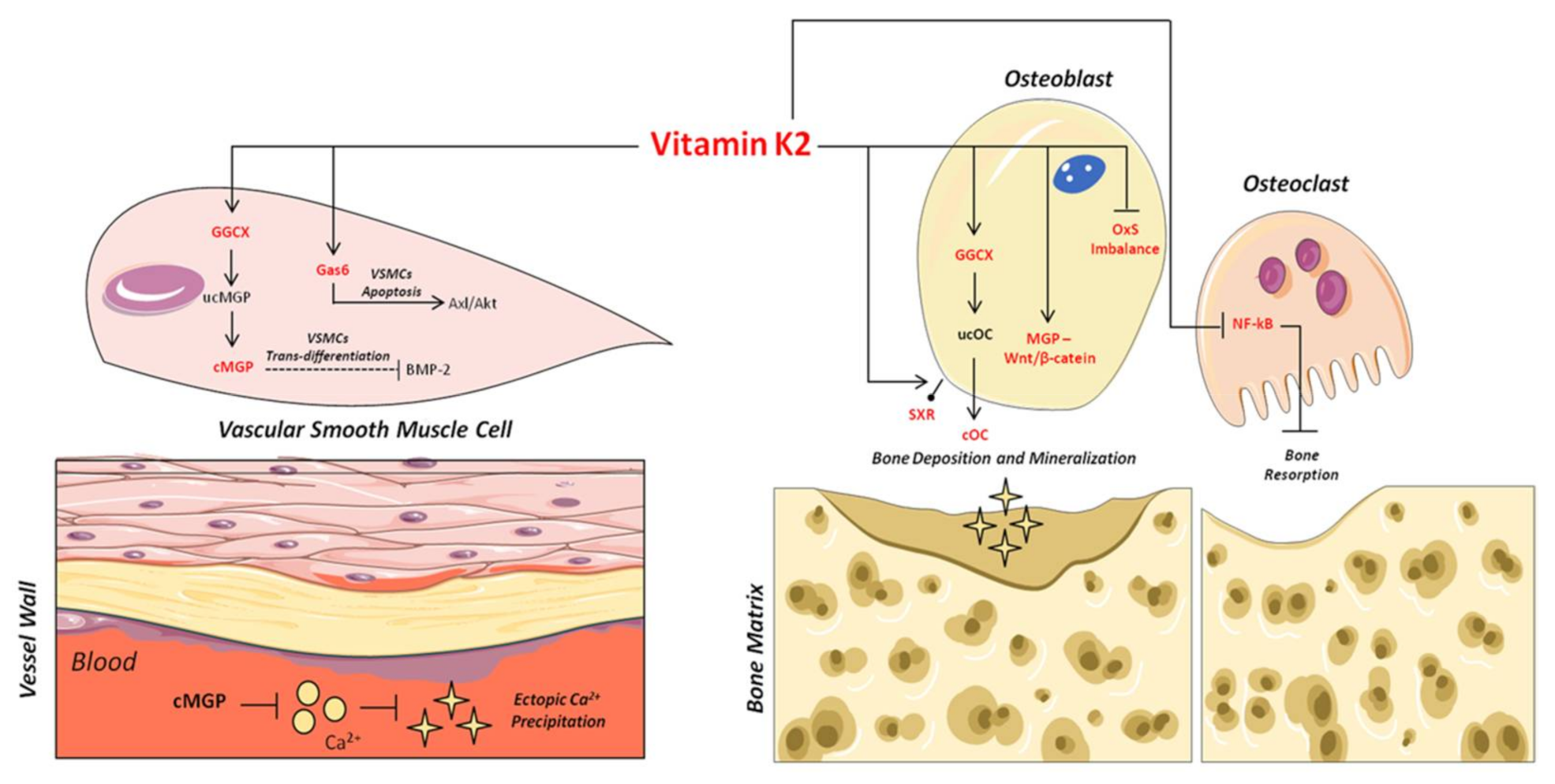
The Dual Role of Vitamin K2 in “Bone-Vascular Crosstalk”: Opposite Effects on Bone Loss and Vascular Calcification
Abstract
:1. Introduction
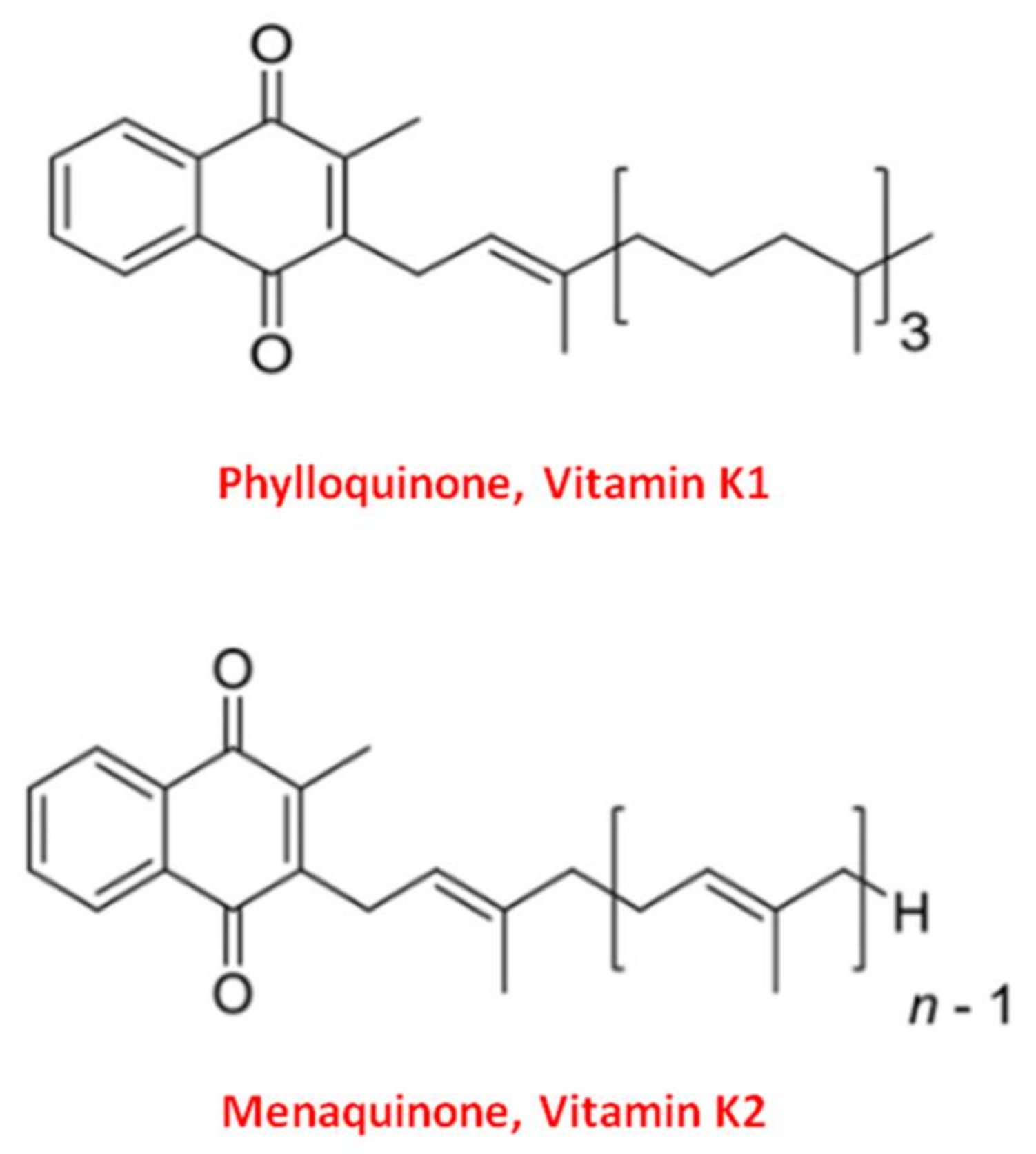
2. Vitamin K, a Family of Essential Fat-Soluble Compounds
3. Vitamin K2 and Its Biomolecular Mechanisms of Action
4. Vitamin K2 and Bone Health
5. Vitamin K2 and Vascular Health
| Study | Type of the Study | Number of Patients Enrolled | Key Findings |
|---|---|---|---|
| [120] | Meta-analysis of Prospect-EPIC cohort study | Healthy 16,057 women (49–70 years) | Menaquinone’s intake reduces the incidence of coronary heart disease |
| [121] | RCT | 564 post-menopausal women | Menaquinone’s intake decreased coronary calcification |
| [122] | Prospective cohort | 35,476 healthy subjects | Menaquinone’s dietary intake was not associated with reduced stroke risk |
| [106] | RCT | 244 post-menopausal women | Vitamin K2 (MK-7; 180 μg/day) supplementation improves arterial stiffness |
| [123] | Prospective cohort study | 7216 participants (Mediterranean population at high cardiovascular disease risk) | Vitamin K2 dietary intake was associated with a reduced risk of cardiovascular events and mortality |
| [124] | RCT | Patients with coronary artery disease (number not specified) | MK-7 (360 μg/day) supplementation arrested coronary artery calcification progression |
| [125] | Prospective cohort study | 36,629 participants with PAD | Vitamin K2 intake was associated with a reduced risk of PAD |
| [126] | Prospective cohort study | 2987 (Norwegian men and women) | Vitamin K2 intake was associated with a reduced risk of coronary artery disease |
| [127] | Prospective cohort study | 33,289 participants from the EPIC-NL cohort | Higher intake Menaquinones was borderline significantly associated with lower CVD mortality |
| [128] | RCT | 68 Type II diabetes and CVD patients | MK-7 (360 μg/day) was not associated with arterial calcification |
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Eriksen, E.F.; Díez-Pérez, A.; Boonen, S. Update on long-term treatment with bisphosphonates for postmenopausal osteoporosis: A systematic review. Bone 2014, 58, 126–135. [Google Scholar] [CrossRef] [PubMed]
- Hendrickx, G.; Boudin, E.; Van Hul, W. A look behind the scenes: The risk and pathogenesis of primary osteoporosis. Nat. Rev. Rheumatol. 2015, 11, 462–474. [Google Scholar] [CrossRef] [PubMed]
- Rochette, L.; Meloux, A.; Rigal, E.; Zeller, M.; Cottin, Y.; Vergely, C. The role of osteoprotegerin in the crosstalk between vessels and bone: Its potential utility as a marker of cardiometabolic diseases. Pharmacol. Ther. 2018, 182, 115–132. [Google Scholar] [CrossRef] [PubMed]
- Nagy, E.E.; Nagy-Finna, C.; Popoviciu, H.-V.; Kovács, B. Soluble Biomarkers of Osteoporosis and Osteoarthritis, from Pathway Mapping to Clinical Trials: An Update. Clin. Interv. Aging 2020, 15, 501–518. [Google Scholar] [CrossRef] [Green Version]
- Speer, M.Y.; Yang, H.-Y.; Brabb, T.; Leaf, E.; Look, A.; Lin, W.-L.; Frutkin, A.; Dichek, D.; Giachelli, C.M. Smooth Muscle Cells Give Rise to Osteochondrogenic Precursors and Chondrocytes in Calcifying Arteries. Circ. Res. 2009, 104, 733–741. [Google Scholar] [CrossRef]
- Lampropoulos, C.E.; Papaioannou, I.; D’Cruz, D.P. Osteoporosis—A risk factor for cardiovascular disease? Nat. Rev. Rheumatol. 2012, 8, 587–598. [Google Scholar] [CrossRef]
- Karwowski, W.; Naumnik, B.; Szczepański, M.; Myśliwiec, M. The mechanism of vascular calcification—A systematic review. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2012, 18, RA1–RA11. [Google Scholar] [CrossRef] [Green Version]
- Wu, M.; Rementer, C.; Giachelli, C.M. Vascular Calcification: An Update on Mechanisms and Challenges in Treatment. Calcif. Tissue Int. 2013, 93, 365–373. [Google Scholar] [CrossRef]
- Boström, K.I.; Jumabay, M.; Matveyenko, A.; Nicholas, S.B.; Yao, Y. Activation of Vascular Bone Morphogenetic Protein Signaling in Diabetes Mellitus. Circ. Res. 2011, 108, 446–457. [Google Scholar] [CrossRef] [Green Version]
- Francesco, V.; Vasuri, F.; Fittipaldi, S.; Pasquinelli, G. Arterial calcification: Finger-pointing at resident and circulating stem cells. World J. Stem Cells 2014, 6, 540–551. [Google Scholar] [CrossRef]
- Shanahan, C.M.; Cary, N.R. Medial localization of mineralization-regulating proteins in association with Monckeberg’s sclerosis: Evidence for smooth muscle cell-mediated vascular calcification. Circulation 1999, 100, 2168–2176. [Google Scholar] [CrossRef] [Green Version]
- Moe, S.M.; Chen, N.X. A rat model of chronic kidney disease-mineral bone disorder. Kidney Int. 2009, 75, 176–184. [Google Scholar] [CrossRef] [Green Version]
- Tyson, K.L.; Reynolds, J.L. Osteo/chondrocytic transcription factors and their target genes exhibit distinct patterns of expression in human arterial calcification. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 489–494. [Google Scholar] [CrossRef] [Green Version]
- Bobryshev, Y.V. Transdifferentiation of smooth muscle cells into chondrocytes in atherosclerotic arteries in situ: Implications for diffuse intimal calcification. J. Pathol. 2005, 205, 641–650. [Google Scholar] [CrossRef]
- Bostrom, K.I.; Rajamannan, N.M. The regulation of valvular and vascular sclerosis by osteogenic morphogens. Circ. Res. 2011, 109, 564–577. [Google Scholar] [CrossRef]
- Leopold, J.A. Vascular calcification: Mechanisms of vascular smooth muscle cell calcification. Trends Cardiovasc. Med. 2015, 25, 267–274. [Google Scholar] [CrossRef] [Green Version]
- McFarlane, S.I.; Muniyappa, R. Osteoporosis and cardiovascular disease: Brittle bones and boned arteries, is there a link? Endocrine 2004, 23, 1–10. [Google Scholar] [CrossRef]
- Sprini, D.; Rini, G.B. Correlation between osteoporosis and cardiovascular disease. Clin. Cases Miner. Bone Metab. 2014, 11, 117–119. [Google Scholar] [CrossRef]
- Vassalle, C.; Mazzone, A. Bone loss and vascular calcification: A bi-directional interplay? Vascul. Pharmacol. 2016, 86, 77–86. [Google Scholar] [CrossRef]
- Chen, Y.; Zhao, X. Arterial Stiffness: A Focus on Vascular Calcification and Its Link to Bone Mineralization. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 1078–1093. [Google Scholar] [CrossRef]
- Kiel, D.P.; Kauppila, L.I. Bone loss and the progression of abdominal aortic calcification over a 25 year period: The Framingham Heart Study. Calcif. Tissue Int. 2001, 68, 271–276. [Google Scholar] [CrossRef]
- Farhat, G.N.; Strotmeyer, E.S. Volumetric and areal bone mineral density measures are associated with cardiovascular disease in older men and women: The health, aging, and body composition study. Calcif. Tissue Int. 2006, 79, 102–111. [Google Scholar] [CrossRef]
- Hyder, J.A.; Allison, M.A. Association of coronary artery and aortic calcium with lumbar bone density: The MESA Abdominal Aortic Calcium Study. Am. J. Epidemiol. 2009, 169, 186–194. [Google Scholar] [CrossRef] [Green Version]
- Campos-Obando, N.; Kavousi, M. Bone health and coronary artery calcification: The Rotterdam Study. Atherosclerosis 2015, 241, 278–283. [Google Scholar] [CrossRef] [Green Version]
- Trivedi, D.P.; Khaw, K.T. Bone mineral density at the hip predicts mortality in elderly men. Osteoporos. Int. 2001, 12, 259–265. [Google Scholar] [CrossRef]
- Farhat, G.N.; Newman, A.B. The association of bone mineral density measures with incident cardiovascular disease in older adults. Osteoporos. Int. 2007, 18, 999–1008. [Google Scholar] [CrossRef] [Green Version]
- Choi, S.H.; An, J.H. Lower bone mineral density is associated with higher coronary calcification and coronary plaque burdens by multidetector row coronary computed tomography in pre- and postmenopausal women. Clin. Endocrinol. 2009, 71, 644–651. [Google Scholar] [CrossRef]
- Schulz, E.; Arfai, K. Aortic calcification and the risk of osteoporosis and fractures. J. Clin. Endocrinol. Metab. 2004, 89, 4246–4253. [Google Scholar] [CrossRef]
- Bagger, Y.Z.; Tanko, L.B. Radiographic measure of aorta calcification is a site-specific predictor of bone loss and fracture risk at the hip. J. Intern. Med. 2006, 259, 598–605. [Google Scholar] [CrossRef]
- Khalil, Z.; Alam, B. The Medical Benefits of Vitamin K2 on Calcium-Related Disorders. Nutrients 2021, 13, 691. [Google Scholar] [CrossRef] [PubMed]
- Persy, V.; de Broe, M. Bisphosphonates prevent experimental vascular calcification: Treat the bone to cure the vessels? Kidney Int. 2006, 70, 1537–1538. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shearer, M.J.; Newman, P. Metabolism and cell biology of vitamin K. Thromb. Haemost. 2008, 100, 530–547. [Google Scholar] [PubMed]
- Flore, R.; Ponziani, F.R. Something more to say about calcium homeostasis: The role of vitamin K2 in vascular calcification and osteoporosis. Eur. Rev. Med. Pharmacol. Sci. 2013, 17, 2433–2440. [Google Scholar]
- Dam, H.; Schonheyder, F. The occurrence and chemical nature of vitamin K. Biochem. J. 1936, 30, 897–901. [Google Scholar] [CrossRef] [Green Version]
- Dam, H. The formation of coprosterol in the intestine: The action of intestinal bacteria on cholesterol. Biochem. J. 1934, 28, 820–825. [Google Scholar] [CrossRef]
- Dam, H. The antihaemorrhagic vitamin of the chick. Biochem. J. 1935, 29, 1273–1285. [Google Scholar] [CrossRef] [Green Version]
- Willems, B.A.; Vermeer, C. The realm of vitamin K dependent proteins: Shifting from coagulation toward calcification. Mol. Nutr. Food Res. 2014, 58, 1620–1635. [Google Scholar] [CrossRef]
- Vermeer, C. Vitamin K: The effect on health beyond coagulation—An overview. Food Nutr. Res. 2012, 56. [Google Scholar] [CrossRef]
- Tie, J.K.; Stafford, D.W. Structural and functional insights into enzymes of the vitamin K cycle. J. Thromb. Haemost. 2016, 14, 236–247. [Google Scholar] [CrossRef] [Green Version]
- Hamidi, M.S.; Cheung, A.M. Vitamin K and musculoskeletal health in postmenopausal women. Mol. Nutr. Food Res. 2014, 58, 1647–1657. [Google Scholar] [CrossRef]
- Schurgers, L.J. Vitamin K: Key vitamin in controlling vascular calcification in chronic kidney disease. Kidney Int. 2013, 83, 782–784. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simes, D.C.; Viegas, C.S.B. Vitamin K as a Powerful Micronutrient in Aging and Age-Related Diseases: Pros and Cons from Clinical Studies. Int. J. Mol. Sci. 2019, 20, 4150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grober, U.; Reichrath, J. Vitamin K: An old vitamin in a new perspective. Dermatoendocrinology 2014, 6, e968490. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palermo, A.; Tuccinardi, D. Vitamin K and osteoporosis: Myth or reality? Metabolism 2017, 70, 57–71. [Google Scholar] [CrossRef] [Green Version]
- Sato, T.; Inaba, N. MK-7 and Its Effects on Bone Quality and Strength. Nutrients 2020, 12, 965. [Google Scholar] [CrossRef] [Green Version]
- Conly, J.M.; Stein, K. Quantitative and qualitative measurements of K vitamins in human intestinal contents. Am. J. Gastroenterol. 1992, 87, 311–316. [Google Scholar]
- Morishita, T.; Tamura, N. Production of menaquinones by lactic acid bacteria. J. Dairy Sci. 1999, 82, 1897–1903. [Google Scholar] [CrossRef]
- Suttie, J.W. The importance of menaquinones in human nutrition. Annu. Rev. Nutr. 1995, 15, 399–417. [Google Scholar] [CrossRef]
- Walther, B.; Karl, J.P. Menaquinones, bacteria, and the food supply: The relevance of dairy and fermented food products to vitamin K requirements. Adv. Nutr. 2013, 4, 463–473. [Google Scholar] [CrossRef] [Green Version]
- Iwamoto, J. Vitamin K(2) therapy for postmenopausal osteoporosis. Nutrients 2014, 6, 1971–1980. [Google Scholar] [CrossRef] [Green Version]
- Gijsbers, B.L.; Jie, K.S. Effect of food composition on vitamin K absorption in human volunteers. Br. J. Nutr. 1996, 76, 223–229. [Google Scholar] [CrossRef] [Green Version]
- Young, M.F. Bone matrix proteins: Their function, regulation, and relationship to osteoporosis. Osteoporos. Int. 2003, 14 (Suppl. S3), S35–S42. [Google Scholar] [CrossRef]
- Gorski, J.P. Biomineralization of bone: A fresh view of the roles of non-collagenous proteins. Front. Biosci. 2011, 16, 2598–2621. [Google Scholar] [CrossRef] [Green Version]
- Gundberg, C.M.; Lian, J.B. Vitamin K-dependent carboxylation of osteocalcin: Friend or foe? Adv. Nutr. 2012, 3, 149–157. [Google Scholar] [CrossRef] [Green Version]
- Booth, S.L.; Centi, A. The role of osteocalcin in human glucose metabolism: Marker or mediator? Nat. Rev. Endocrinol. 2013, 9, 43–55. [Google Scholar] [CrossRef] [Green Version]
- Ferron, M.; Wei, J. Insulin signaling in osteoblasts integrates bone remodeling and energy metabolism. Cell 2010, 142, 296–308. [Google Scholar] [CrossRef] [Green Version]
- Zoch, M.L.; Clemens, T.L. New insights into the biology of osteocalcin. Bone 2016, 82, 42–49. [Google Scholar] [CrossRef] [Green Version]
- Lacombe, J.; Karsenty, G. In vivo analysis of the contribution of bone resorption to the control of glucose metabolism in mice. Mol. Metab. 2013, 2, 498–504. [Google Scholar] [CrossRef]
- Kalaiselvi, V.S.; Prabhu, K. The association of serum osteocalcin with the bone mineral density in post menopausal women. J. Clin. Diagn. Res. 2013, 7, 814–816. [Google Scholar]
- Eastell, R.; Pigott, T. Diagnosis of endocrine disease: Bone turnover markers: Are they clinically useful? Eur. J. Endocrinol. 2018, 178, R19–R31. [Google Scholar] [CrossRef] [Green Version]
- Lee, N.K.; Sowa, H. Endocrine regulation of energy metabolism by the skeleton. Cell 2007, 130, 456–469. [Google Scholar] [CrossRef] [Green Version]
- Karsenty, G.; Oury, F. Regulation of male fertility by the bone-derived hormone osteocalcin. Mol. Cell. Endocrinol. 2014, 382, 521–526. [Google Scholar] [CrossRef] [Green Version]
- Kanazawa, I. Osteocalcin as a hormone regulating glucose metabolism. World J. Diabetes 2015, 6, 1345–1354. [Google Scholar] [CrossRef]
- Ducy, P. The role of osteocalcin in the endocrine cross-talk between bone remodelling and energy metabolism. Diabetologia 2011, 54, 1291–1297. [Google Scholar] [CrossRef] [Green Version]
- Yan, M.K.; Khalil, H. Vitamin supplements in type 2 diabetes mellitus management: A review. Diabetes Metab. Syndr. 2017, 11 (Suppl. S2), S589–S595. [Google Scholar] [CrossRef]
- Schurgers, L.J.; Cranenburg, E.C. Matrix Gla-protein: The calcification inhibitor in need of vitamin K. Thromb. Haemost. 2008, 100, 593–603. [Google Scholar]
- Schurgers, L.J.; Uitto, J. Vitamin K-dependent carboxylation of matrix Gla-protein: A crucial switch to control ectopic mineralization. Trends Mol. Med. 2013, 19, 217–226. [Google Scholar] [CrossRef]
- Price, P.A.; Rice, J.S. Conserved phosphorylation of serines in the Ser-X-Glu/Ser(P) sequences of the vitamin K-dependent matrix Gla protein from shark, lamb, rat, cow, and human. Protein Sci. 1994, 3, 822–830. [Google Scholar] [CrossRef] [Green Version]
- Wajih, N.; Borras, T. Processing and transport of matrix γ-carboxyglutamic acid protein and bone morphogenetic protein-2 in cultured human vascular smooth muscle cells: Evidence for an uptake mechanism for serum fetuin. J. Biol. Chem. 2004, 279, 43052–43060. [Google Scholar] [CrossRef] [Green Version]
- Chatrou, M.L.; Winckers, K. Vascular calcification: The price to pay for anticoagulation therapy with vitamin K-antagonists. Blood Rev. 2012, 26, 155–166. [Google Scholar] [CrossRef]
- Scheiber, D.; Veulemans, V. High-Dose Menaquinone-7 Supplementation Reduces Cardiovascular Calcification in a Murine Model of Extraosseous Calcification. Nutrients 2015, 7, 6991–7011. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, G.; Ducy, P. Spontaneous calcification of arteries and cartilage in mice lacking matrix GLA protein. Nature 1997, 386, 78–81. [Google Scholar] [CrossRef] [PubMed]
- Munroe, P.B.; Olgunturk, R.O. Mutations in the gene encoding the human matrix Gla protein cause Keutel syndrome. Nat. Genet. 1999, 21, 142–144. [Google Scholar] [CrossRef] [PubMed]
- Hur, D.J.; Raymond, G.V. A novel MGP mutation in a consanguineous family: Review of the clinical and molecular characteristics of Keutel syndrome. Am. J. Med. Genet. A 2005, 135, 36–40. [Google Scholar] [CrossRef] [PubMed]
- O’Young, J.; Liao, Y. Matrix Gla protein inhibits ectopic calcification by a direct interaction with hydroxyapatite crystals. J. Am. Chem. Soc. 2011, 133, 18406–18412. [Google Scholar] [CrossRef]
- Steitz, S.A.; Speer, M.Y. Smooth muscle cell phenotypic transition associated with calcification: Upregulation of Cbfa1 and downregulation of smooth muscle lineage markers. Circ. Res. 2001, 89, 1147–1154. [Google Scholar] [CrossRef]
- Engelse, M.A.; Neele, J.M. Vascular calcification: Expression patterns of the osteoblast-specific gene core binding factor alpha-1 and the protective factor matrix gla protein in human atherogenesis. Cardiovasc. Res. 2001, 52, 281–289. [Google Scholar] [CrossRef] [Green Version]
- Shioi, A.; Taniwaki, H. Monckeberg’s medial sclerosis and inorganic phosphate in uremia. Am. J. Kidney Dis. 2001, 38, S47–S49. [Google Scholar] [CrossRef]
- Wallin, R.; Cain, D. Modulation of the binding of matrix Gla protein (MGP) to bone morphogenetic protein-2 (BMP-2). Thromb. Haemost. 2000, 84, 1039–1044. [Google Scholar]
- Bostrom, K.; Tsao, D. Matrix GLA protein modulates differentiation induced by bone morphogenetic protein-2 in C3H10T1/2 cells. J. Biol. Chem. 2001, 276, 14044–14052. [Google Scholar] [CrossRef] [Green Version]
- Zebboudj, A.F.; Shin, V. Matrix GLA protein and BMP-2 regulate osteoinduction in calcifying vascular cells. J. Cell. Biochem. 2003, 90, 756–765. [Google Scholar] [CrossRef]
- Puzantian, H.; Akers, S.R. Circulating Dephospho-Uncarboxylated Matrix Gla-Protein Is Associated with Kidney Dysfunction and Arterial Stiffness. Am. J. Hypertens. 2018, 31, 988–994. [Google Scholar] [CrossRef]
- Emaus, N.; Gjesdal, C.G. Vitamin K2 supplementation does not influence bone loss in early menopausal women: A randomised double-blind placebo-controlled trial. Osteoporos. Int. 2010, 21, 1731–1740. [Google Scholar] [CrossRef]
- Chan, R.; Leung, J. No association between dietary vitamin K intake and fracture risk in chinese community-dwelling older men and women: A prospective study. Calcif. Tissue Int. 2012, 90, 396–403. [Google Scholar] [CrossRef]
- Kanellakis, S.; Moschonis, G. Changes in parameters of bone metabolism in postmenopausal women following a 12-month intervention period using dairy products enriched with calcium, vitamin D, and phylloquinone (vitamin K(1)) or menaquinone-7 (vitamin K (2)): The Postmenopausal Health Study II. Calcif. Tissue Int. 2012, 90, 251–262. [Google Scholar]
- Knapen, M.H.; Drummen, N.E. Three-year low-dose menaquinone-7 supplementation helps decrease bone loss in healthy postmenopausal women. Osteoporos. Int. 2013, 24, 2499–2507. [Google Scholar] [CrossRef]
- Huang, Z.B.; Wan, S.L. Does vitamin K2 play a role in the prevention and treatment of osteoporosis for postmenopausal women: A meta-analysis of randomized controlled trials. Osteoporos. Int. 2015, 26, 1175–1186. [Google Scholar] [CrossRef]
- Mott, A.; Bradley, T. Effect of vitamin K on bone mineral density and fractures in adults: An updated systematic review and meta-analysis of randomised controlled trials. Osteoporos. Int. 2019, 30, 1543–1559. [Google Scholar] [CrossRef]
- van Summeren, M.J.; Braam, L.A. The effect of menaquinone-7 (vitamin K2) supplementation on osteocalcin carboxylation in healthy prepubertal children. Br. J. Nutr. 2009, 102, 1171–1178. [Google Scholar] [CrossRef] [Green Version]
- Nakamura, E.; Aoki, M. Low-dose menaquinone-4 improves γ-carboxylation of osteocalcin in young males: A non-placebo-controlled dose-response study. Nutr. J. 2014, 13, 85. [Google Scholar] [CrossRef] [Green Version]
- Koitaya, N.; Sekiguchi, M. Low-dose vitamin K2 (MK-4) supplementation for 12 months improves bone metabolism and prevents forearm bone loss in postmenopausal Japanese women. J. Bone Miner. Metab. 2014, 32, 142–150. [Google Scholar] [CrossRef]
- Inaba, N.; Sato, T. Low-Dose Daily Intake of Vitamin K(2) (Menaquinone-7) Improves Osteocalcin γ-carboxylation: A Double-Blind, Randomized Controlled Trials. J. Nutr. Sci. Vitaminol. 2015, 61, 471–480. [Google Scholar] [CrossRef] [Green Version]
- Atkins, G.J.; Welldon, K.J. Vitamin K promotes mineralization, osteoblast-to-osteocyte transition, and an anticatabolic phenotype by γ-carboxylation-dependent and -independent mechanisms. Am. J. Physiol. Cell Physiol. 2009, 297, C1358–C1367. [Google Scholar] [CrossRef] [Green Version]
- Rangel, L.B.A.; de Siqueira, D. Vitamin K Supplementation Modulates Bone Metabolism and Ultra-Structure of Ovariectomized Mice. Cell Physiol. Biochem. 2018, 51, 356–374. [Google Scholar] [CrossRef]
- Weng, S.J.; Xie, Z.J. Effects of combined menaquinone-4 and PTH1-34 treatment on osetogenesis and angiogenesis in calvarial defect in osteopenic rats. Endocrine 2019, 63, 376–384. [Google Scholar] [CrossRef]
- Akbari, S.; Rasouli-Ghahroudi, A.A. Vitamin K and Bone Metabolism: A Review of the Latest Evidence in Preclinical Studies. Biomed. Res. Int. 2018, 2018, 4629383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Ma, Z. Matrix Gla Protein Promotes the Bone Formation by Up-Regulating Wnt/beta-Catenin Signaling Pathway. Front. Endocrinol. 2019, 10, 891. [Google Scholar] [CrossRef] [PubMed]
- Ye, Z.W.; Zhang, J. Oxidative stress, redox regulation and diseases of cellular differentiation. Biochim. Biophys. Acta 2015, 1850, 1607–1621. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muszynska, M.; Ambrozewicz, E. Protective Effects of Vitamin K Compounds on the Proteomic Profile of Osteoblasts under Oxidative Stress Conditions. Molecules 2020, 25, 1990. [Google Scholar] [CrossRef] [PubMed]
- Tabb, M.M.; Sun, A. Vitamin K2 regulation of bone homeostasis is mediated by the steroid and xenobiotic receptor SXR. J. Biol. Chem. 2003, 278, 43919–43927. [Google Scholar] [CrossRef] [Green Version]
- Ichikawa, T.; Horie-Inoue, K. Steroid and xenobiotic receptor SXR mediates vitamin K2-activated transcription of extracellular matrix-related genes and collagen accumulation in osteoblastic cells. J. Biol. Chem. 2006, 281, 16927–16934. [Google Scholar] [CrossRef] [Green Version]
- Koshihara, Y.; Hoshi, K. Vitamin K stimulates osteoblastogenesis and inhibits osteoclastogenesis in human bone marrow cell culture. J. Endocrinol. 2003, 176, 339–348. [Google Scholar] [CrossRef] [Green Version]
- Yamaguchi, M.; Weitzmann, M.N. Vitamin K2 stimulates osteoblastogenesis and suppresses osteoclastogenesis by suppressing NF-kappaB activation. Int. J. Mol. Med. 2011, 27, 3–14. [Google Scholar]
- Wu, W.J.; Kim, M.S. The inhibitory effect of vitamin K on RANKL-induced osteoclast differentiation and bone resorption. Food Funct. 2015, 6, 3351–3358. [Google Scholar] [CrossRef]
- Mandatori, D.; Penolazzi, L. Menaquinone-4 enhances osteogenic potential of human amniotic fluid mesenchymal stem cells cultured in 2D and 3D dynamic culture systems. J. Tissue Eng. Regen. Med. 2017. [Google Scholar] [CrossRef]
- Knapen, M.H.; Braam, L.A. Menaquinone-7 supplementation improves arterial stiffness in healthy postmenopausal women. A double-blind randomised clinical trial. Thromb. Haemost. 2015, 113, 1135–1144. [Google Scholar] [CrossRef]
- Barrett, H.; O’Keeffe, M. Is Matrix Gla Protein Associated with Vascular Calcification? A Systematic Review. Nutrients 2018, 10, 415. [Google Scholar] [CrossRef] [Green Version]
- Schurgers, L.J.; Joosen, I.A. Vitamin K-antagonists accelerate atherosclerotic calcification and induce a vulnerable plaque phenotype. PLoS ONE 2012, 7, e43229. [Google Scholar] [CrossRef] [Green Version]
- Shea, M.K.; Holden, R.M. Vitamin K status and vascular calcification: Evidence from observational and clinical studies. Adv. Nutr. 2012, 3, 158–165. [Google Scholar] [CrossRef] [Green Version]
- Sheng, K.; Zhang, P. Association of Matrix Gla protein gene (rs1800801, rs1800802, rs4236) polymorphism with vascular calcification and atherosclerotic disease: A meta-analysis. Sci. Rep. 2017, 7, 8713. [Google Scholar] [CrossRef]
- Gallieni, M.; Fusaro, M. Vitamin K and cardiovascular calcification in CKD: Is patient supplementation on the horizon? Kidney Int. 2014, 86, 232–234. [Google Scholar] [CrossRef] [Green Version]
- van Gorp, R.H.; Dijkgraaf, I. Off-target effects of oral anticoagulants—Vascular effects of vitamin K antagonist and non-vitamin K antagonist oral anticoagulant dabigatran etexilate. J. Thromb. Haemost. 2021. [Google Scholar] [CrossRef]
- Petsophonsakul, P.; Furmanik, M. Role of Vascular Smooth Muscle Cell Phenotypic Switching and Calcification in Aortic Aneurysm Formation. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 1351–1368. [Google Scholar] [CrossRef]
- Yaghini, F.A.; Song, C.Y. Angiotensin II-induced vascular smooth muscle cell migration and growth are mediated by cytochrome P450 1B1-dependent superoxide generation. Hypertension 2010, 55, 1461–1467. [Google Scholar] [CrossRef] [Green Version]
- Rennenberg, R.J.; van Varik, B.J. Chronic coumarin treatment is associated with increased extracoronary arterial calcification in humans. Blood 2010, 115, 5121–5123. [Google Scholar] [CrossRef] [Green Version]
- Ueland, T.; Gullestad, L. Undercarboxylated matrix Gla protein is associated with indices of heart failure and mortality in symptomatic aortic stenosis. J. Intern. Med. 2010, 268, 483–492. [Google Scholar] [CrossRef]
- Cranenburg, E.C.; Koos, R. Characterisation and potential diagnostic value of circulating matrix Gla protein (MGP) species. Thromb. Haemost. 2010, 104, 811–822. [Google Scholar] [CrossRef]
- Donaldson, C.J.; Harrington, D.J. Therapeutic warfarin use and the extrahepatic functions of vitamin K-dependent proteins. Br. J. Biomed. Sci. 2017, 74, 163–169. [Google Scholar] [CrossRef]
- Geleijnse, J.M.; Vermeer, C. Dietary intake of menaquinone is associated with a reduced risk of coronary heart disease: The Rotterdam Study. J. Nutr. 2004, 134, 3100–3105. [Google Scholar] [CrossRef]
- Gast, G.C.; de Roos, N.M. A high menaquinone intake reduces the incidence of coronary heart disease. Nutr. Metab. Cardiovasc. Dis. 2009, 19, 504–510. [Google Scholar] [CrossRef]
- Beulens, J.W.; Bots, M.L. High dietary menaquinone intake is associated with reduced coronary calcification. Atherosclerosis 2009, 203, 489–493. [Google Scholar] [CrossRef] [PubMed]
- Vissers, L.E.; Dalmeijer, G.W. Intake of dietary phylloquinone and menaquinones and risk of stroke of article. J. Am. Heart Assoc. 2013, 2, e000455. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Juanola-Falgarona, M.; Salas-Salvado, J. Dietary intake of vitamin K is inversely associated with mortality risk. J. Nutr. 2014, 144, 743–750. [Google Scholar] [CrossRef] [PubMed]
- Vossen, L.M.; Schurgers, L.J. Menaquinone-7 Supplementation to Reduce Vascular Calcification in Patients with Coronary Artery Disease: Rationale and Study Protocol (VitaK-CAC Trial). Nutrients 2015, 7, 8905–8915. [Google Scholar] [CrossRef] [Green Version]
- Vissers, L.E.; Dalmeijer, G.W. The relationship between vitamin K and peripheral arterial disease of article. Atherosclerosis 2016, 252, 15–20. [Google Scholar] [CrossRef]
- Haugsgjerd, T.R.; Egeland, G.M. Association of dietary vitamin K and risk of coronary heart disease in middle-age adults: The Hordaland Health Study Cohort. BMJ Open 2020, 10, e035953. [Google Scholar] [CrossRef]
- Zwakenberg, S.R.; den Braver, N.R. Vitamin K intake and all-cause and cause specific mortality of article. Clin. Nutr. 2017, 36, 1294–1300. [Google Scholar] [CrossRef]
- Bartstra, J.W.; Draaisma, F. Six months vitamin K treatment does not affect systemic arterial calcification or bone mineral density in diabetes mellitus 2. Eur. J. Nutr. 2020. [Google Scholar] [CrossRef]
- Zwakenberg, S.R.; Burgess, S. Circulating phylloquinone, inactive Matrix Gla protein and coronary heart disease risk: A two-sample Mendelian Randomization study of article. Clin. Nutr. 2020, 39, 1131–1136. [Google Scholar] [CrossRef]
- Van den Heuvel, E.G.; van Schoor, N.M. Circulating uncarboxylated matrix Gla protein, a marker of vitamin K status, as a risk factor of cardiovascular disease. Maturitas 2014, 77, 137–141. [Google Scholar] [CrossRef]
- Pivin, E.; Ponte, B. Inactive Matrix Gla-Protein Is Associated with Arterial Stiffness in an Adult Population-Based Study. Hypertension 2015, 66, 85–92. [Google Scholar] [CrossRef] [Green Version]
- Liabeuf, S.; Bourron, O. Vascular calcification in patients with type 2 diabetes: The involvement of matrix Gla protein. Cardiovasc. Diabetol. 2014, 13, 85. [Google Scholar] [CrossRef] [Green Version]
- Cheung, C.L.; Sahni, S. Vitamin K intake and mortality in people with chronic kidney disease from NHANES III. Clin. Nutr. 2015, 34, 235–240. [Google Scholar] [CrossRef]
- Westenfeld, R.; Krueger, T. Effect of vitamin K2 supplementation on functional vitamin K deficiency in hemodialysis patients: A randomized trial. Am. J. Kidney Dis. 2012, 59, 186–195. [Google Scholar] [CrossRef]
- Dalmeijer, G.W.; van der Schouw, Y.T. The effect of menaquinone-7 supplementation on circulating species of matrix Gla protein. Atherosclerosis 2012, 225, 397–402. [Google Scholar] [CrossRef]
- Caluwe, R.; Vandecasteele, S. Vitamin K2 supplementation in haemodialysis patients: A randomized dose-finding study. Nephrol. Dial. Transplant. 2014, 29, 1385–1390. [Google Scholar] [CrossRef]
- Aoun, M.; Makki, M. High Dephosphorylated-Uncarboxylated MGP in Hemodialysis patients: Risk factors and response to vitamin K2, A pre-post intervention clinical trial. BMC Nephrol. 2017, 18, 191. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, Z. Vitamin K2 can suppress the expression of Toll-like receptor 2 (TLR2) and TLR4, and inhibit calcification of aortic intima in ApoE(-/-) mice as well as smooth muscle cells. Vascular 2018, 26, 18–26. [Google Scholar] [CrossRef] [Green Version]
- Shanahan, C.M.; Crouthamel, M.H. Arterial calcification in chronic kidney disease: Key roles for calcium and phosphate. Circ. Res. 2011, 109, 697–711. [Google Scholar] [CrossRef] [Green Version]
- Mandatori, D.; Pipino, C. Osteogenic transdifferentiation of vascular smooth muscle cells isolated from spontaneously hypertensive rats and potential menaquinone-4 inhibiting effect. J. Cell Physiol. 2019, 234, 19761–19773. [Google Scholar] [CrossRef]
- Hasanbasic, I.; Rajotte, I. The role of γ-carboxylation in the anti-apoptotic function of gas6. J. Thromb. Haemost. 2005, 3, 2790–2797. [Google Scholar] [CrossRef] [PubMed]
- Qiu, C.; Zheng, H. Vitamin K2 inhibits rat vascular smooth muscle cell calcification by restoring the Gas6/Axl/Akt anti-apoptotic pathway. Mol. Cell Biochem. 2017. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Tao, H. Vitamin K2 regression aortic calcification induced by warfarin via Gas6/Axl survival pathway in rats. Eur. J. Pharmacol. 2016, 786, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Shea, M.K.; Berkner, K.L. Perspective: Evidence before Enthusiasm—A Critical Review of the Potential Cardiovascular Benefits of Vitamin K. Adv. Nutr. 2021. [Google Scholar] [CrossRef]
| Study | Name of the Study | Number of Patients Enrolled | Key Findings |
|---|---|---|---|
| [21] | Framingham Heart Study | 364 women and 190 men (28–62 years old) | Bone loss was associated with progression of aortic calcification in women over 25 years |
| [22] | Women’s Health Across the Nation Study | 90 women (45–58 years old) | Lower BMD was related to high aortic calcification |
| [23] | MESA Study | 946 women (mean age 65.5 years old) and 963 men (mean age 64.1 years old) | Lower BMD was associated with greater coronary artery and abdominal aortic calcium score |
| [24] | Rotterdam Study | 582 men and 694 women all >55 years old | BMD loss was significantly associated with higher follow-up coronary artery calcification |
| Study | Type of the Study | Number of Patients Enrolled | Key Findings |
|---|---|---|---|
| [85] | RCT | 219 post-menopausal women | BMD increase following one year of vitamin K2 supplementation (100 μg/day) |
| [86] | RCT | 244 healthy post-menopausal women | Decrease bone loss following three years MK-7 supplement (180 μg/day) |
| [87] | Meta-analysis of 19 RCTs | 6759 participants (post-menopausal women) | BMD improvement and low incidence of fracture in osteoporotic subjects following K2 treatment |
| [88] | Meta-analysis of 36 RCTs | 11,122 participants (post-menopausal women) | Vitamin K2 treatment (MK-4: 45 mg/day) reduce fracture, increase cOC and decrease ucOC serum concentration |
| [89] | RCT | 55 healthy children | 8 weeks MK-7 supplementation increase cOC serum concentration |
| [90] | Non-placebo-controlled dose-examination study | 55 healthy males | MK-4 supplementation (600 and 900 μg/day) decrease ucOC and increase cOC level respectively |
| [91] | RCT | 48 healthy post-menopausal women | Serum ucOC concentrations were significantly lower following 6–12 months MK-4 treatment (1.5 mg/day) |
| [92] | RCT | 60 postmenopausal women | MK-7 treatment (100 μg/day) significantly decrease ucOC and increase cOC/ucOC ratio |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mandatori, D.; Pelusi, L.; Schiavone, V.; Pipino, C.; Di Pietro, N.; Pandolfi, A. The Dual Role of Vitamin K2 in “Bone-Vascular Crosstalk”: Opposite Effects on Bone Loss and Vascular Calcification. Nutrients 2021, 13, 1222. https://doi.org/10.3390/nu13041222
Mandatori D, Pelusi L, Schiavone V, Pipino C, Di Pietro N, Pandolfi A. The Dual Role of Vitamin K2 in “Bone-Vascular Crosstalk”: Opposite Effects on Bone Loss and Vascular Calcification. Nutrients. 2021; 13(4):1222. https://doi.org/10.3390/nu13041222
Chicago/Turabian StyleMandatori, Domitilla, Letizia Pelusi, Valeria Schiavone, Caterina Pipino, Natalia Di Pietro, and Assunta Pandolfi. 2021. "The Dual Role of Vitamin K2 in “Bone-Vascular Crosstalk”: Opposite Effects on Bone Loss and Vascular Calcification" Nutrients 13, no. 4: 1222. https://doi.org/10.3390/nu13041222