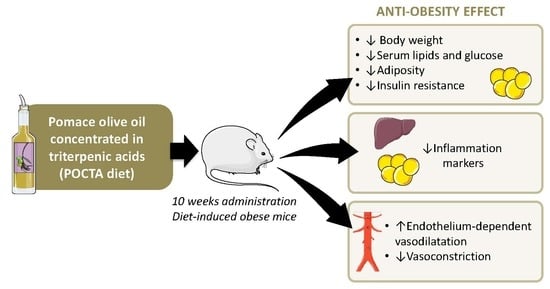
Pomace Olive Oil Concentrated in Triterpenic Acids Restores Vascular Function, Glucose Tolerance and Obesity Progression in Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Diets
2.2. Blood Biochemical Assays
2.3. Liver Triglycerides Quantification
2.4. Glucose Tolerance and Insulin Resistance Test
2.5. RNA Extraction and Quantitative Real time-Polymerase Chain Reaction (RT-PCR) on Anti-Inflammatory Markers
2.6. Arterial Preparation and Vascular Reactivity Experiments
2.7. Data Analysis and Statistics
3. Results
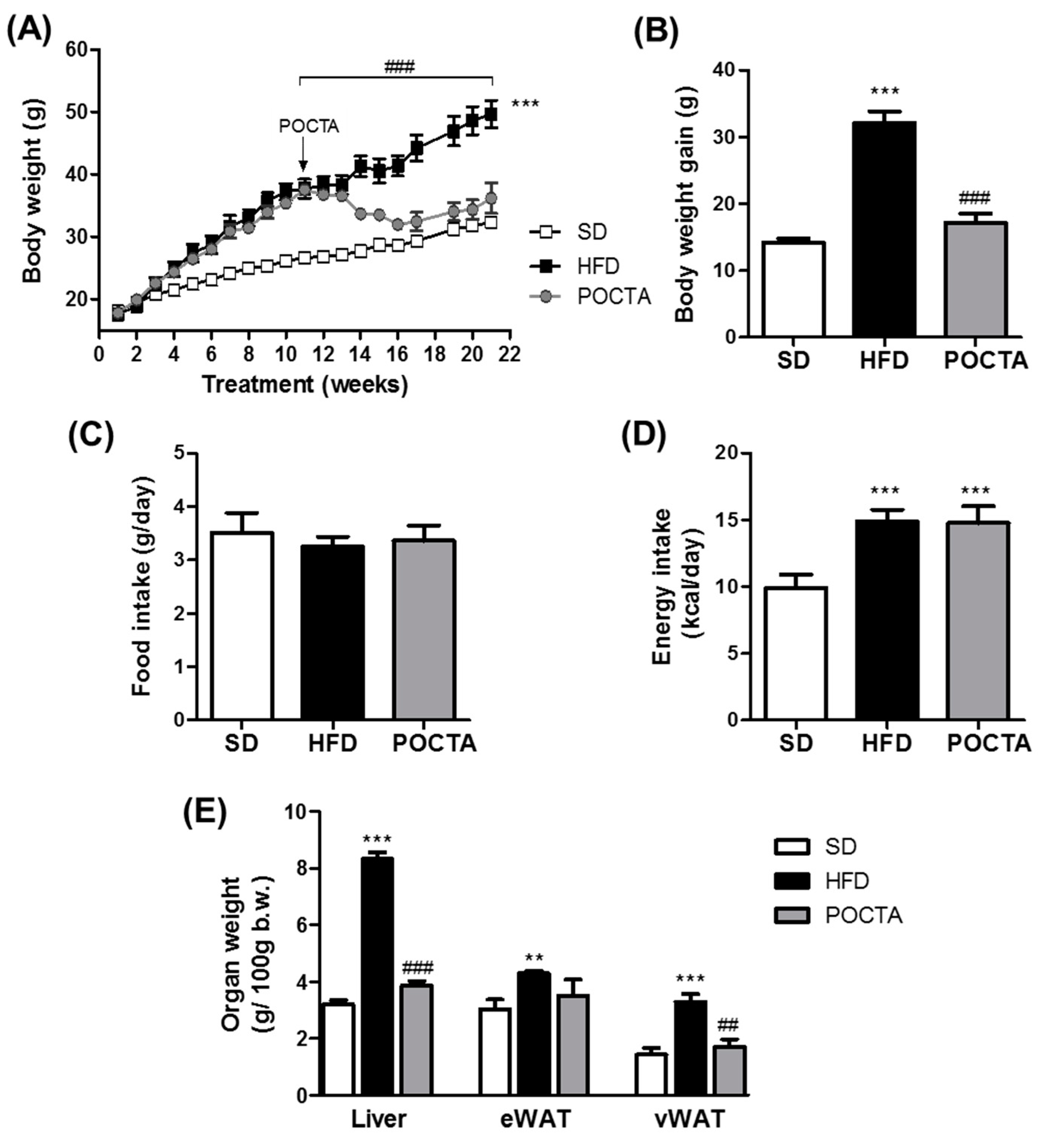
3.1. POCTA Attenuated Body Weight Gain and Organ Weight in Obese Mice
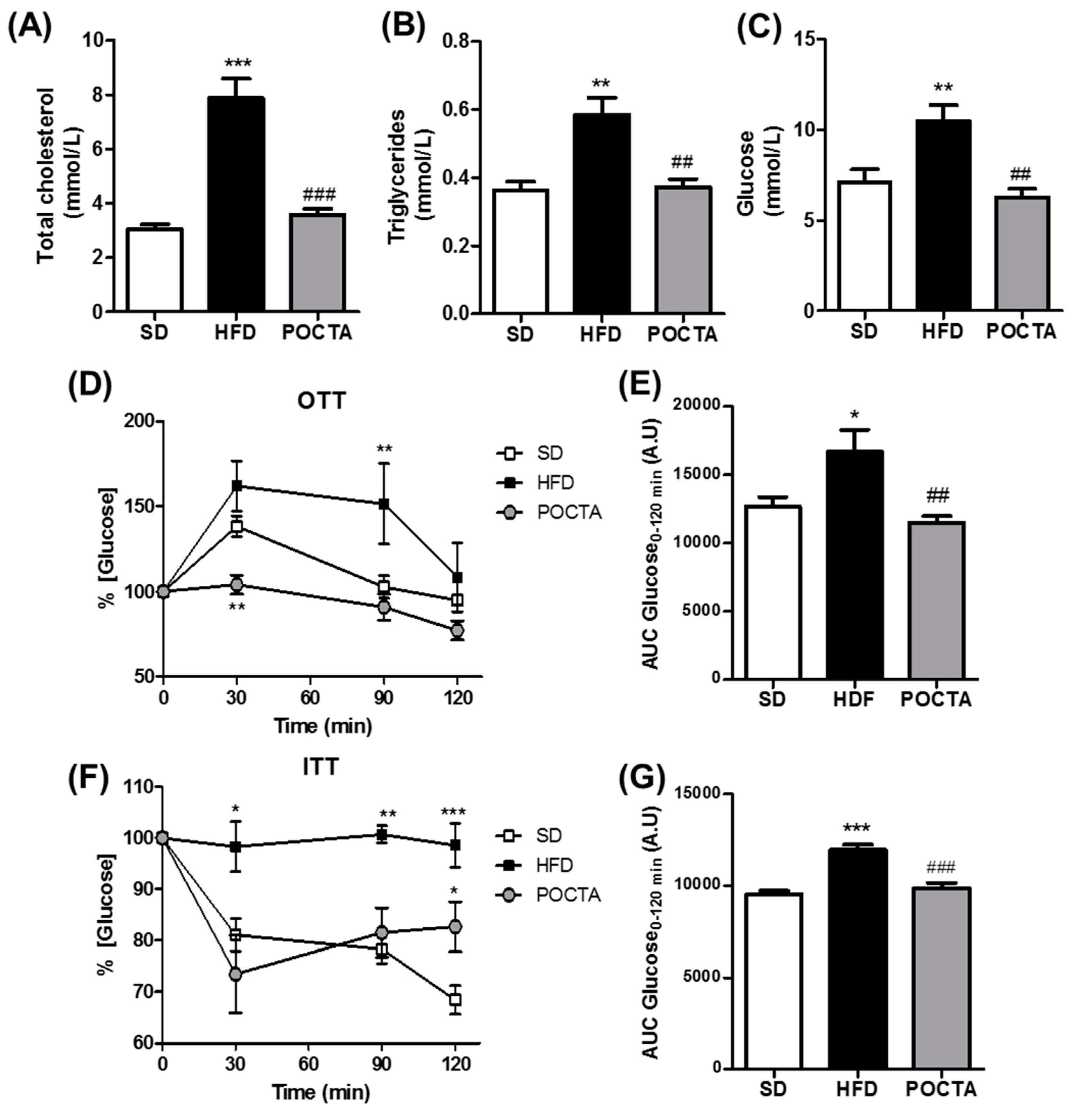
3.2. POCTA Improved Serum Cholesterol, Triglycerides, Glucose, and Insulin Resistance
3.3. POCTA Reduced the Expression of Inflammatory Markers in White Adipose Tissues
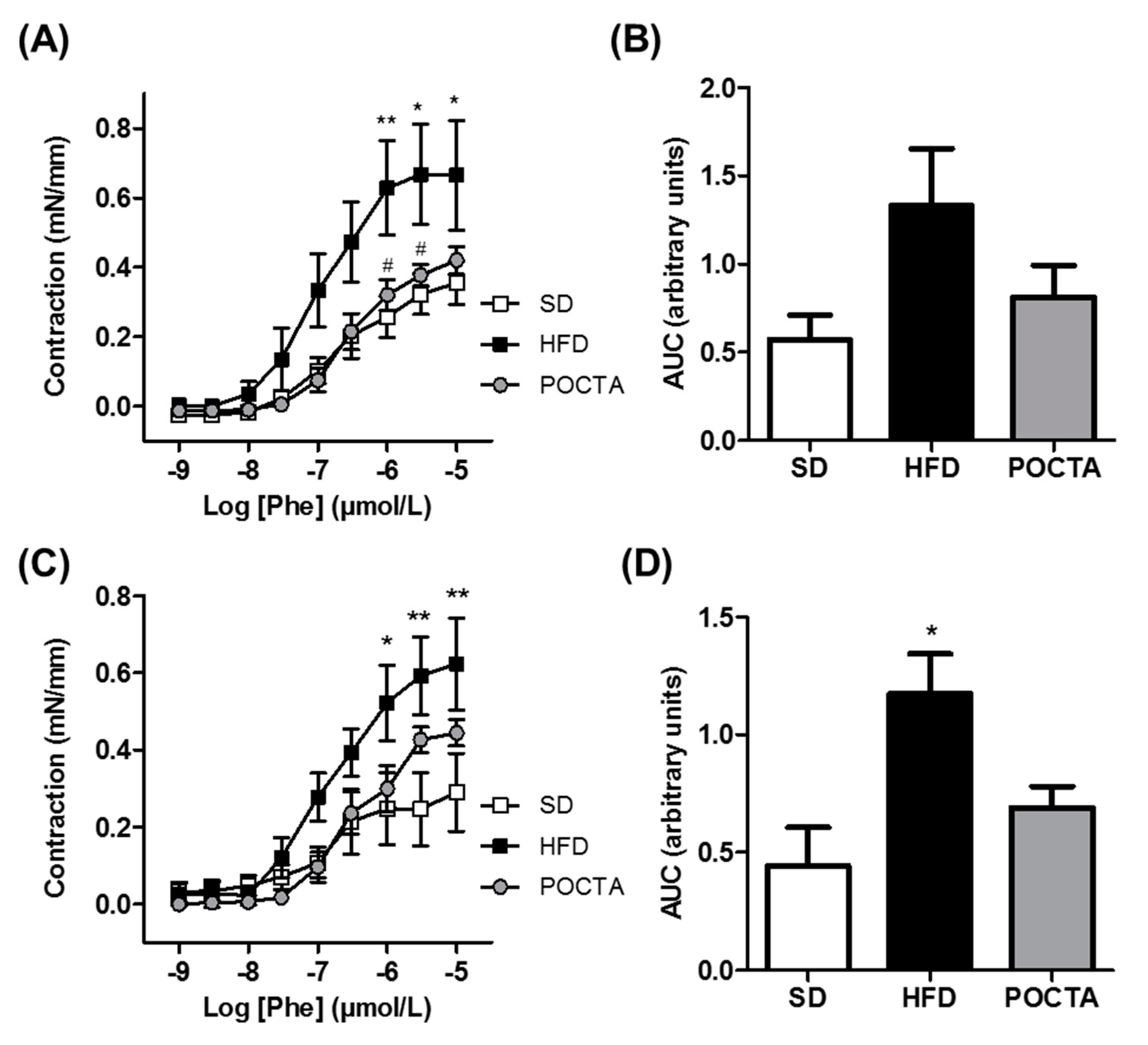
3.4. POCTA Restored Vascular Reactivity in Obese Mice
3.4.1. Vasodilatation
3.4.2. Vasoconstriction
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Malik, V.S.; Willett, W.C.; Hu, F.B. Global obesity: Trends, risk factors and policy implications. Nat. Rev. Endocrinol. 2013, 9, 13–27. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Obesity and Overweight. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 19 November 2019).
- Bhupathiraju, S.N.; Hu, F.B. Epidemiology of Obesity and Diabetes and Their Cardiovascular Complications. Circ. Res. 2016, 118, 1723–1735. [Google Scholar] [CrossRef] [PubMed]
- Lavie, C.J.; Arena, R.; Alpert, M.A.; Milani, R.V.; Ventura, H.O. Management of cardiovascular diseases in patients with obesity. Nat. Rev. Cardiol. 2018, 15, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Grosso, G.; Mistretta, A.; Marventano, S.; Purrello, A.; Vitaglione, P.; Calabrese, G.; Drago, F.; Galvano, F. Beneficial effects of the Mediterranean diet on metabolic syndrome. Curr. Pharm. Des. 2014, 20, 5039–5044. [Google Scholar] [CrossRef]
- Ríos-Hoyo, A.; Cortés, M.J.; Ríos-Ontiveros, H.; Meaney, E.; Ceballos, G.; Gutiérrez-Salmeán, G. Obesity, Metabolic Syndrome, and Dietary Therapeutical Approaches with a Special Focus on Nutraceuticals (Polyphenols): A Mini-Review. Int. J. Vitam Nutr. Res. 2014, 84, 113–123. [Google Scholar]
- Zhao, D.; Qi, Y.; Zheng, Z.; Wang, Y.; Zhang, X.-Y.; Li, H.-J.; Liu, H.-H.; Zhang, X.-T.; Du, J.; Liu, J. Dietary factors associated with hypertension. Nat. Rev. Cardiol. 2011, 8, 456–465. [Google Scholar] [CrossRef]
- Conroy, K.P.; Davidson, I.M.; Warnock, M. Pathogenic obesity and nutraceuticals. Proc. Nutr. Soc. 2011, 70, 426–438. [Google Scholar] [CrossRef] [Green Version]
- Buckland, G.; Travier, N.; Cottet, V.; González, C.A.; Luján-Barroso, L.; Agudo, A.; Trichopoulou, A.; Lagiou, P.; Trichopoulos, D.; Peeters, P.H.; et al. Adherence to the mediterranean diet and risk of breast cancer in the European prospective investigation into cancer and nutrition cohort study. Int. J. Cancer 2013, 132, 2918–2927. [Google Scholar] [CrossRef]
- Beulen, Y.; Martínez-González, M.A.; van de Rest, O.; Salas-Salvadó, J.; Sorlí, J.V.; Gómez-Gracia, E.; Fiol, M.; Estruch, R.; Santos-Lozano, J.M.; Schröder, H.; et al. Quality of dietary fat intake and body weight and obesity in a mediterranean population: Secondary analyses within the PREDIMED trial. Nutrients 2018, 10, 2011. [Google Scholar] [CrossRef] [Green Version]
- Yubero-Serrano, E.M.; Lopez-Moreno, J.; Gomez-Delgado, F.; Lopez-Miranda, J. Extra virgin olive oil: More than a healthy fat. Eur. J. Clin. Nutr. 2019, 72, 8–17. [Google Scholar] [CrossRef] [Green Version]
- Marcelino, G.; Hiane, P.A.; Freitas, K. de C.; Santana, L.F.; Pott, A.; Donadon, J.R.; Guimarães, R.c.A. Effects of Olive Oil and Its Minor Components on Cardiovascular Diseases, Inflammation, and Gut Microbiota. Nutrients 2019, 11, 1826. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodriguez-Rodriguez, R. Oleanolic acid and related triterpenoids from olives on vascular function: Molecular mechanisms and therapeutic perspectives. Curr. Med. Chem. 2015, 22, 1414–1425. [Google Scholar] [CrossRef]
- Fernández-Aparicio, A.; Schmidt-RioValle, J.; Perona, J.S.; Correa-Rodríguez, M.; Castellano, J.M.; González-Jiménez, E. Potential Protective Effect of Oleanolic Acid on the Components of Metabolic Syndrome: A Systematic Review. J. Clin. Med. 2019, 8, 1294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liou, C.-J.; Dai, Y.-W.; Wang, C.-L.; Fang, L.-W.; Huang, W.-C. Maslinic acid protects against obesity-induced nonalcoholic fatty liver disease in mice through regulation of the Sirt1/AMPK signaling pathway. FASEB J. 2019, 33, 11791–11803. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Žiberna, L.; Šamec, D.; Mocan, A.; Nabavi, S.; Bishayee, A.; Farooqi, A.; Sureda, A.; Nabavi, S. Oleanolic Acid Alters Multiple Cell Signaling Pathways: Implication in Cancer Prevention and Therapy. Int. J. Mol. Sci. 2017, 18, 643. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allouche, Y.; Uceda, M.; Jiménez, A.; Aguilera, M.P.; Gaforio, J.J.; Beltrán, G. Fruit quality and olive leaf and stone addition affect Picual virgin olive oil triterpenic content. J. Agric. Food Chem. 2009, 57, 8998–9001. [Google Scholar] [CrossRef]
- Pérez-Camino, M.C.; Cert, A. Quantitative Determination of Hydroxy Pentacyclic Triterpene Acids in Vegetable Oils. J. Agric. Food Chem. 1999, 47, 1558–1562. [Google Scholar] [CrossRef]
- Rodriguez-Rodriguez, R.; Perona, J.S.; Herrera, M.D.; Ruiz-Gutierrez, V. Triterpenic compounds from “Orujo” olive oil elicit vasorelaxation in aorta from spontaneously hypertensive rats. J. Agric. Food Chem. 2006, 54, 2096–2102. [Google Scholar] [CrossRef]
- Rodríguez-Rodríguez, R.; Herrera, M.D.; Perona, J.S.; Ruiz-Gutiérrez, V. Potential vasorelaxant effects of oleanolic acid and erythrodiol, two triterpenoids contained in “orujo” olive oil, on rat aorta. Br. J. Nutr. 2004, 92, 635–642. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez-Rodriguez, R.; Stankevicius, E.; Herrera, M.D.; Østergaard, L.; Andersen, M.R.; Ruiz-Gutierrez, V.; Simonsen, U. Oleanolic acid induces relaxation and calcium-independent release of endothelium-derived nitric oxide. Br. J. Pharmacol. 2008, 155, 535–546. [Google Scholar] [CrossRef]
- Martínez-González, J.; Rodríguez-Rodríguez, R.; González-Díez, M.; Rodríguez, C.; Herrera, M.D.; Ruiz-Gutierrez, V.; Badimon, L. Oleanolic acid induces prostacyclin release in human vascular smooth muscle cells through a cyclooxygenase-2-dependent mechanism. J. Nutr. 2008, 138, 443–448. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Su, S.; Wu, G.; Cheng, X.; Fan, J.; Peng, J.; Su, H.; Xu, Z.; Cao, M.; Long, Z.; Hao, Y.; et al. Oleanolic acid attenuates PCBs-induced adiposity and insulin resistance via HNF1b-mediated regulation of redox and PPARγ signaling. Free Radic. Biol. Med. 2018, 124, 122–134. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Sung, H.-Y.; Kim, M.S.; Kim, J.-L.; Kang, M.-K.; Gong, J.-H.; Park, H.-S.; Kang, Y.-H. Oleanolic acid suppresses resistin induction in adipocytes by modulating Tyk-STAT signaling. Nutr. Res. 2013, 33, 144–153. [Google Scholar] [CrossRef]
- de Melo, C.L.; Queiroz, M.G.R.; Fonseca, S.G.C.; Bizerra, A.M.C.; Lemos, T.L.G.; Melo, T.S.; Santos, F.A.; Rao, V.S. Oleanolic acid, a natural triterpenoid improves blood glucose tolerance in normal mice and ameliorates visceral obesity in mice fed a high-fat diet. Chem. Biol. Interact. 2010, 185, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Djeziri, F.Z.; Belarbi, M.; Murtaza, B.; Hichami, A.; Benammar, C.; Khan, N.A. Oleanolic acid improves diet-induced obesity by modulating fat preference and inflammation in mice. Biochimie 2018, 152, 110–120. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Rodriguez, R.; Herrera, M.D.; de Sotomayor, M.A.; Ruiz-Gutierrez, V. Pomace Olive Oil Improves Endothelial Function in Spontaneously Hypertensive Rats by Increasing Endothelial Nitric Oxide Synthase Expression. Am. J. Hypertens. 2007, 20, 728–734. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodriguez-Rodriguez, R.; Herrera, M.D.; De Sotomayor, M.A.; Ruiz-Gutierrez, V. Effects of pomace olive oil-enriched diets on endothelial function of small mesenteric arteries from spontaneously hypertensive rats. Br. J. Nutr. 2009, 102, 1435–1444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valero-Muñoz, M.; Martín-Fernández, B.; Ballesteros, S.; de la Fuente, E.; Quintela, J.C.; Lahera, V.; De las Heras, N. Protective effect of a pomace olive oil concentrated in triterpenic acids in alterations related to hypertension in rats: Mechanisms involved. Mol. Nutr. Food Res. 2014, 58, 376–383. [Google Scholar] [CrossRef]
- Justo, M.L.; Rodriguez-Rodriguez, R.; Claro, C.M.; Alvarez De Sotomayor, M.; Parrado, J.; Herrera, M.D. Water-soluble rice bran enzymatic extract attenuates dyslipidemia, hypertension and insulin resistance in obese Zucker rats. Eur. J. Nutr. 2013, 52, 789–797. [Google Scholar] [CrossRef]
- Justo, M.L.; Claro, C.; Zeyda, M.; Stulnig, T.M.; Herrera, M.D.; Rodríguez-Rodríguez, R. Rice bran prevents high-fat diet-induced inflammation and macrophage content in adipose tissue. Eur. J. Nutr. 2016, 55, 2011–2019. [Google Scholar] [CrossRef]
- Rodríguez-Rodríguez, R.; Miralpeix, C.; Fosch, A.; Pozo, M.; Calderón-Domínguez, M.; Perpinyà, X.; Vellvehí, M.; López, M.; Herrero, L.; Serra, D.; et al. CPT1C in the ventromedial nucleus of the hypothalamus is necessary for brown fat thermogenesis activation in obesity. Mol. Metab. 2019, 19, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Claro, C.; Ogalla, E.; Rodriguez-Rodriguez, R.; Herrera, M.D.; Alvarez de Sotomayor, M. Phenolic content of extra virgin olive oil is essential to restore endothelial dysfunction but not to prevent vascular inflammation in atherosclerotic lesions of Apo E deficient mice. J. Funct. Foods 2015, 15, 126–136. [Google Scholar] [CrossRef]
- Konstantinidi, M.; Koutelidakis, A.E. Functional Foods and Bioactive Compounds: A Review of Its Possible Role on Weight Management and Obesity’s Metabolic Consequences. Medicines 2019, 6, 94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Unamuno, X.; Gómez-Ambrosi, J.; Rodríguez, A.; Becerril, S.; Frühbeck, G.; Catalán, V. Adipokine dysregulation and adipose tissue inflammation in human obesity. Eur. J. Clin. Invest. 2018, 48, e12997. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, H.; Kumar, P.; Deshmukh, R.R.; Bishayee, A.; Kumar, S. Pentacyclic triterpenes: New tools to fight metabolic syndrome. Phytomedicine 2018, 50, 166–177. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.-Y.; Kang, S.-W.; Kim, J.-L.; Li, J.; Lee, E.-S.; Gong, J.-H.; Han, S.J.; Kang, Y.-H. Oleanolic acid reduces markers of differentiation in 3T3-L1 adipocytes. Nutr. Res. 2010, 30, 831–839. [Google Scholar] [CrossRef]
- Pérez-Jiménez, A.; Rufino-Palomares, E.E.; Fernández-Gallego, N.; Ortuño-Costela, M.C.; Reyes-Zurita, F.J.; Peragón, J.; García-Salguero, L.; Mokhtari, K.; Medina, P.P.; Lupiáñez, J.A. Target molecules in 3T3-L1 adipocytes differentiation are regulated by maslinic acid, a natural triterpene from Olea europaea. Phytomedicine 2016, 23, 1301–1311. [Google Scholar] [CrossRef]
- Silva, F.S.G.; Oliveira, P.J.; Duarte, M.F. Oleanolic, Ursolic, and Betulinic Acids as Food Supplements or Pharmaceutical Agents for Type 2 Diabetes: Promise or Illusion? J. Agric. Food Chem. 2016, 64, 2991–3008. [Google Scholar] [CrossRef]
- Anouar, E.H.; Zakaria, N.S.S.; Alsalme, A.; Shah, S.A.A. α-Glucosidase activity of oleanolic acid and its oxidative metabolites: DFT and Docking studies. Mini Rev. Med. Chem. 2015, 15, 1148–1158. [Google Scholar] [CrossRef]
- Tesauro, M.; Cardillo, C. Obesity, blood vessels and metabolic syndrome. Acta Physiol. 2011, 203, 279–286. [Google Scholar] [CrossRef]
- Iantorno, M.; Campia, U.; Di Daniele, N.; Nistico, S.; Forleo, G.B.; Cardillo, C.; Tesauro, M. Obesity, inflammation and endothelial dysfunction. J. Biol. Regul. Homeost. Agents 2014, 28, 169–176. [Google Scholar] [PubMed]
- Huang, P.L. Unraveling the Links Between Diabetes, Obesity, and Cardiovascular Disease. Circ. Res. 2005, 96, 1129–1131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.; Montagnani, M.; Koh, K.K.; Quon, M.J. Reciprocal Relationships Between Insulin Resistance and Endothelial Dysfunction. Circulation 2006, 113, 1888–1904. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.Q.; Hopfner, R.L.; McNeill, J.R.; Wilson, T.W.; Gopalakrishnan, V. Altered paracrine effect of endothelin in blood vessels of the hyperinsulinemic, insulin resistant obese Zucker rat. Cardiovasc. Res. 2000, 45, 994–1000. [Google Scholar] [CrossRef] [Green Version]
- Stepp, D.W.; Frisbee, J.C. Augmented adrenergic vasoconstriction in hypertensive diabetic obese Zucker rats. Am. J. Physiol. Heart Circ. Physiol. 2002, 282, H816–H820. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mateos, R.; Sarria, B.; Bravo, L. Nutritional and other health properties of olive pomace oil. Crit. Rev. Food Sci. Nutr. 2019, 1–16. [Google Scholar] [CrossRef]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Claro-Cala, C.M.; Quintela, J.C.; Pérez-Montero, M.; Miñano, J.; Alvarez de Sotomayor, M.; Herrera, M.D.; Rodríguez-Rodríguez, R. Pomace Olive Oil Concentrated in Triterpenic Acids Restores Vascular Function, Glucose Tolerance and Obesity Progression in Mice. Nutrients 2020, 12, 323. https://doi.org/10.3390/nu12020323
Claro-Cala CM, Quintela JC, Pérez-Montero M, Miñano J, Alvarez de Sotomayor M, Herrera MD, Rodríguez-Rodríguez R. Pomace Olive Oil Concentrated in Triterpenic Acids Restores Vascular Function, Glucose Tolerance and Obesity Progression in Mice. Nutrients. 2020; 12(2):323. https://doi.org/10.3390/nu12020323
Chicago/Turabian StyleClaro-Cala, Carmen Maria, Jose Carlos Quintela, Marta Pérez-Montero, Javier Miñano, María Alvarez de Sotomayor, María Dolores Herrera, and Rosalía Rodríguez-Rodríguez. 2020. "Pomace Olive Oil Concentrated in Triterpenic Acids Restores Vascular Function, Glucose Tolerance and Obesity Progression in Mice" Nutrients 12, no. 2: 323. https://doi.org/10.3390/nu12020323