Source and Composition in Amino Acid of Dietary Proteins in the Primary Prevention and Treatment of CKD
Abstract
:1. Introduction
2. Clinical Evidence of Plant-Based Diets in CKD Incidence, Progression, and Complications
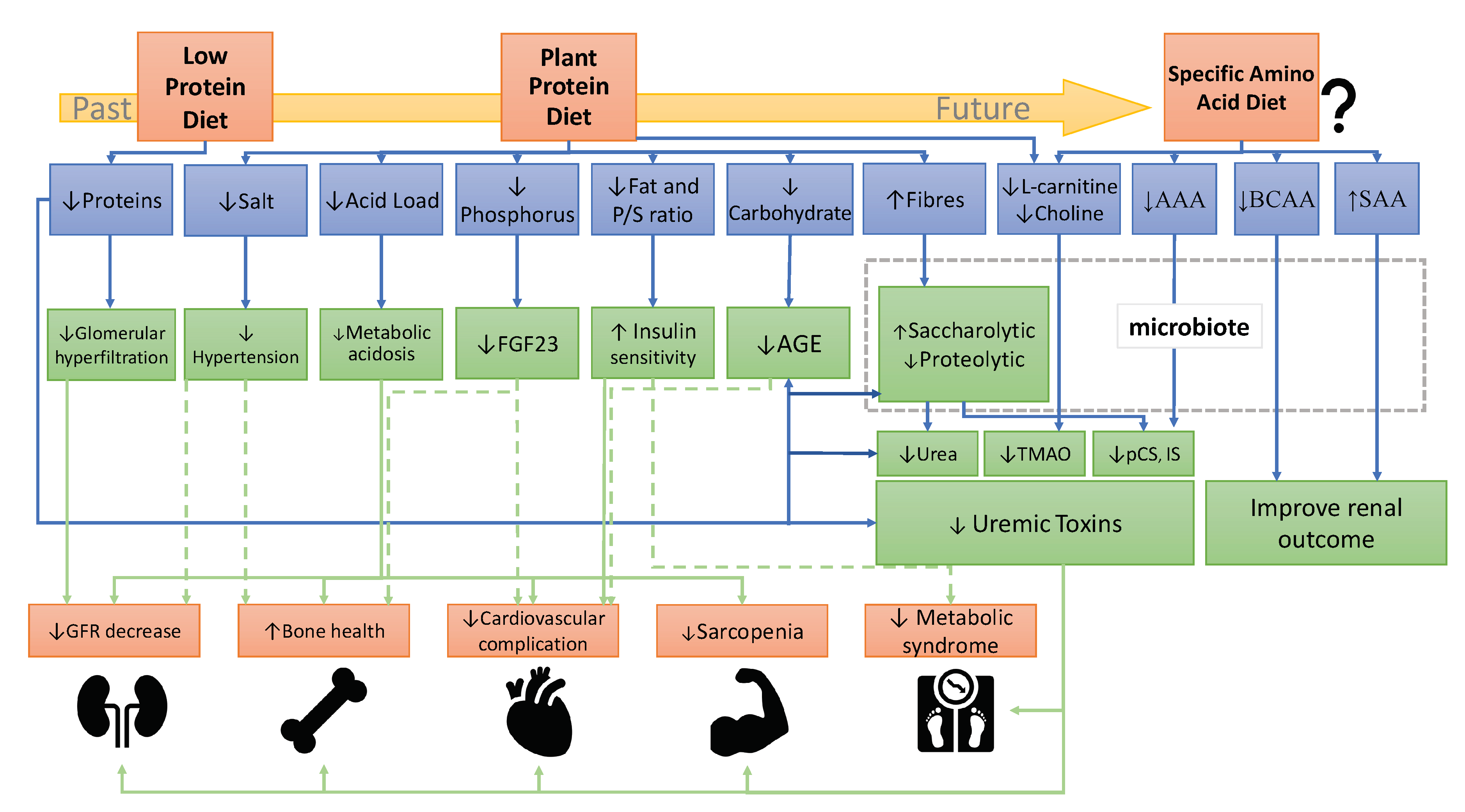
3. Presumed Mechanisms of Plant-Diets Beneficial Effects on CKD Progression
4. Limitations of Plant-Diets in CKD
4.1. Denutrition/Malnutrition
4.2. Bone Disorder
4.3. Hyperkalemia
5. Does the AA Composition Influence the Progression of CKD and Its Complications?
5.1. Plasma Concentrations of Amino Acids in the Context of Plants and Animal Diets
5.2. Influence of Specific Amino Acids on Renal Hemodynamics
5.3. Influence of Specific Amino Acids on Uremic Toxins Generation and Intestinal Microbiota
5.3.1. Aromatic Amino Acids (AAAs)
5.3.2. Sulfur-Containing Amino Acids (SAAs)
5.3.3. L-carnitine and Choline
5.4. Amino Acids Composition and Metabolic Complications
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Koppe, L.; Mafra, D.; Fouque, D. Probiotics and Chronic Kidney Disease. Kidney Int. 2015, 88, 958–966. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koppe, L.; Fouque, D.; Soulage, C.O. The Role of Gut Microbiota and Diet on Uremic Retention Solutes Production in the Context of Chronic Kidney Disease. Toxins 2018, 10, 155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brenner, B.M.; Meyer, T.W.; Hostetter, T.H. Dietary Protein Intake and the Progressive Nature of Kidney Disease: The Role of Hemodynamically Mediated Glomerular Injury in the Pathogenesis of Progressive Glomerular Sclerosis in Aging, Renal Ablation, and Intrinsic Renal Disease. N. Engl. J. Med. 1982, 307, 652–659. [Google Scholar] [CrossRef] [PubMed]
- Metzger, M.; Yuan, W.L.; Haymann, J.-P.; Flamant, M.; Houillier, P.; Thervet, E.; Boffa, J.-J.; Vrtovsnik, F.; Froissart, M.; Bankir, L.; et al. Association of a Low-Protein Diet With Slower Progression of CKD. Kidney Int. Rep. 2018, 3, 105–114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ko, G.-J.; Rhee, C.M.; Kalantar-Zadeh, K.; Joshi, S. The Effects of High-Protein Diets on Kidney Health and Longevity. J. Am. Soc. Nephrol. JASN 2020, 31, 1667–1679. [Google Scholar] [CrossRef] [PubMed]
- Ikizler, T.A.; Burrowes, J.D.; Byham-Gray, L.D.; Campbell, K.L.; Carrero, J.-J.; Chan, W.; Fouque, D.; Friedman, A.N.; Ghaddar, S.; Goldstein-Fuchs, D.J.; et al. KDOQI Clinical Practice Guideline for Nutrition in CKD: 2020 Update. Am. J. Kidney Dis. Off. J. Natl. Kidney Found. 2020, 76, S1–S107. [Google Scholar] [CrossRef]
- Block, R.J.; Bolling, D. The Amino Acid Composition of Proteins and Foods. Science 1946, 103, 431. [Google Scholar] [CrossRef]
- Moore, L.W.; Byham-Gray, L.D.; Scott Parrott, J.; Rigassio-Radler, D.; Mandayam, S.; Jones, S.L.; Mitch, W.E.; Osama Gaber, A. The Mean Dietary Protein Intake at Different Stages of Chronic Kidney Disease Is Higher than Current Guidelines. Kidney Int. 2013, 83, 724–732. [Google Scholar] [CrossRef] [Green Version]
- Athinarayanan, S.J.; Adams, R.N.; Hallberg, S.J.; McKenzie, A.L.; Bhanpuri, N.H.; Campbell, W.W.; Volek, J.S.; Phinney, S.D.; McCarter, J.P. Long-Term Effects of a Novel Continuous Remote Care Intervention Including Nutritional Ketosis for the Management of Type 2 Diabetes: A 2-Year Non-Randomized Clinical Trial. Front. Endocrinol. 2019, 10. [Google Scholar] [CrossRef] [Green Version]
- Joshi, S.; Hashmi, S.; Shah, S.; Kalantar-Zadeh, K. Plant-Based Diets for Prevention and Management of Chronic Kidney Disease. Curr. Opin. Nephrol. Hypertens. 2020, 29, 16–21. [Google Scholar] [CrossRef]
- Carrero, J.J.; González-Ortiz, A.; Avesani, C.M.; Bakker, S.J.L.; Bellizzi, V.; Chauveau, P.; Clase, C.M.; Cupisti, A.; Espinosa-Cuevas, A.; Molina, P.; et al. Plant-Based Diets to Manage the Risks and Complications of Chronic Kidney Disease. Nat. Rev. Nephrol. 2020, 16, 525–542. [Google Scholar] [CrossRef] [PubMed]
- Chauveau, P.; Koppe, L.; Combe, C.; Lasseur, C.; Trolonge, S.; Aparicio, M. Vegetarian Diets and Chronic Kidney Disease. Nephrol. Dial. Transplant. Off. Publ. Eur. Dial. Transpl. Assoc. Eur. Ren. Assoc. 2018. [Google Scholar] [CrossRef] [PubMed]
- Melina, V.; Craig, W.; Levin, S. Position of the Academy of Nutrition and Dietetics: Vegetarian Diets. J. Acad. Nutr. Diet. 2016, 116, 1970–1980. [Google Scholar] [CrossRef] [PubMed]
- Sinha, R.; Cross, A.J.; Graubard, B.I.; Leitzmann, M.F.; Schatzkin, A. Meat Intake and Mortality: A Prospective Study of over Half a Million People. Arch. Intern. Med. 2009, 169, 562–571. [Google Scholar] [CrossRef]
- Haring, B.; Selvin, E.; Liang, M.; Coresh, J.; Grams, M.E.; Petruski-Ivleva, N.; Steffen, L.M.; Rebholz, C.M. Dietary Protein Sources and Risk for Incident Chronic Kidney Disease: Results from the Atherosclerosis Risk in Communities (ARIC) Study. J. Ren. Nutr. Off. J. Counc. Ren. Nutr. Natl. Kidney Found. 2017, 27, 233–242. [Google Scholar] [CrossRef]
- Oosterwijk, M.M.; Soedamah-Muthu, S.S.; Geleijnse, J.M.; Bakker, S.J.L.; Navis, G.; Binnenmars, S.H.; Gant, C.M.; Laverman, G.D. High Dietary Intake of Vegetable Protein Is Associated with Lower Prevalence of Renal Function Impairment: Results of the Dutch DIALECT-1 Cohort. Kidney Int. Rep. 2019, 4, 710–719. [Google Scholar] [CrossRef] [Green Version]
- Vernooij, R.W.M.; Zeraatkar, D.; Han, M.A.; El Dib, R.; Zworth, M.; Milio, K.; Sit, D.; Lee, Y.; Gomaa, H.; Valli, C.; et al. Patterns of Red and Processed Meat Consumption and Risk for Cardiometabolic and Cancer Outcomes: A Systematic Review and Meta-Analysis of Cohort Studies. Ann. Intern. Med. 2019, 171, 732–741. [Google Scholar] [CrossRef] [Green Version]
- Kalantar-Zadeh, K.; Joshi, S.; Schlueter, R.; Cooke, J.; Brown-Tortorici, A.; Donnelly, M.; Schulman, S.; Lau, W.-L.; Rhee, C.M.; Streja, E.; et al. Plant-Dominant Low-Protein Diet for Conservative Management of Chronic Kidney Disease. Nutrients 2020, 12, 1931. [Google Scholar] [CrossRef]
- Kelly, J.T.; Palmer, S.C.; Wai, S.N.; Ruospo, M.; Carrero, J.-J.; Campbell, K.L.; Strippoli, G.F.M. Healthy Dietary Patterns and Risk of Mortality and ESRD in CKD: A Meta-Analysis of Cohort Studies. Clin. J. Am. Soc. Nephrol. 2017, 12, 272–279. [Google Scholar] [CrossRef]
- Kamper, A.-L.; Strandgaard, S. Long-Term Effects of High-Protein Diets on Renal Function. Annu. Rev. Nutr. 2017, 37, 347–369. [Google Scholar] [CrossRef]
- Lin, J.; Hu, F.B.; Curhan, G.C. Associations of Diet with Albuminuria and Kidney Function Decline. Clin. J. Am. Soc. Nephrol. CJASN 2010, 5, 836–843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gutiérrez, O.M.; Muntner, P.; Rizk, D.V.; McClellan, W.M.; Warnock, D.G.; Newby, P.K.; Judd, S.E. Dietary Patterns and Risk of Death and Progression to ESRD in Individuals with CKD: A Cohort Study. Am. J. Kidney Dis. Off. J. Natl. Kidney Found. 2014, 64, 204–213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, X.; Wei, G.; Jalili, T.; Metos, J.; Giri, A.; Cho, M.E.; Boucher, R.; Greene, T.; Beddhu, S. The Associations of Plant Protein Intake with All-Cause Mortality in CKD. Am. J. Kidney Dis. Off. J. Natl. Kidney Found. 2016, 67, 423–430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herber-Gast, G.-C.M.; Boersma, M.; Verschuren, W.M.M.; Stehouwer, C.D.A.; Gansevoort, R.T.; Bakker, S.J.L.; Spijkerman, A.M.W. Consumption of Whole Grains, Fruit and Vegetables Is Not Associated with Indices of Renal Function in the Population-Based Longitudinal Doetinchem Study. Br. J. Nutr. 2017, 118, 375–382. [Google Scholar] [CrossRef] [Green Version]
- Asghari, G.; Momenan, M.; Yuzbashian, E.; Mirmiran, P.; Azizi, F. Dietary Pattern and Incidence of Chronic Kidney Disease among Adults: A Population-Based Study. Nutr. Metab. 2018, 15. [Google Scholar] [CrossRef]
- Liu, H.-W.; Tsai, W.-H.; Liu, J.-S.; Kuo, K.-L. Association of Vegetarian Diet with Chronic Kidney Disease. Nutrients 2019, 11, 279. [Google Scholar] [CrossRef] [Green Version]
- Jhee, J.H.; Kee, Y.K.; Park, J.T.; Chang, T.-I.; Kang, E.W.; Yoo, T.-H.; Kang, S.-W.; Han, S.H. A Diet Rich in Vegetables and Fruit and Incident CKD: A Community-Based Prospective Cohort Study. Am. J. Kidney Dis. Off. J. Natl. Kidney Found. 2019, 74, 491–500. [Google Scholar] [CrossRef]
- Kim, H.; Caulfield, L.E.; Garcia-Larsen, V.; Steffen, L.M.; Grams, M.E.; Coresh, J.; Rebholz, C.M. Plant-Based Diets and Incident CKD and Kidney Function. Clin. J. Am. Soc. Nephrol. CJASN 2019, 14, 682–691. [Google Scholar] [CrossRef] [Green Version]
- Saglimbene, V.M.; Wong, G.; Ruospo, M.; Palmer, S.C.; Garcia-Larsen, V.; Natale, P.; Teixeira-Pinto, A.; Campbell, K.L.; Carrero, J.-J.; Stenvinkel, P.; et al. Fruit and Vegetable Intake and Mortality in Adults Undergoing Maintenance Hemodialysis. Clin. J. Am. Soc. Nephrol. CJASN 2019, 14, 250–260. [Google Scholar] [CrossRef] [Green Version]
- Fanti, P.; Asmis, R.; Stephenson, T.J.; Sawaya, B.P.; Franke, A.A. Positive Effect of Dietary Soy in ESRD Patients with Systemic Inflammation—Correlation between Blood Levels of the Soy Isoflavones and the Acute-Phase Reactants. Nephrol. Dial. Transplant. 2006, 21, 2239–2246. [Google Scholar] [CrossRef] [Green Version]
- Soroka, N.; Silverberg, D.S.; Greemland, M.; Birk, Y.; Blum, M.; Peer, G.; Iaina, A. Comparison of a Vegetable-Based (Soya) and an Animal-Based Low-Protein Diet in Predialysis Chronic Renal Failure Patients. Nephron 1998, 79, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Tabibi, H.; Imani, H.; Hedayati, M.; Atabak, S.; Rahmani, L. Effects of Soy Consumption on Serum Lipids and Apoproteins in Peritoneal Dialysis Patients: A Randomized Controlled Trial: Perit. Dial. Int. 2009. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moe, S.M.; Zidehsarai, M.P.; Chambers, M.A.; Jackman, L.A.; Radcliffe, J.S.; Trevino, L.L.; Donahue, S.E.; Asplin, J.R. Vegetarian Compared with Meat Dietary Protein Source and Phosphorus Homeostasis in Chronic Kidney Disease. Clin. J. Am. Soc. Nephrol. CJASN 2011, 6, 257–264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goraya, N.; Simoni, J.; Jo, C.-H.; Wesson, D.E. A Comparison of Treating Metabolic Acidosis in CKD Stage 4 Hypertensive Kidney Disease with Fruits and Vegetables or Sodium Bicarbonate. Clin. J. Am. Soc. Nephrol. CJASN 2013, 8, 371–381. [Google Scholar] [CrossRef] [PubMed]
- Goraya, N.; Simoni, J.; Jo, C.-H.; Wesson, D.E. Treatment of Metabolic Acidosis in Patients with Stage 3 Chronic Kidney Disease with Fruits and Vegetables or Oral Bicarbonate Reduces Urine Angiotensinogen and Preserves Glomerular Filtration Rate. Kidney Int. 2014, 86, 1031–1038. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goraya, N.; Munoz-Maldonado, Y.; Simoni, J.; Wesson, D.E. Fruit and Vegetable Treatment of Chronic Kidney Disease-Related Metabolic Acidosis Reduces Cardiovascular Risk Better than Sodium Bicarbonate. Am. J. Nephrol. 2019, 49, 438–448. [Google Scholar] [CrossRef] [PubMed]
- Kontessis, P.; Jones, S.; Dodds, R.; Trevisan, R.; Nosadini, R.; Fioretto, P.; Borsato, M.; Sacerdoti, D.; Viberti, G. Renal, Metabolic and Hormonal Responses to Ingestion of Animal and Vegetable Proteins. Kidney Int. 1990, 38, 136–144. [Google Scholar] [CrossRef] [Green Version]
- Iwasaki, K.; Gleiser, C.A.; Masoro, E.J.; McMahan, C.A.; Seo, E.J.; Yu, B.P. The Influence of Dietary Protein Source on Longevity and Age-Related Disease Processes of Fischer Rats. J. Gerontol. 1988, 43, B5–B12. [Google Scholar] [CrossRef]
- Bozzetto, L.; Costabile, G.; Della Pepa, G.; Ciciola, P.; Vetrani, C.; Vitale, M.; Rivellese, A.A.; Annuzzi, G. Dietary Fibre as a Unifying Remedy for the Whole Spectrum of Obesity-Associated Cardiovascular Risk. Nutrients 2018, 10, 943. [Google Scholar] [CrossRef] [Green Version]
- Anderson, J.W.; O’Neal, D.S.; Riddell-Mason, S.; Floore, T.L.; Dillon, D.W.; Oeltgen, P.R. Postprandial Serum Glucose, Insulin, and Lipoprotein Responses to High- and Low-Fiber Diets. Metabolism 1995, 44, 848–854. [Google Scholar] [CrossRef]
- Ferdowsian, H.R.; Barnard, N.D. Effects of Plant-Based Diets on Plasma Lipids. Am. J. Cardiol. 2009, 104, 947–956. [Google Scholar] [CrossRef] [PubMed]
- Austel, A.; Ranke, C.; Wagner, N.; Görge, J.; Ellrott, T. Weight Loss with a Modified Mediterranean-Type Diet Using Fat Modification: A Randomized Controlled Trial. Eur. J. Clin. Nutr. 2015, 69, 878–884. [Google Scholar] [CrossRef]
- Goraya, N.; Simoni, J.; Jo, C.; Wesson, D.E. Dietary Acid Reduction with Fruits and Vegetables or Bicarbonate Attenuates Kidney Injury in Patients with a Moderately Reduced Glomerular Filtration Rate Due to Hypertensive Nephropathy. Kidney Int. 2012, 81, 86–93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wesson, D.E.; Simoni, J. Acid Retention during Kidney Failure Induces Endothelin and Aldosterone Production Which Lead to Progressive GFR Decline, a Situation Ameliorated by Alkali Diet. Kidney Int. 2010, 78, 1128–1135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wesson, D.E. Endogenous Endothelins Mediate Increased Acidification in Remnant Kidneys. J. Am. Soc. Nephrol. JASN 2001, 12, 1826–1835. [Google Scholar] [PubMed]
- Sacks, F.M.; Svetkey, L.P.; Vollmer, W.M.; Appel, L.J.; Bray, G.A.; Harsha, D.; Obarzanek, E.; Conlin, P.R.; Miller, E.R.; Simons-Morton, D.G.; et al. Effects on Blood Pressure of Reduced Dietary Sodium and the Dietary Approaches to Stop Hypertension (DASH) Diet. DASH-Sodium Collaborative Research Group. N. Engl. J. Med. 2001, 344, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Moorthi, R.N.; Armstrong, C.L.H.; Janda, K.; Ponsler-Sipes, K.; Asplin, J.R.; Moe, S.M. The Effect of a Diet Containing 70% Protein from Plants on Mineral Metabolism and Musculoskeletal Health in Chronic Kidney Disease. Am. J. Nephrol. 2014, 40, 582–591. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, H.; Huang, X.; Risérus, U.; Krishnamurthy, V.M.; Cederholm, T.; Ärnlöv, J.; Lindholm, B.; Sjögren, P.; Carrero, J.J. Dietary Fiber, Kidney Function, Inflammation, and Mortality Risk. Clin. J. Am. Soc. Nephrol. CJASN 2014, 9, 2104–2110. [Google Scholar] [CrossRef]
- Krishnamurthy, V.M.R.; Wei, G.; Baird, B.C.; Murtaugh, M.; Chonchol, M.B.; Raphael, K.L.; Greene, T.; Beddhu, S. High Dietary Fiber Intake Is Associated with Decreased Inflammation and All-Cause Mortality in Patients with Chronic Kidney Disease. Kidney Int. 2012, 81, 300–306. [Google Scholar] [CrossRef] [Green Version]
- Garneata, L.; Stancu, A.; Dragomir, D.; Stefan, G.; Mircescu, G. Ketoanalogue-Supplemented Vegetarian Very Low-Protein Diet and CKD Progression. J. Am. Soc. Nephrol. JASN 2016, 27, 2164–2176. [Google Scholar] [CrossRef] [Green Version]
- Hahn, D.; Hodson, E.M.; Fouque, D. Low Protein Diets for Non-Diabetic Adults with Chronic Kidney Disease. Cochrane Database Syst. Rev. 2018, 10, CD001892. [Google Scholar] [CrossRef] [PubMed]
- Poesen, R.; Mutsaers, H.A.M.; Windey, K.; van den Broek, P.H.; Verweij, V.; Augustijns, P.; Kuypers, D.; Jansen, J.; Evenepoel, P.; Verbeke, K.; et al. The Influence of Dietary Protein Intake on Mammalian Tryptophan and Phenolic Metabolites. PLOS ONE 2015, 10, e0140820. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patel, K.P.; Luo, F.J.-G.; Plummer, N.S.; Hostetter, T.H.; Meyer, T.W. The Production of P-Cresol Sulfate and Indoxyl Sulfate in Vegetarians versus Omnivores. Clin. J. Am. Soc. Nephrol. CJASN 2012, 7, 982–988. [Google Scholar] [CrossRef] [PubMed]
- Marzocco, S.; Dal Piaz, F.; Di Micco, L.; Torraca, S.; Sirico, M.L.; Tartaglia, D.; Autore, G.; Di Iorio, B. Very Low Protein Diet Reduces Indoxyl Sulfate Levels in Chronic Kidney Disease. Blood Purif. 2013, 35, 196–201. [Google Scholar] [CrossRef]
- Di Iorio, B.R.; Marzocco, S.; Bellasi, A.; De Simone, E.; Dal Piaz, F.; Rocchetti, M.T.; Cosola, C.; Di Micco, L.; Gesualdo, L. Nutritional Therapy Reduces Protein Carbamylation through Urea Lowering in Chronic Kidney Disease. Nephrol. Dial. Transplant. Off. Publ. Eur. Dial. Transpl. Assoc. Eur. Ren. Assoc. 2018, 33, 804–813. [Google Scholar] [CrossRef]
- Di Iorio, B.R.; Rocchetti, M.T.; De Angelis, M.; Cosola, C.; Marzocco, S.; Di Micco, L.; di Bari, I.; Accetturo, M.; Vacca, M.; Gobbetti, M.; et al. Nutritional Therapy Modulates Intestinal Microbiota and Reduces Serum Levels of Total and Free Indoxyl Sulfate and P-Cresyl Sulfate in Chronic Kidney Disease (Medika Study). J. Clin. Med. 2019, 8, 1424. [Google Scholar] [CrossRef] [Green Version]
- Sirich, T.L.; Plummer, N.S.; Gardner, C.D.; Hostetter, T.H.; Meyer, T.W. Effect of Increasing Dietary Fiber on Plasma Levels of Colon-Derived Solutes in Hemodialysis Patients. Clin. J. Am. Soc. Nephrol. CJASN 2014, 9, 1603–1610. [Google Scholar] [CrossRef] [Green Version]
- Xie, L.-M.; Ge, Y.-Y.; Huang, X.; Zhang, Y.-Q.; Li, J.-X. Effects of Fermentable Dietary Fiber Supplementation on Oxidative and Inflammatory Status in Hemodialysis Patients. Int. J. Clin. Exp. Med. 2015, 8, 1363–1369. [Google Scholar]
- Xu, H.; Rossi, M.; Campbell, K.L.; Sencion, G.L.; Ärnlöv, J.; Cederholm, T.; Sjögren, P.; Risérus, U.; Lindholm, B.; Carrero, J.J. Excess Protein Intake Relative to Fiber and Cardiovascular Events in Elderly Men with Chronic Kidney Disease. Nutr. Metab. Cardiovasc. Dis. NMCD 2016, 26, 597–602. [Google Scholar] [CrossRef] [Green Version]
- Rossi, M.; Johnson, D.W.; Xu, H.; Carrero, J.J.; Pascoe, E.; French, C.; Campbell, K.L. Dietary Protein-Fiber Ratio Associates with Circulating Levels of Indoxyl Sulfate and p-Cresyl Sulfate in Chronic Kidney Disease Patients. Nutr. Metab. Cardiovasc. Dis. NMCD 2015, 25, 860–865. [Google Scholar] [CrossRef]
- Wu, G.D.; Chen, J.; Hoffmann, C.; Bittinger, K.; Chen, Y.-Y.; Keilbaugh, S.A.; Bewtra, M.; Knights, D.; Walters, W.A.; Knight, R.; et al. Linking Long-Term Dietary Patterns with Gut Microbial Enterotypes. Science 2011, 334, 105–108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garibotto, G.; Sofia, A.; Parodi, E.L.; Ansaldo, F.; Bonanni, A.; Picciotto, D.; Signori, A.; Vettore, M.; Tessari, P.; Verzola, D. Effects of Low-Protein, and Supplemented Very Low–Protein Diets, on Muscle Protein Turnover in Patients With CKD. Kidney Int. Rep. 2018, 3, 701–710. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chauveau, P.; Combe, C.; Fouque, D.; Aparicio, M. Vegetarianism: Advantages and Drawbacks in Patients with Chronic Kidney Diseases. J. Ren. Nutr. Off. J. Counc. Ren. Nutr. Natl. Kidney Found. 2013, 23, 399–405. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.-T.; Chang, C.-Y.; Hsu, W.-M.; Wang, I.-K.; Hsu, C.-H.; Cheng, S.-H.; Liang, C.-C.; Chang, C.-T.; Huang, C.-C. Nutritional Status of Vegetarians on Maintenance Haemodialysis. Nephrol. Carlton Vic 2011, 16, 582–587. [Google Scholar] [CrossRef] [PubMed]
- Weaver, C.M. Calcium Bioavailability and Its Relation to Osteoporosis. Proc. Soc. Exp. Biol. Med. Soc. Exp. Biol. Med. N. Y. N 1992, 200, 157–160. [Google Scholar] [CrossRef] [PubMed]
- Weaver, C.M.; Plawecki, K.L. Dietary Calcium: Adequacy of a Vegetarian Diet. Am. J. Clin. Nutr. 1994, 59, 1238S–1241S. [Google Scholar] [CrossRef]
- Shams-White, M.M.; Chung, M.; Du, M.; Fu, Z.; Insogna, K.L.; Karlsen, M.C.; LeBoff, M.S.; Shapses, S.A.; Sackey, J.; Wallace, T.C.; et al. Dietary Protein and Bone Health: A Systematic Review and Meta-Analysis from the National Osteoporosis Foundation. Am. J. Clin. Nutr. 2017, 105, 1528–1543. [Google Scholar] [CrossRef] [Green Version]
- Shams-White, M.M.; Chung, M.; Fu, Z.; Insogna, K.L.; Karlsen, M.C.; LeBoff, M.S.; Shapses, S.A.; Sackey, J.; Shi, J.; Wallace, T.C.; et al. Animal versus Plant Protein and Adult Bone Health: A Systematic Review and Meta-Analysis from the National Osteoporosis Foundation. PloS ONE 2018, 13, e0192459. [Google Scholar] [CrossRef] [Green Version]
- Movassagh, E.Z.; Baxter-Jones, A.D.G.; Kontulainen, S.; Whiting, S.; Szafron, M.; Vatanparast, H. Vegetarian-Style Dietary Pattern during Adolescence Has Long-Term Positive Impact on Bone from Adolescence to Young Adulthood: A Longitudinal Study. Nutr. J. 2018, 17, 36. [Google Scholar] [CrossRef] [Green Version]
- Berg, J.; Seyedsadjadi, N.; Grant, R. Increased Consumption of Plant Foods Is Associated with Increased Bone Mineral Density. J. Nutr. Health Aging 2020, 24, 388–397. [Google Scholar] [CrossRef]
- Hu, D.; Cheng, L.; Jiang, W. Fruit and Vegetable Consumption and the Risk of Postmenopausal Osteoporosis: A Meta-Analysis of Observational Studies. Food Funct. 2018, 9, 2607–2616. [Google Scholar] [CrossRef] [PubMed]
- Hsu, E. Plant-Based Diets and Bone Health: Sorting through the Evidence. Curr. Opin. Endocrinol. Diabetes Obes. 2020, 27, 248–252. [Google Scholar] [CrossRef] [PubMed]
- Kieneker, L.M.; Bakker, S.J.L.; de Boer, R.A.; Navis, G.J.; Gansevoort, R.T.; Joosten, M.M. Low Potassium Excretion but Not High Sodium Excretion Is Associated with Increased Risk of Developing Chronic Kidney Disease. Kidney Int. 2016, 90, 888–896. [Google Scholar] [CrossRef] [PubMed]
- St-Jules, D.E.; Goldfarb, D.S.; Sevick, M.A. Nutrient Non-Equivalence: Does Restricting High-Potassium Plant Foods Help to Prevent Hyperkalemia in Hemodialysis Patients? J. Ren. Nutr. Off. J. Counc. Ren. Nutr. Natl. Kidney Found. 2016, 26, 282–287. [Google Scholar] [CrossRef] [Green Version]
- Chiu, S.; Bergeron, N.; Williams, P.T.; Bray, G.A.; Sutherland, B.; Krauss, R.M. Comparison of the DASH (Dietary Approaches to Stop Hypertension) Diet and a Higher-Fat DASH Diet on Blood Pressure and Lipids and Lipoproteins: A Randomized Controlled Trial123. Am. J. Clin. Nutr. 2016, 103, 341–347. [Google Scholar] [CrossRef] [Green Version]
- Aburto, N.J.; Hanson, S.; Gutierrez, H.; Hooper, L.; Elliott, P.; Cappuccio, F.P. Effect of Increased Potassium Intake on Cardiovascular Risk Factors and Disease: Systematic Review and Meta-Analyses. BMJ 2013, 346, f1378. [Google Scholar] [CrossRef] [Green Version]
- Smyth, A.; Dunkler, D.; Gao, P.; Teo, K.K.; Yusuf, S.; O’Donnell, M.J.; Mann, J.F.E.; Clase, C.M. ONTARGET and TRANSCEND investigators The Relationship between Estimated Sodium and Potassium Excretion and Subsequent Renal Outcomes. Kidney Int. 2014, 86, 1205–1212. [Google Scholar] [CrossRef] [Green Version]
- Nagata, T.; Sobajima, H.; Ohashi, N.; Hirakawa, A.; Katsuno, T.; Yasuda, Y.; Matsuo, S.; Tsuboi, N.; Maruyama, S. Association between 24h Urinary Sodium and Potassium Excretion and Estimated Glomerular Filtration Rate (EGFR) Decline or Death in Patients with Diabetes Mellitus and EGFR More than 30 Ml/Min/1.73m2. PloS ONE 2016, 11, e0152306. [Google Scholar] [CrossRef]
- Tyson, C.C.; Lin, P.-H.; Corsino, L.; Batch, B.C.; Allen, J.; Sapp, S.; Barnhart, H.; Nwankwo, C.; Burroughs, J.; Svetkey, L.P. Short-Term Effects of the DASH Diet in Adults with Moderate Chronic Kidney Disease: A Pilot Feeding Study. Clin. Kidney J. 2016, 9, 592–598. [Google Scholar] [CrossRef] [Green Version]
- Barsotti, G.; Morelli, E.; Cupisti, A.; Meola, M.; Dani, L.; Giovannetti, S. A Low-Nitrogen Low-Phosphorus Vegan Diet for Patients with Chronic Renal Failure. Nephron 1996, 74, 390–394. [Google Scholar] [CrossRef]
- Cupisti, A.; Kovesdy, C.P.; D’Alessandro, C.; Kalantar-Zadeh, K. Dietary Approach to Recurrent or Chronic Hyperkalaemia in Patients with Decreased Kidney Function. Nutrients 2018, 10, 261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Picard, K.; Griffiths, M.; Mager, D.R.; Richard, C. Handouts for Low-Potassium Diets Disproportionately Restrict Fruits and Vegetables. J. Ren. Nutr. Off. J. Counc. Ren. Nutr. Natl. Kidney Found. 2020. [Google Scholar] [CrossRef] [PubMed]
- Potassium Supplementation in CKD-Full Text View-ClinicalTrials. Available online: https://clinicaltrials.gov/ct2/show/NCT03253172 (accessed on 21 September 2020).
- Koppe, L.; Fouque, D. The Role for Protein Restriction in Addition to Renin-Angiotensin-Aldosterone System Inhibitors in the Management of CKD. Am. J. Kidney Dis. Off. J. Natl. Kidney Found. 2019, 73, 248–257. [Google Scholar] [CrossRef] [PubMed]
- Treviño-Villarreal, J.H.; Reynolds, J.S.; Bartelt, A.; Langston, P.K.; MacArthur, M.R.; Arduini, A.; Tosti, V.; Veronese, N.; Bertozzi, B.; Brace, L.E.; et al. Dietary Protein Restriction Reduces Circulating VLDL Triglyceride Levels via CREBH-APOA5-Dependent and -Independent Mechanisms. JCI Insight 2018, 3. [Google Scholar] [CrossRef]
- Maida, A.; Zota, A.; Sjøberg, K.A.; Schumacher, J.; Sijmonsma, T.P.; Pfenninger, A.; Christensen, M.M.; Gantert, T.; Fuhrmeister, J.; Rothermel, U.; et al. A Liver Stress-Endocrine Nexus Promotes Metabolic Integrity during Dietary Protein Dilution. J. Clin. Invest. 2016, 126, 3263–3278. [Google Scholar] [CrossRef] [Green Version]
- Gorissen, S.H.M.; Crombag, J.J.R.; Senden, J.M.G.; Waterval, W.A.H.; Bierau, J.; Verdijk, L.B.; van Loon, L.J.C. Protein Content and Amino Acid Composition of Commercially Available Plant-Based Protein Isolates. Amino Acids 2018, 50, 1685–1695. [Google Scholar] [CrossRef] [Green Version]
- Millward, D.J. Identifying Recommended Dietary Allowances for Protein and Amino Acids: A Critique of the 2007 WHO/FAO/UNU Report. Br. J. Nutr. 2012, 108 (Suppl. 2), S3–S21. [Google Scholar] [CrossRef] [Green Version]
- Millward, D.J. The Nutritional Value of Plant-Based Diets in Relation to Human Amino Acid and Protein Requirements. Proc. Nutr. Soc. 1999, 58, 249–260. [Google Scholar] [CrossRef]
- Young, V.R.; Pellett, P.L. Plant Proteins in Relation to Human Protein and Amino Acid Nutrition. Am. J. Clin. Nutr. 1994, 59, 1203S–1212S. [Google Scholar] [CrossRef]
- Schmidt, J.A.; Rinaldi, S.; Scalbert, A.; Ferrari, P.; Achaintre, D.; Gunter, M.J.; Appleby, P.N.; Key, T.J.; Travis, R.C. Plasma Concentrations and Intakes of Amino Acids in Male Meat-Eaters, Fish-Eaters, Vegetarians and Vegans: A Cross-Sectional Analysis in the EPIC-Oxford Cohort. Eur. J. Clin. Nutr. 2016, 70, 306–312. [Google Scholar] [CrossRef] [Green Version]
- Guebre-Egziabher, F.; Juillard, L.; Boirie, Y.; Laville, M.; Beaufrère, B.; Fouque, D. Short-Term Administration of a Combination of Recombinant Growth Hormone and Insulin-like Growth Factor-I Induces Anabolism in Maintenance Hemodialysis. J. Clin. Endocrinol. Metab. 2009, 94, 2299–2305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lim, V.S.; Ikizler, T.A.; Raj, D.S.C.; Flanigan, M.J. Does Hemodialysis Increase Protein Breakdown? Dissociation between Whole-Body Amino Acid Turnover and Regional Muscle Kinetics. J. Am. Soc. Nephrol. JASN 2005, 16, 862–868. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kontessis, P.A.; Bossinakou, I.; Sarika, L.; Iliopoulou, E.; Papantoniou, A.; Trevisan, R.; Roussi, D.; Stipsanelli, K.; Grigorakis, S.; Souvatzoglou, A. Renal, Metabolic, and Hormonal Responses to Proteins of Different Origin in Normotensive, Nonproteinuric Type I Diabetic Patients. Diabetes Care 1995, 18, 1233. [Google Scholar] [CrossRef] [PubMed]
- Claris-Appiani, A.; Assael, B.M.; Tirelli, A.S.; Marra, G.; Cavanna, G. Lack of Glomerular Hemodynamic Stimulation after Infusion of Branched-Chain Amino Acids. Kidney Int. 1988, 33, 91–94. [Google Scholar] [CrossRef] [Green Version]
- Pillai, S.M.; Herzog, B.; Seebeck, P.; Pellegrini, G.; Roth, E.; Verrey, F. Differential Impact of Dietary Branched Chain and Aromatic Amino Acids on Chronic Kidney Disease Progression in Rats. Front. Physiol. 2019, 10. [Google Scholar] [CrossRef] [Green Version]
- Gao, X.; Huang, L.; Grosjean, F.; Esposito, V.; Wu, J.; Fu, L.; Hu, H.; Tan, J.; He, C.; Gray, S.; et al. Low-Protein Diet Supplemented with Ketoacids Reduces the Severity of Renal Disease in 5/6 Nephrectomized Rats: A Role for KLF15. Kidney Int. 2011, 79, 987–996. [Google Scholar] [CrossRef] [Green Version]
- Barba, C.; Soulage, C.; Glorieux, G.; Picard, C.; Fouque, D.; Koppe, L. P0922A Low Aromatic Amino-Acid Diet Improves Renal Function and Prevents Kidney Fibrosis iN Mice with Chronic Kidney Disease. Nephrol. Dial. Transplant. 2020, 35. [Google Scholar] [CrossRef]
- de Brito, J.S.; Borges, N.A.; Dolenga, C.J.R.; Carraro-Eduardo, J.C.; Nakao, L.S.; Mafra, D. Is there a relationship between tryptophan dietary intake and plasma levels of indoxyl sulfate in chronic kidney disease patients on hemodialysis? J. Bras. Nefrol. Orgao Of. Soc. Bras. E Lat.-Am. Nefrol. 2016, 38, 396–402. [Google Scholar] [CrossRef]
- Wikoff, W.R.; Anfora, A.T.; Liu, J.; Schultz, P.G.; Lesley, S.A.; Peters, E.C.; Siuzdak, G. Metabolomics Analysis Reveals Large Effects of Gut Microflora on Mammalian Blood Metabolites. Proc. Natl. Acad. Sci. USA 2009, 106, 3698–3703. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Yang, S.; Li, S.; Zhao, L.; Hao, Y.; Qin, J.; Zhang, L.; Zhang, C.; Bian, W.; Zuo, L.; et al. Aberrant Gut Microbiota Alters Host Metabolome and Impacts Renal Failure in Humans and Rodents. Gut 2020. [Google Scholar] [CrossRef]
- Magee, E.A.; Richardson, C.J.; Hughes, R.; Cummings, J.H. Contribution of Dietary Protein to Sulfide Production in the Large Intestine: An in Vitro and a Controlled Feeding Study in Humans. Am. J. Clin. Nutr. 2000, 72, 1488–1494. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thielemann, L.E.; Oberhauser, E.W.; Rosenblut, G.; Videla, L.A.; Valenzuela, A. Sulfur-Containing Amino Acids That Increase Renal Glutathione Protect the Kidney against Papillary Necrosis Induced by 2-Bromoethylamine. Cell Biochem. Funct. 1990, 8, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Trachtman, H.; Sturman, J.A. Taurine: A Therapeutic Agent in Experimental Kidney Disease. Amino Acids 1996, 11, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Lobel, L.; Cao, Y.G.; Fenn, K.; Glickman, J.N.; Garrett, W.S. Diet Posttranslationally Modifies the Mouse Gut Microbial Proteome to Modulate Renal Function. Science 2020, 369, 1518–1524. [Google Scholar] [CrossRef] [PubMed]
- Koppe, L.; Nyam, E.; Vivot, K.; Manning Fox, J.E.; Dai, X.-Q.; Nguyen, B.N.; Trudel, D.; Attané, C.; Moullé, V.S.; MacDonald, P.E.; et al. Urea Impairs β Cell Glycolysis and Insulin Secretion in Chronic Kidney Disease. J. Clin. Investig. 2016, 126, 3598–3612. [Google Scholar] [CrossRef] [Green Version]
- Desai, M.S.; Seekatz, A.M.; Koropatkin, N.M.; Kamada, N.; Hickey, C.A.; Wolter, M.; Pudlo, N.A.; Kitamoto, S.; Terrapon, N.; Muller, A.; et al. A Dietary Fiber-Deprived Gut Microbiota Degrades the Colonic Mucus Barrier and Enhances Pathogen Susceptibility. Cell 2016, 167, 1339–1353.e21. [Google Scholar] [CrossRef] [Green Version]
- Fletcher, C.M.; Coyne, M.J.; Villa, O.F.; Chatzidaki-Livanis, M.; Comstock, L.E. A General O-Glycosylation System Important to the Physiology of a Major Human Intestinal Symbiont. Cell 2009, 137, 321–331. [Google Scholar] [CrossRef] [Green Version]
- Mafra, D.; Borges, N.A.; Lindholm, B.; Shiels, P.G.; Evenepoel, P.; Stenvinkel, P. Food as Medicine: Targeting the Uraemic Phenotype in Chronic Kidney Disease. Nat. Rev. Nephrol. 2020. [Google Scholar] [CrossRef]
- Tang, W.H.W.; Wang, Z.; Levison, B.S.; Koeth, R.A.; Britt, E.B.; Fu, X.; Wu, Y.; Hazen, S.L. Intestinal Microbial Metabolism of Phosphatidylcholine and Cardiovascular Risk. N. Engl. J. Med. 2013, 368, 1575–1584. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Roberts, A.B.; Buffa, J.A.; Levison, B.S.; Zhu, W.; Org, E.; Gu, X.; Huang, Y.; Zamanian-Daryoush, M.; Culley, M.K.; et al. Non-Lethal Inhibition of Gut Microbial Trimethylamine Production for the Treatment of Atherosclerosis. Cell 2015, 163, 1585–1595. [Google Scholar] [CrossRef] [Green Version]
- Tang, W.H.W.; Wang, Z.; Kennedy, D.J.; Wu, Y.; Buffa, J.A.; Agatisa-Boyle, B.; Li, X.S.; Levison, B.S.; Hazen, S.L. Gut Microbiota-Dependent Trimethylamine N-Oxide (TMAO) Pathway Contributes to Both Development of Renal Insufficiency and Mortality Risk in Chronic Kidney Disease. Circ. Res. 2015, 116, 448–455. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ye, J.-Z.; Li, Y.-T.; Wu, W.-R.; Shi, D.; Fang, D.-Q.; Yang, L.-Y.; Bian, X.-Y.; Wu, J.-J.; Wang, Q.; Jiang, X.-W.; et al. Dynamic Alterations in the Gut Microbiota and Metabolome during the Development of Methionine-Choline-Deficient Diet-Induced Nonalcoholic Steatohepatitis. World J. Gastroenterol. 2018, 24, 2468–2481. [Google Scholar] [CrossRef] [PubMed]
- DiNicolantonio, J.J.; Lavie, C.J.; Fares, H.; Menezes, A.R.; O’Keefe, J.H. L-Carnitine in the Secondary Prevention of Cardiovascular Disease: Systematic Review and Meta-Analysis. Mayo Clin. Proc. 2013, 88, 544–551. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Enoki, Y.; Watanabe, H.; Arake, R.; Fujimura, R.; Ishiodori, K.; Imafuku, T.; Nishida, K.; Sugimoto, R.; Nagao, S.; Miyamura, S.; et al. Potential Therapeutic Interventions for Chronic Kidney Disease-Associated Sarcopenia via Indoxyl Sulfate-Induced Mitochondrial Dysfunction. J. Cachexia Sarcopenia Muscle 2017. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Bergeron, N.; Levison, B.S.; Li, X.S.; Chiu, S.; Jia, X.; Koeth, R.A.; Li, L.; Wu, Y.; Tang, W.H.W.; et al. Impact of Chronic Dietary Red Meat, White Meat, or Non-Meat Protein on Trimethylamine N-Oxide Metabolism and Renal Excretion in Healthy Men and Women. Eur. Heart J. 2019, 40, 583–594. [Google Scholar] [CrossRef] [PubMed]
- Koeth, R.A.; Wang, Z.; Levison, B.S.; Buffa, J.A.; Org, E.; Sheehy, B.T.; Britt, E.B.; Fu, X.; Wu, Y.; Li, L.; et al. Intestinal Microbiota Metabolism of L-Carnitine, a Nutrient in Red Meat, Promotes Atherosclerosis. Nat. Med. 2013, 19, 576–585. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kandouz, S.; Mohamed, A.S.; Zheng, Y.; Sandeman, S.; Davenport, A. Reduced Protein Bound Uraemic Toxins in Vegetarian Kidney Failure Patients Treated by Haemodiafiltration. Hemodial. Int. Int. Symp. Home Hemodial. 2016, 20, 610–617. [Google Scholar] [CrossRef] [Green Version]
- Smits, L.P.; Kootte, R.S.; Levin, E.; Prodan, A.; Fuentes, S.; Zoetendal, E.G.; Wang, Z.; Levison, B.S.; Cleophas, M.C.P.; Kemper, E.M.; et al. Effect of Vegan Fecal Microbiota Transplantation on Carnitine- and Choline-Derived Trimethylamine-N-Oxide Production and Vascular Inflammation in Patients With Metabolic Syndrome. J. Am. Heart Assoc. 2018, 7. [Google Scholar] [CrossRef]
- Arias, N.; Arboleya, S.; Allison, J.; Kaliszewska, A.; Higarza, S.G.; Gueimonde, M.; Arias, J.L. The Relationship between Choline Bioavailability from Diet, Intestinal Microbiota Composition, and Its Modulation of Human Diseases. Nutrients 2020, 12, 2340. [Google Scholar] [CrossRef]
- Wu, Q.; Zhao, Y.; Zhang, X.; Yang, X. A Faster and Simpler UPLC-MS/MS Method for the Simultaneous Determination of Trimethylamine N-Oxide, Trimethylamine and Dimethylamine in Different Types of Biological Samples. Food Funct. 2019, 10, 6484–6491. [Google Scholar] [CrossRef]
- Jaworska, K.; Hering, D.; Mosieniak, G.; Bielak-Zmijewska, A.; Pilz, M.; Konwerski, M.; Gasecka, A.; Kapłon-Cieślicka, A.; Filipiak, K.; Sikora, E.; et al. TMA, A Forgotten Uremic Toxin, but Not TMAO, Is Involved in Cardiovascular Pathology. Toxins 2019, 11, 490. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rigalleau, V.; Combe, C.; Blanchetier, V.; Aubertin, J.; Aparicio, M.; Gin, H. Low Protein Diet in Uremia: Effects on Glucose Metabolism and Energy Production Rate. Kidney Int. 1997, 51, 1222–1227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, F.; Cavener, D.R. The GCN2 EIF2alpha Kinase Regulates Fatty-Acid Homeostasis in the Liver during Deprivation of an Essential Amino Acid. Cell Metab. 2007, 5, 103–114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pissios, P.; Hong, S.; Kennedy, A.R.; Prasad, D.; Liu, F.-F.; Maratos-Flier, E. Methionine and Choline Regulate the Metabolic Phenotype of a Ketogenic Diet. Mol. Metab. 2013, 2, 306–313. [Google Scholar] [CrossRef] [PubMed]
- Velmurugan, G.; Dinakaran, V.; Rajendhran, J.; Swaminathan, K. Blood Microbiota and Circulating Microbial Metabolites in Diabetes and Cardiovascular Disease. Trends Endocrinol. Metab. TEM 2020, 31, 835–847. [Google Scholar] [CrossRef] [PubMed]
- Fontana, L.; Cummings, N.E.; Arriola Apelo, S.I.; Neuman, J.C.; Kasza, I.; Schmidt, B.A.; Cava, E.; Spelta, F.; Tosti, V.; Syed, F.A.; et al. Decreased Consumption of Branched-Chain Amino Acids Improves Metabolic Health. Cell Rep. 2016, 16, 520–530. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.; Sood, S.; McIntire, K.; Roth, R.; Rabkin, R. Leucine-Stimulated MTOR Signaling Is Partly Attenuated in Skeletal Muscle of Chronically Uremic Rats. Am. J. Physiol. Endocrinol. Metab. 2011, 301, E873–E881. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fuchs, C.J.; Hermans, W.J.H.; Holwerda, A.M.; Smeets, J.S.J.; Senden, J.M.; van Kranenburg, J.; Gijsen, A.P.; Wodzig, W.K.H.W.; Schierbeek, H.; Verdijk, L.B.; et al. Branched-Chain Amino Acid and Branched-Chain Ketoacid Ingestion Increases Muscle Protein Synthesis Rates in Vivo in Older Adults: A Double-Blind, Randomized Trial. Am. J. Clin. Nutr. 2019, 110, 862–872. [Google Scholar] [CrossRef] [Green Version]
- Paddon-Jones, D.; Layman, D.K. Branched-Chain Ketoacid Ingestion: An Alternative to Efficiently Increase Skeletal Muscle Protein Synthesis. Am. J. Clin. Nutr. 2019, 110, 799–800. [Google Scholar] [CrossRef]
- Yap, Y.W.; Rusu, P.M.; Chan, A.Y.; Fam, B.C.; Jungmann, A.; Solon-Biet, S.M.; Barlow, C.K.; Creek, D.J.; Huang, C.; Schittenhelm, R.B.; et al. Restriction of Essential Amino Acids Dictates the Systemic Metabolic Response to Dietary Protein Dilution. Nat. Commun. 2020, 11, 2894. [Google Scholar] [CrossRef]
- Koppe, L.; Pillon, N.J.; Vella, R.E.; Croze, M.L.; Pelletier, C.C.; Chambert, S.; Massy, Z.; Glorieux, G.; Vanholder, R.; Dugenet, Y.; et al. P-Cresyl Sulfate Promotes Insulin Resistance Associated with CKD. J. Am. Soc. Nephrol. JASN 2013, 24, 88–99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Green, C.L.; Lamming, D.W. Regulation of Metabolic Health by Essential Dietary Amino Acids. Mech. Ageing Dev. 2019, 177, 186–200. [Google Scholar] [CrossRef] [PubMed]
| Study | Type | Intervention/Assessment | Population | Follow Up | Outcomes | Results/Comments |
|---|---|---|---|---|---|---|
| Lin et al. 2010 [21] | Observational | FFQ: Salt, animal fat, several nutrients | n = 3348 General population | 11 years | eGFR decline Microalbuminuria | ↗ animal fat consumption was associated with ↗ risk of microalbuminuria (OR, 1.51; 95% CI, 1.01 to 2.26) ↗ β-carotene intake appeared protective against eGFR decline (OR, 0.56; 95% CI, 0.40 to 0.78) |
| Gutiérrez et al. 2014 [22] | Observational | FFQ: Plant-Based pattern score | n = 3972 CKD G3-G5 | 6 years | All-cause mortality Kidney failure | ↘ risk of mortality with higher adherence of plant-based diet (HR, 0.77; 95% CI; 0.61 to 0.97, p < 0.05) No negative association between plant diet and risk of ESKD |
| Chen et al. 2016 [23] | Observational | Dietary interview: Plant protein–total protein ratio and total plant protein intake | n = 14,866 including 728 patients with CKD | 6.2 to 8.6 years | All-cause mortality | ↗ plant protein–total protein ratio is associated with a significant ↘ risk of death in CKD population with eGFR < 60 mL/min/1.73 m² (HR, 0.77; 95% CI, 0.61 to 0.96) |
| Haring et al. 2017 [15] | Observational | FFQ: Plant protein intake | n = 11,952 General population | 23 years | Incident CKD | ↘ risk of developing CKD with ↗ consumption of nuts (HR, 0.75; 95% CI, 0.65 to 0.85, p < 0.001), low-fat dairy products, or legumes (HR, 0.83; 95% CI, 0.72 to 0.95, p = 0.03) |
| Herber-Gast et al. 2017 [24] | Observational | FFQ: consumption of whole grains, fruit, and plants | n = 3787 General population | 15 years | eGFR decline Albuminuria creatinine ratio | No association on multivariate model of fruit and plant intakes with changes in renal function or albuminuria creatinine ratio |
| Asghari et al. 2018 [25] | Observational | FFQ: Lacto-vegetarian, traditional Iranian, and high fat, high sugar dietary pattern | n = 1630 General population | 6.1 years | Incident CKD | ↘ CKD incidence (OR, 0.57; 95% CI, 0.41 to 0.8, p = 0.002) with ↗ adherence to the vegetarian dietary pattern |
| Liu et al. 2019 [26] | Observational | FFQ: vegan, ovo-lacto vegetarian, or omnivore diets | n = 55,113 General population | Cross-sectional | Prevalence of CKD | ↘ CKD in vegan diet adherent (OR, 0.87; 95% CI, 0.75 to 0.97, p = 0.018) and ovo-lacto vegetarian diet adherent (OR, 0.84; 95% CI 0.77 to 0.88, p < 0.001) |
| Jhee et al. 2019 [27] | Observational | FFQ: nonfermented and fermented plant and fruit | n = 9229 General population | 8.2 years | Incidence of CKD Incident proteinuria | ↘ CKD incidence (HR, 0.86; 95% CI, 0.76 to 0.98, p < 0.05) and proteinuria incidence (HR, 0.68; 95% CI, 0.59 to 0.78, p < 0.05) with highest versus lowest intake of nonfermented plants |
| Kim et al. 2019 [28] | Observational | FFQ: Healthy pro-vegetarian diet to less healthy | n = 14,686 General population | 24 years | Incident CKD eGFR decline | ↘ CKD incidence with higher adherence to the healthy plant-based diet (OR, 0.86; 95% CI, 0.78 to 0.96, p = 0.001) and pro-vegetarian diets (OR, 0.9; 95% CI, 0.82 to 0.99, p = 0.03) ↘ eGFR annual decline with healthy plant-based diet (OR, −1.46; 95% CI, −1.50 to −1.43 p <0.001) |
| Oosterwijk et al. 2019 [16] | Observational | FFQ: protein intake, including types and sources of protein | n = 420 Type 2 diabetes population | Cross-sectional | Prevalence of CKD | ↗ intake of vegetable protein is associated with ↘ prevalence of CKD in higher tertile (OR, 0.47: 95% CI, 0.23 to 0.98, p = 0.04) |
| Saglimbene et al. 2019 [29] | Observational | FFQ: fruit and plant intake | n = 8078 Adults on maintenance hemodialysis | 2.7 years | Mortality | In the hemodialysis population, ↗ consumption of fruit and plant is associated with ↘ all-cause (OR, 0.8; 95% CI, 0.71 to 0.91, p = 0.002) and non-cardiovascular death (OR, 0.84; 95% CI, 0.70 to 1.00, p = 0.14) |
| Study | Type | Intervention /Assessment | Population | Follow Up | Outcomes | Results/Comments |
|---|---|---|---|---|---|---|
| INTERVENTIONAL STUDIES | ||||||
| Fanti et al. 2006 [30] | Randomized controlled trial | Isoflavone-containing soy-based nutritional supplements (soy group) or isoflavone-free milk protein (control group) | n = 25 ESKD on chronic hemodialysis with systemic inflammation | 8 weeks | Impact on inflammatory markers and nutrition markers | ↗ serum isoflavone levels associated with ↘ CRP (HR = −0.599, p = 0.02) and ↗ albumin (HR = 0.522, p < 0.05) No significant decrease of CRP between the two groups but a trend in the soy protein group |
| Soroka et al. 1998 [31] | Randomized cross-over trial | Plant protein diet versus animal protein diet | n = 9 CKD G3-G4 | 1 year | eGFR decline | Failed to find a difference between APD versus VPD but it was underpowered and short trial A better degree of compliance with caloric, protein, and phosphate intakes |
| Tabibi et al. 2009 [32] | Randomized controlled trial | Soy flour (14 g of soy protein) versus usual diet | n = 40 ESKD on peritoneal dialysis | 8 weeks | Serum lipid profile | ↘ serum Lipoprotein A concentration in the soy protein group (p < 0.05) |
| Moe et al. 2011 [33] | Cross-over trial | Vegetarian versus meat diet comparison | n = 8 CKD G3-G4 | 7 days | Impact on phosphorus homeostasis | ↘ phosphorus serum concentration (p = 0.02) and ↘ FGF23 (p = 0.008) in the vegetarian diet |
| Goraya et al. 2013 [34] | Randomized controlled trial | Oral NaHCO3 compared with fruit and plant diet with a controlled arm | n = 106 CKD G4 with metabolic acidosis | 1 year | Metabolic acidosis | Fruit and plant diet are as effective as oral bicarbonate to improve metabolic acidosis (19.9 versus 19.3 mM; p= 0.01), without an increase of hyperkaliemia risk. |
| Goraya et al. 2014 [35] | Randomized controlled trial | Oral NaHCO3 compared with fruit and p diet with a controlled arm | n = 108 CKD G3 A > 1 | 3 years | Urine excretion of angiotensinogen eGFR decline | Fruit and plant diet are as effective as oral bicarbonate decrease angiotensinogen urine excretion (p < 0.05) and preserve eGFR (p < 0.01) versus usual care |
| Goraya et al. 2019 [36] | Randomized controlled trial | Oral NaHCO3 compared with fruit and plant diet with a controlled arm | n = 108 CKD G3-4 A > 1 Nondiabetic | 5 years | Metabolic acidosis, eGFR decline and CVD risk factors | Fruit and plant diet are as effective as oral bicarbonate to correct metabolic acidosis (p < 0.01), eGFR decline (−10.0, 95% CI −10.6 to −9.4 mL/min/1.73 m2 versus −18.8, 95% CI −19.5 to −18.2 mL/min/1.73 m2 in usual care group), p < 0.01.) and was better than bicarbonate to reduce systolic blood pressure (p < 0.01) It was more effective to lower low-density lipoprotein and increase serum vitamin K1 |
| Amino Acid | Requirements, mg/kg per day | mg/g Protein (1) |
|---|---|---|
| Histidine | 10 | 15 |
| Isoleucine | 20 | 30 |
| Leucine | 39 | 59 |
| Lysine | 30 | 45 |
| Methionine | 10 | 16 |
| Cysteine | 4 | 6 |
| Phenylalanine plus tyrosine | 25 | 38 |
| Threonine | 15 | 23 |
| Tryptophan | 4 | 6 |
| Valine | 26 | 39 |
| Total | 184 | 277 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Letourneau, P.; Bataille, S.; Chauveau, P.; Fouque, D.; Koppe, L. Source and Composition in Amino Acid of Dietary Proteins in the Primary Prevention and Treatment of CKD. Nutrients 2020, 12, 3892. https://doi.org/10.3390/nu12123892
Letourneau P, Bataille S, Chauveau P, Fouque D, Koppe L. Source and Composition in Amino Acid of Dietary Proteins in the Primary Prevention and Treatment of CKD. Nutrients. 2020; 12(12):3892. https://doi.org/10.3390/nu12123892
Chicago/Turabian StyleLetourneau, Pierre, Stanislas Bataille, Philippe Chauveau, Denis Fouque, and Laetitia Koppe. 2020. "Source and Composition in Amino Acid of Dietary Proteins in the Primary Prevention and Treatment of CKD" Nutrients 12, no. 12: 3892. https://doi.org/10.3390/nu12123892





