Omega-3 Fatty Acids for Sport Performance—Are They Equally Beneficial for Athletes and Amateurs? A Narrative Review
Abstract
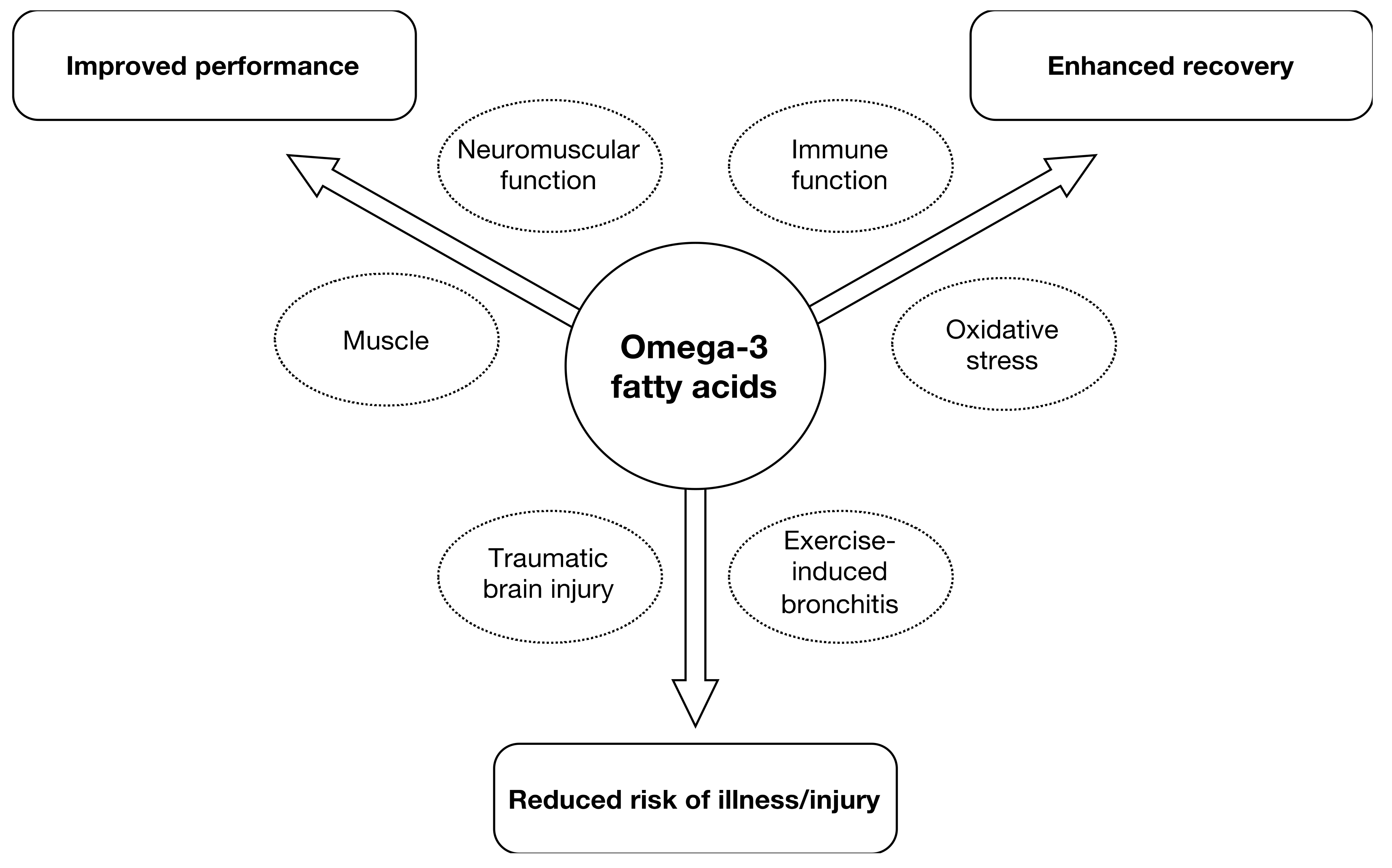
:1. Introduction
2. Materials and Methods
3. Results
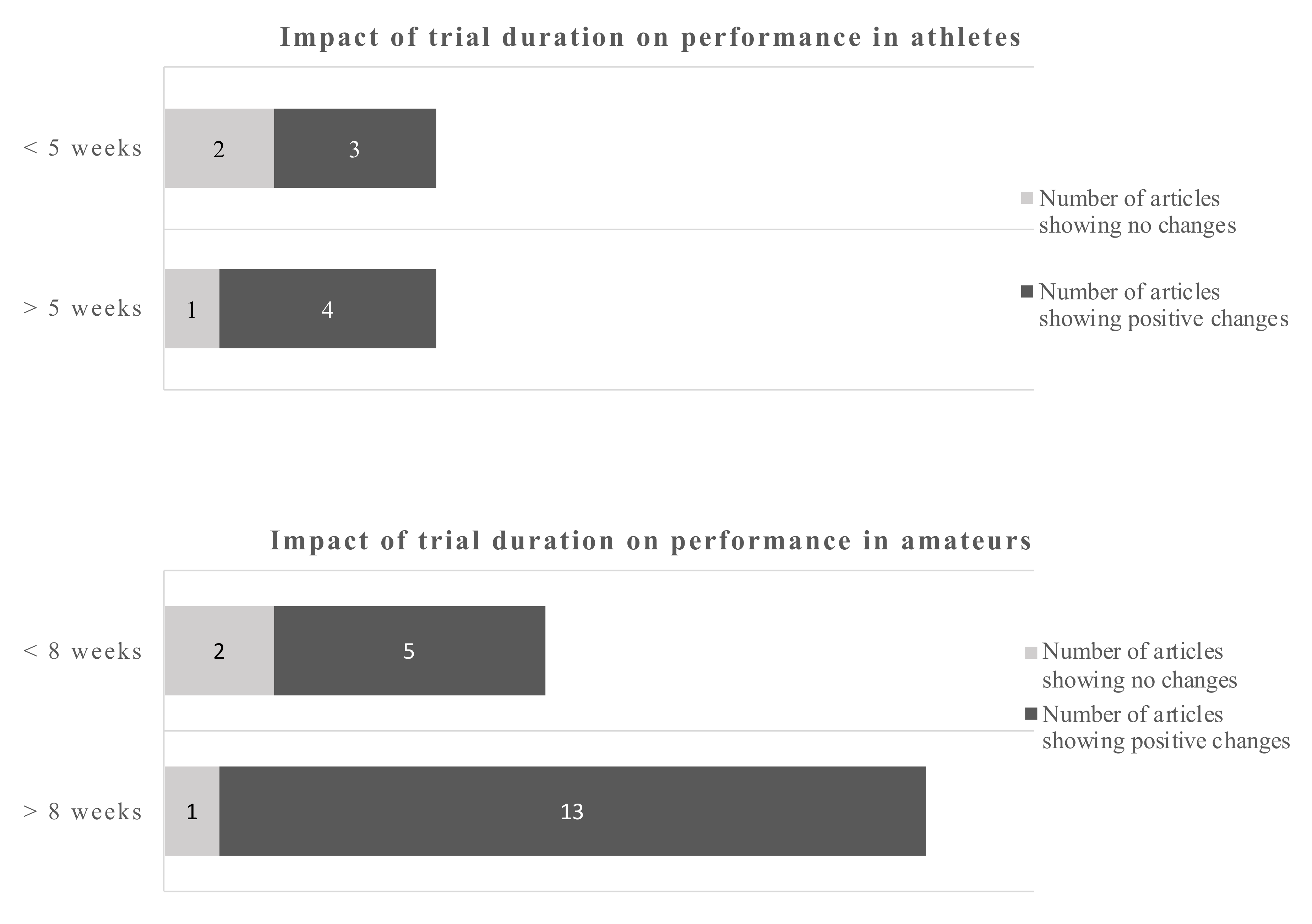
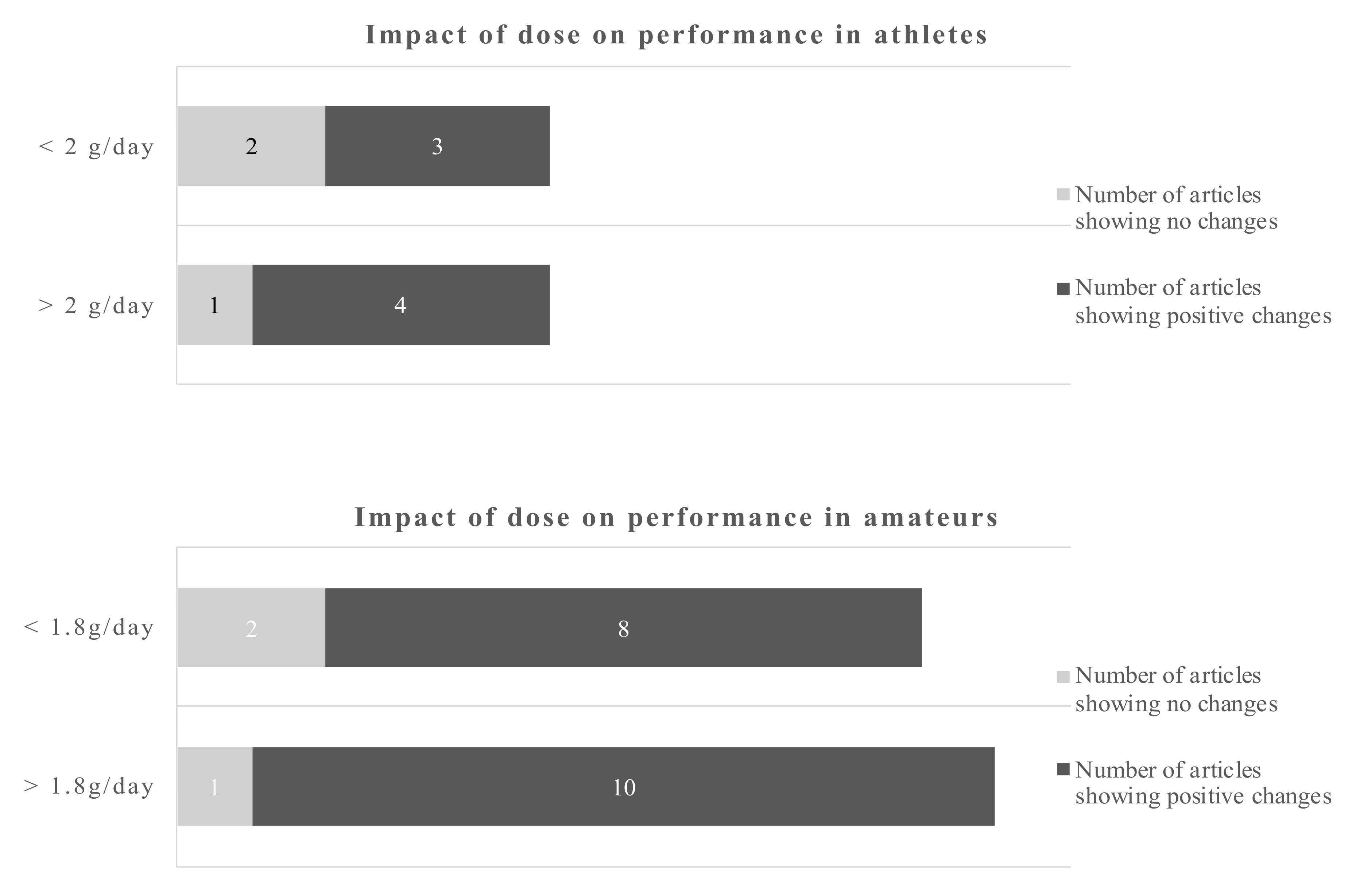
3.1. The Influence of Dose and Study Duration in Athletes and Amateurs on Performance
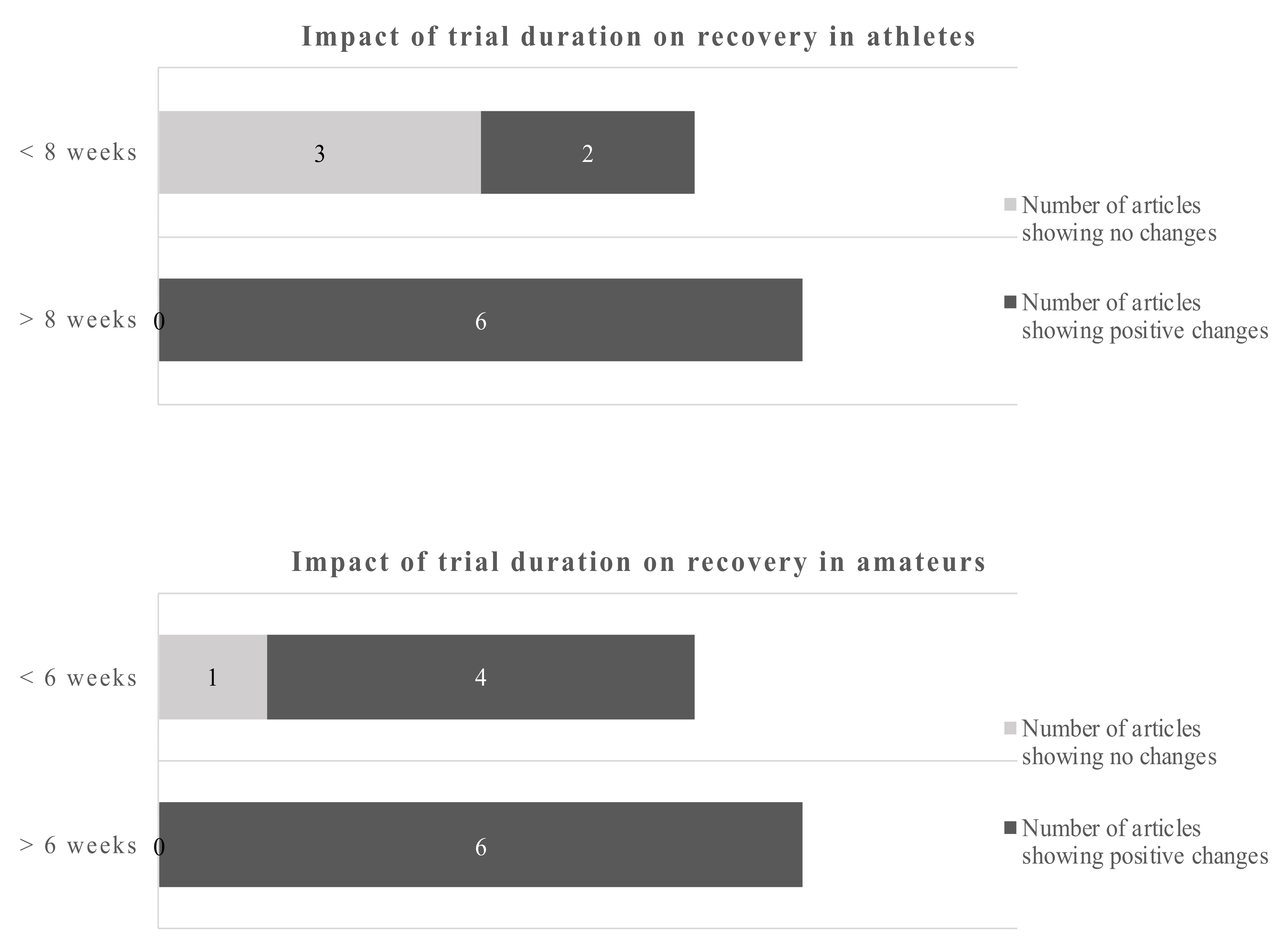
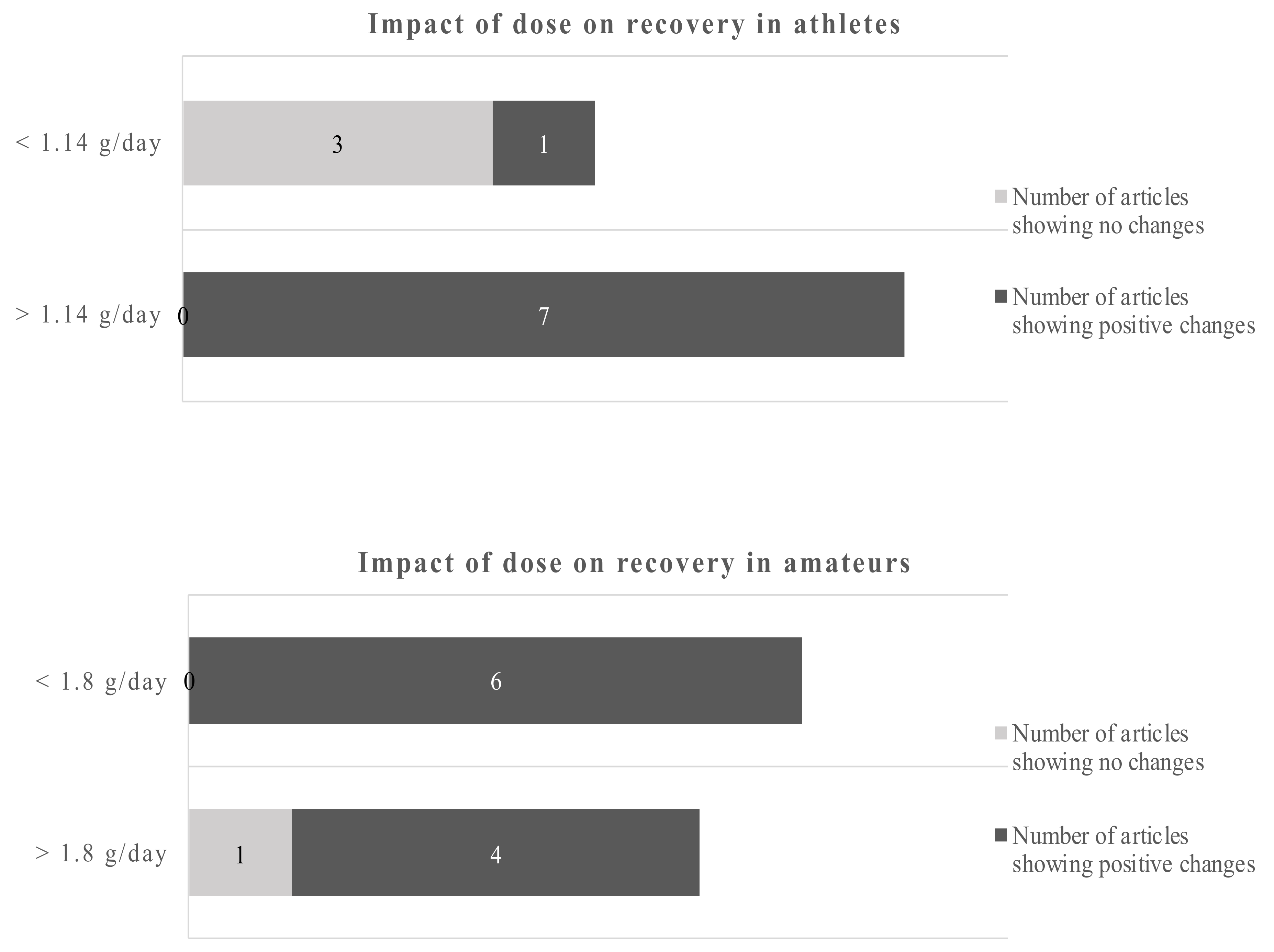
3.2. The Influence of Dose and Study Duration in Athletes and Amateurs on Recovery
3.3. Reduced Risk of Injury/Illness
4. Discussion
4.1. Increased Performance
4.2. Enhanced Recovery
4.3. Reduced Risk of Injury/Illness
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Da Boit, M.; Hunter, A.M.; Gray, S.R. Fit with good fat? The role of n-3 polyunsaturated fatty acids on exercise performance. Metabolism 2017, 66, 45–54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simopoulos, A.P. Omega-3 fatty acids and athletics. Curr. Sports Med. Rep. 2007, 6, 230–236. [Google Scholar] [PubMed]
- Burdge, G.C.; Calder, P.C. Introduction to fatty acids and lipids. World Rev. Nutr. Diet 2015, 112, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C. Very long-chain n-3 fatty acids and human health: Fact, fiction and the future. Proc. Nutr. Soc. 2018, 77, 52–72. [Google Scholar] [CrossRef] [Green Version]
- Arterburn, L.M.; Hall, E.B.; Oken, H. Distribution, interconversion, and dose response of n-3 fatty acids in humans. Am. J. Clin. Nutr. 2006, 83, 1467S–1476S. [Google Scholar] [CrossRef]
- Ameur, A.; Enroth, S.; Johansson, A.; Zaboli, G.; Igl, W.; Johansson, A.C.; Rivas, M.A.; Daly, M.J.; Schmitz, G.; Hicks, A.A.; et al. Genetic adaptation of fatty-acid metabolism: A human-specific haplotype increasing the biosynthesis of long-chain omega-3 and omega-6 fatty acids. Am. J. Hum. Genet. 2012, 90, 809–820. [Google Scholar] [CrossRef] [Green Version]
- EFSA. Scientific Opinion on Dietary Reference Values for fats, including saturated fatty acids, polyunsaturated fatty acids, monounsaturated fatty acids, trans fatty acids, and cholesterol. EFSA J. 2010, 8, 1461. [Google Scholar] [CrossRef] [Green Version]
- Sioen, I.; van Lieshout, L.; Eilander, A.; Fleith, M.; Lohner, S.; Szommer, A.; Petisca, C.; Eussen, S.; Forsyth, S.; Calder, P.C.; et al. Systematic Review on N-3 and N-6 Polyunsaturated Fatty Acid Intake in European Countries in Light of the Current Recommendations - Focus on Specific Population Groups. Ann. Nutr. Metab. 2017, 70, 39–50. [Google Scholar] [CrossRef]
- Agriculture U.D.o.H.a.H.S.a.U.D.o. 2015–2020 Dietary Guidelines for Americans; US Government Printing Office: Washington, DC, USA, 2015. [Google Scholar]
- EFSA. Scientific Opinion on the Tolerable Upper Intake Level of eicosapentaenoic acid (EPA), docosahexaenoic acid (DHA) and docosapentaenoic acid (DPA). EFSA J. 2012, 10. [Google Scholar] [CrossRef]
- Baranauskas, M.; Stukas, R.; Tubelis, L.; Zagminas, K.; Surkiene, G.; Svedas, E.; Giedraitis, V.R.; Dobrovolskij, V.; Abaravicius, J.A. Nutritional habits among high-performance endurance athletes. Medicina (Kaunas) 2015, 51, 351–362. [Google Scholar] [CrossRef]
- Von Schacky, C.; Kemper, M.; Haslbauer, R.; Halle, M. Low Omega-3 Index in 106 German elite winter endurance athletes: A pilot study. Int. J. Sport Nutr. Exerc. Metab. 2014, 24, 559–564. [Google Scholar] [CrossRef] [Green Version]
- Anzalone, A.; Carbuhn, A.; Jones, L.; Gallop, A.; Smith, A.; Johnson, P.; Swearingen, L.; Moore, C.; Rimer, E.; McBeth, J.; et al. The Omega-3 Index in National Collegiate Athletic Association Division I Collegiate Football Athletes. J. Athletic Train. 2019, 54, 7–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Philpott, J.D.; Witard, O.C.; Galloway, S.D.R. Applications of omega-3 polyunsaturated fatty acid supplementation for sport performance. Res. Sports Med. 2019, 27, 219–237. [Google Scholar] [CrossRef]
- Cohen, J. The cost of dichotomization. Appl. Psychol.l Measur. 1983, 7, 249–253. [Google Scholar] [CrossRef]
- Peoples, G.E.; McLennan, P.L.; Howe, P.R.; Groeller, H. Fish oil reduces heart rate and oxygen consumption during exercise. J. Cardiovasc. Pharmacol. 2008, 52, 540–547. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nieman, D.C.; Henson, D.A.; McAnulty, S.R.; Jin, F.; Maxwell, K.R. n-3 polyunsaturated fatty acids do not alter immune and inflammation measures in endurance athletes. Int. J. Sport Nutr. Exerc. Metab. 2009, 19, 536–546. [Google Scholar] [CrossRef]
- Black, K.E.; Witard, O.C.; Baker, D.; Healey, P.; Lewis, V.; Tavares, F.; Christensen, S.; Pease, T.; Smith, B. Adding omega-3 fatty acids to a protein-based supplement during pre-season training results in reduced muscle soreness and the better maintenance of explosive power in professional Rugby Union players. Eur. J. Sport Sci. 2018, 18, 1357–1367. [Google Scholar] [CrossRef]
- Buckley, J.D.; Burgess, S.; Murphy, K.J.; Howe, P.R. DHA-rich fish oil lowers heart rate during submaximal exercise in elite Australian Rules footballers. J. Sci. Med. Sport 2009, 12, 503–507. [Google Scholar] [CrossRef]
- Gravina, L.; Brown, F.F.; Alexander, L.; Dick, J.; Bell, G.; Witard, O.C.; Galloway, S.D.R. n-3 Fatty Acid Supplementation During 4 Weeks of Training Leads to Improved Anaerobic Endurance Capacity, but not Maximal Strength, Speed, or Power in Soccer Players. Int. J. Sport Nutr. Exerc. Metab. 2017, 27, 305–313. [Google Scholar] [CrossRef] [Green Version]
- Zebrowska, A.; Mizia-Stec, K.; Mizia, M.; Gasior, Z.; Poprzecki, S. Omega-3 fatty acids supplementation improves endothelial function and maximal oxygen uptake in endurance-trained athletes. Eur. J. Sport Sci. 2015, 15, 305–314. [Google Scholar] [CrossRef]
- Oostenbrug, G.S.; Mensink, R.P.; Hardeman, M.R.; De Vries, T.; Brouns, F.; Hornstra, G. Exercise performance, red blood cell deformability, and lipid peroxidation: Effects of fish oil and vitamin E. J. Appl. Physiol. 1997, 83, 746–752. [Google Scholar] [CrossRef] [PubMed]
- Hingley, L.; Macartney, M.J.; Brown, M.A.; McLennan, P.L.; Peoples, G.E. DHA-rich Fish Oil Increases the Omega-3 Index and Lowers the Oxygen Cost of Physiologically Stressful Cycling in Trained Individuals. Int. J. Sport Nutr. Exerc. Metab. 2017, 27, 335–343. [Google Scholar] [CrossRef] [PubMed]
- Jakeman, J.R.; Lambrick, D.M.; Wooley, B.; Babraj, J.A.; Faulkner, J.A. Effect of an acute dose of omega-3 fish oil following exercise-induced muscle damage. Eur. J. Appl. Physiol. 2017, 117, 575–582. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Edholm, P.; Strandberg, E.; Kadi, F. Lower limb explosive strength capacity in elderly women: Effects of resistance training and healthy diet. J. Appl. Physiol. 2017, 123, 190–196. [Google Scholar] [CrossRef] [Green Version]
- Smith, G.I.; Julliand, S.; Reeds, D.N.; Sinacore, D.R.; Klein, S.; Mittendorfer, B. Fish oil-derived n-3 PUFA therapy increases muscle mass and function in healthy older adults. Am. J. Clin. Nutr. 2015, 102, 115–122. [Google Scholar] [CrossRef] [Green Version]
- Rodacki, C.L.; Rodacki, A.L.; Pereira, G.; Naliwaiko, K.; Coelho, I.; Pequito, D.; Fernandes, L.C. Fish-oil supplementation enhances the effects of strength training in elderly women. Am. J. Clin. Nutr. 2012, 95, 428–436. [Google Scholar] [CrossRef]
- Da Boit, M.; Sibson, R.; Sivasubramaniam, S.; Meakin, J.R.; Greig, C.A.; Aspden, R.M.; Thies, F.; Jeromson, S.; Hamilton, D.L.; Speakman, J.R.; et al. Sex differences in the effect of fish-oil supplementation on the adaptive response to resistance exercise training in older people: A randomized controlled trial. Am. J. Clin. Nutr. 2017, 105, 151–158. [Google Scholar] [CrossRef]
- Krzyminska-Siemaszko, R.; Czepulis, N.; Lewandowicz, M.; Zasadzka, E.; Suwalska, A.; Witowski, J.; Wieczorowska-Tobis, K. The Effect of a 12-Week Omega-3 Supplementation on Body Composition, Muscle Strength and Physical Performance in Elderly Individuals with Decreased Muscle Mass. Int. J. Environ. Res. Public Health 2015, 12, 10558–10574. [Google Scholar] [CrossRef] [Green Version]
- Ninio, D.M.; Hill, A.M.; Howe, P.R.; Buckley, J.D.; Saint, D.A. Docosahexaenoic acid-rich fish oil improves heart rate variability and heart rate responses to exercise in overweight adults. Br. J. Nutr. 2008, 100, 1097–1103. [Google Scholar] [CrossRef]
- McGlory, C.; Wardle, S.L.; Macnaughton, L.S.; Witard, O.C.; Scott, F.; Dick, J.; Bell, J.G.; Phillips, S.M.; Galloway, S.D.; Hamilton, D.L.; et al. Fish oil supplementation suppresses resistance exercise and feeding-induced increases in anabolic signaling without affecting myofibrillar protein synthesis in young men. Physiol. Rep. 2016, 4. [Google Scholar] [CrossRef] [Green Version]
- Kawabata, F.; Neya, M.; Hamazaki, K.; Watanabe, Y.; Kobayashi, S.; Tsuji, T. Supplementation with eicosapentaenoic acid-rich fish oil improves exercise economy and reduces perceived exertion during submaximal steady-state exercise in normal healthy untrained men. Biosci. Biotechnol. Biochem. 2014, 78, 2081–2088. [Google Scholar] [CrossRef] [PubMed]
- Ochi, E.; Tsuchiya, Y.; Yanagimoto, K. Effect of eicosapentaenoic acids-rich fish oil supplementation on motor nerve function after eccentric contractions. J. Int. Soc. Sports Nutr. 2017, 14, 23. [Google Scholar] [CrossRef]
- Tsuchiya, Y.; Yanagimoto, K.; Nakazato, K.; Hayamizu, K.; Ochi, E. Eicosapentaenoic and docosahexaenoic acids-rich fish oil supplementation attenuates strength loss and limited joint range of motion after eccentric contractions: A randomized, double-blind, placebo-controlled, parallel-group trial. Eur. J. Appl. Physiol. 2016, 116, 1179–1188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Macartney, M.J.; Hingley, L.; Brown, M.A.; Peoples, G.E.; McLennan, P.L. Intrinsic heart rate recovery after dynamic exercise is improved with an increased omega-3 index in healthy males. Br. J. Nutr. 2014, 112, 1984–1992. [Google Scholar] [CrossRef] [PubMed]
- Smith, G.I.; Atherton, P.; Reeds, D.N.; Mohammed, B.S.; Rankin, D.; Rennie, M.J.; Mittendorfer, B. Dietary omega-3 fatty acid supplementation increases the rate of muscle protein synthesis in older adults: A randomized controlled trial. Am. J. Clin. Nutr. 2011, 93, 402–412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, G.I.; Atherton, P.; Reeds, D.N.; Mohammed, B.S.; Rankin, D.; Rennie, M.J.; Mittendorfer, B. Omega-3 polyunsaturated fatty acids augment the muscle protein anabolic response to hyperinsulinaemia-hyperaminoacidaemia in healthy young and middle-aged men and women. Clin. Sci. (Lond.) 2011, 121, 267–278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haghravan, S.; Keshavarz, S.A.; Mazaheri, R.; Alizadeh, Z.; Mansournia, M.A. Effect of Omega-3 PUFAs Supplementation with Lifestyle Modification on Anthropometric Indices and Vo2 max in Overweight Women. Arch. Iran Med. 2016, 19, 342–347. [Google Scholar]
- Lembke, P.; Capodice, J.; Hebert, K.; Swenson, T. Influence of omega-3 (n3) index on performance and wellbeing in young adults after heavy eccentric exercise. J. Sports Sci. Med. 2014, 13, 151–156. [Google Scholar]
- Mickleborough, T.D.; Sinex, J.A.; Platt, D.; Chapman, R.F.; Hirt, M. The effects PCSO-524(R), a patented marine oil lipid and omega-3 PUFA blend derived from the New Zealand green lipped mussel (Perna canaliculus), on indirect markers of muscle damage and inflammation after muscle damaging exercise in untrained men: A randomized, placebo controlled trial. J. Int. Soc. Sports Nutr. 2015, 12, 10. [Google Scholar] [CrossRef] [Green Version]
- Lewis, E.J.; Radonic, P.W.; Wolever, T.M.; Wells, G.D. 21 days of mammalian omega-3 fatty acid supplementation improves aspects of neuromuscular function and performance in male athletes compared to olive oil placebo. J. Int. Soc. Sports Nutr. 2015, 12, 28. [Google Scholar] [CrossRef] [Green Version]
- Lewis, E.J.H.; Stucky, F.; Radonic, P.W.; Metherel, A.H.; Wolever, T.M.S.; Wells, G.D. Neuromuscular adaptations to sprint interval training and the effect of mammalian omega-3 fatty acid supplementation. Eur. J. Appl. Physiol. 2017, 117, 469–482. [Google Scholar] [CrossRef] [PubMed]
- Bortolotti, M.; Tappy, L.; Schneiter, P. Fish oil supplementation does not alter energy efficiency in healthy males. Clin. Nutr. 2007, 26, 225–230. [Google Scholar] [CrossRef] [PubMed]
- Tinsley, G.M.; Gann, J.J.; Huber, S.R.; Andre, T.L.; La Bounty, P.M.; Bowden, R.G.; Gordon, P.M.; Grandjean, P.W. Effects of Fish Oil Supplementation on Postresistance Exercise Muscle Soreness. J. Diet Suppl. 2016. [Google Scholar] [CrossRef] [PubMed]
- Rontoyanni, V.G.; Hall, W.L.; Pombo-Rodrigues, S.; Appleton, A.; Chung, R.; Sanders, T.A. A comparison of the changes in cardiac output and systemic vascular resistance during exercise following high-fat meals containing DHA or EPA. Br. J. Nutr. 2012, 108, 492–499. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jouris, K.B.; McDaniel, J.L.; Weiss, E.P. The Effect of Omega-3 Fatty Acid Supplementation on the Inflammatory Response to eccentric strength exercise. J. Sports Sci. Med. 2011, 10, 432–438. [Google Scholar]
- Busquets-Cortes, C.; Capo, X.; Martorell, M.; Tur, J.A.; Sureda, A.; Pons, A. Training Enhances Immune Cells Mitochondrial Biosynthesis, Fission, Fusion, and Their Antioxidant Capabilities Synergistically with Dietary Docosahexaenoic Supplementation. Oxid Med Cell Longev 2016, 2016, 8950384. [Google Scholar] [CrossRef] [Green Version]
- Capo, X.; Martorell, M.; Sureda, A.; Llompart, I.; Tur, J.A.; Pons, A. Diet supplementation with DHA-enriched food in football players during training season enhances the mitochondrial antioxidant capabilities in blood mononuclear cells. Eur. J. Nutr. 2015, 54, 35–49. [Google Scholar] [CrossRef]
- Capo, X.; Martorell, M.; Sureda, A.; Tur, J.A.; Pons, A. Effects of dietary Docosahexaenoic, training and acute exercise on lipid mediators. J. Int. Soc. Sports Nutr. 2016, 13, 16. [Google Scholar] [CrossRef] [Green Version]
- Capo, X.; Martorell, M.; Sureda, A.; Batle, J.M.; Tur, J.A.; Pons, A. Docosahexaenoic diet supplementation, exercise and temperature affect cytokine production by lipopolysaccharide-stimulated mononuclear cells. J. Physiol. Biochem. 2016, 72, 421–434. [Google Scholar] [CrossRef]
- Martorell, M.; Capo, X.; Sureda, A.; Batle, J.M.; Llompart, I.; Argelich, E.; Tur, J.A.; Pons, A. Effect of DHA on plasma fatty acid availability and oxidative stress during training season and football exercise. Food Funct. 2014, 5, 1920–1931. [Google Scholar] [CrossRef]
- Martorell, M.; Capo, X.; Bibiloni, M.M.; Sureda, A.; Mestre-Alfaro, A.; Batle, J.M.; Llompart, I.; Tur, J.A.; Pons, A. Docosahexaenoic acid supplementation promotes erythrocyte antioxidant defense and reduces protein nitrosative damage in male athletes. Lipids 2015, 50, 131–148. [Google Scholar] [CrossRef] [PubMed]
- Filaire, E.; Massart, A.; Rouveix, M.; Portier, H.; Rosado, F.; Durand, D. Effects of 6 weeks of n-3 fatty acids and antioxidant mixture on lipid peroxidation at rest and postexercise. Eur. J. Appl. Physiol. 2011, 111, 1829–1839. [Google Scholar] [CrossRef] [PubMed]
- Philpott, J.D.; Donnelly, C.; Walshe, I.H.; MacKinley, E.E.; Dick, J.; Galloway, S.D.R.; Tipton, K.D.; Witard, O.C. Adding Fish Oil to Whey Protein, Leucine, and Carbohydrate Over a Six-Week Supplementation Period Attenuates Muscle Soreness Following Eccentric Exercise in Competitive Soccer Players. Int. J. Sport Nutr. Exerc. Metab. 2018, 28, 26–36. [Google Scholar] [CrossRef] [Green Version]
- Capo, X.; Martorell, M.; Busquets-Cortes, C.; Sureda, A.; Riera, J.; Drobnic, F.; Tur, J.A.; Pons, A. Effects of dietary almond- and olive oil-based docosahexaenoic acid- and vitamin E-enriched beverage supplementation on athletic performance and oxidative stress markers. Food Funct. 2016, 7, 4920–4934. [Google Scholar] [CrossRef] [PubMed]
- Capo, X.; Martorell, M.; Sureda, A.; Riera, J.; Drobnic, F.; Tur, J.A.; Pons, A. Effects of Almond- and Olive Oil-Based Docosahexaenoic- and Vitamin E-Enriched Beverage Dietary Supplementation on Inflammation Associated to Exercise and Age. Nutrients 2016, 8, 619. [Google Scholar] [CrossRef] [PubMed]
- Delfan, M.; Ebrahim, K.; Baesi, F.; Mirakhori, Z.; Ghalamfarsa, G.; Bakhshaei, P.; Saboor-Yaraghi, A.A.; Razavi, A.; Setayesh, M.; Yousefi, M.; et al. The immunomodulatory effects of fish-oil supplementation in elite paddlers: A pilot randomized double blind placebo-controlled trial. Prostaglandins Leukot. Essent. Fatty Acids 2015, 99, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Tartibian, B.; Maleki, B.H.; Abbasi, A. The effects of ingestion of omega-3 fatty acids on perceived pain and external symptoms of delayed onset muscle soreness in untrained men. Clin. J. Sport Med. 2009, 19, 115–119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poprzecki, S.; Zajac, A.; Chalimoniuk, M.; Waskiewicz, Z.; Langfort, J. Modification of blood antioxidant status and lipid profile in response to high-intensity endurance exercise after low doses of omega-3 polyunsaturated fatty acids supplementation in healthy volunteers. Int. J. Food Sci. Nutr. 2009, 60 Suppl. 2, 67–79. [Google Scholar] [CrossRef]
- Gray, P.; Gabriel, B.; Thies, F.; Gray, S.R. Fish oil supplementation augments post-exercise immune function in young males. Brain Behav. Immun. 2012, 26, 1265–1272. [Google Scholar] [CrossRef]
- Gray, P.; Chappell, A.; Jenkinson, A.M.; Thies, F.; Gray, S.R. Fish oil supplementation reduces markers of oxidative stress but not muscle soreness after eccentric exercise. Int. J. Sport Nutr. Exerc. Metab. 2014, 24, 206–214. [Google Scholar] [CrossRef] [Green Version]
- Da Boit, M.; Mastalurova, I.; Brazaite, G.; McGovern, N.; Thompson, K.; Gray, S.R. The Effect of Krill Oil Supplementation on Exercise Performance and Markers of Immune Function. PLoS One 2015, 10, e0139174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Corder, K.E.; Newsham, K.R.; McDaniel, J.L.; Ezekiel, U.R.; Weiss, E.P. Effects of Short-Term Docosahexaenoic Acid Supplementation on Markers of Inflammation after Eccentric Strength Exercise in Women. J. Sports Sci. Med. 2016, 15, 176–183. [Google Scholar] [PubMed]
- Tartibian, B.; Maleki, B.H.; Abbasi, A. Omega-3 fatty acids supplementation attenuates inflammatory markers after eccentric exercise in untrained men. Clin. J. Sport Med. 2011, 21, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Filaire, E.; Massart, A.; Portier, H.; Rouveix, M.; Rosado, F.; Bage, A.S.; Gobert, M.; Durand, D. Effect of 6 Weeks of n-3 fatty-acid supplementation on oxidative stress in Judo athletes. Int. J. Sport Nutr. Exerc. Metab. 2010, 20, 496–506. [Google Scholar] [CrossRef] [PubMed]
- Oliver, J.M.; Jones, M.T.; Kirk, K.M.; Gable, D.A.; Repshas, J.T.; Johnson, T.A.; Andreasson, U.; Norgren, N.; Blennow, K.; Zetterberg, H. Effect of Docosahexaenoic Acid on a Biomarker of Head Trauma in American Football. Med. Sci. Sports Exerc. 2016, 48, 974–982. [Google Scholar] [CrossRef]
- Williams, N.C.; Hunter, K.A.; Shaw, D.E.; Jackson, K.G.; Sharpe, G.R.; Johnson, M.A. Comparable reductions in hyperpnoea-induced bronchoconstriction and markers of airway inflammation after supplementation with 6.2 and 3.1 g/d of long-chain n-3 PUFA in adults with asthma. Br J Nutr 2017, 117, 1379–1389. [Google Scholar] [CrossRef] [Green Version]
- Mickleborough, T.D.; Vaughn, C.L.; Shei, R.J.; Davis, E.M.; Wilhite, D.P. Marine lipid fraction PCSO-524 (lyprinol/omega XL) of the New Zealand green lipped mussel attenuates hyperpnea-induced bronchoconstriction in asthma. Respir. Med. 2013, 107, 1152–1163. [Google Scholar] [CrossRef] [Green Version]
- Brannan, J.D.; Bood, J.; Alkhabaz, A.; Balgoma, D.; Otis, J.; Delin, I.; Dahlen, B.; Wheelock, C.E.; Nair, P.; Dahlen, S.E.; et al. The effect of omega-3 fatty acids on bronchial hyperresponsiveness, sputum eosinophilia, and mast cell mediators in asthma. Chest 2015, 147, 397–405. [Google Scholar] [CrossRef] [Green Version]
- Adams, S.; Lopata, A.L.; Smuts, C.M.; Baatjies, R.; Jeebhay, M.F. Relationship between Serum Omega-3 Fatty Acid and Asthma Endpoints. Int J Environ Res Public Health 2018, 16, 43. [Google Scholar] [CrossRef] [Green Version]
- Barros, R.; Moreira, A.; Fonseca, J.; Delgado, L.; Castel-Branco, M.G.; Haahtela, T.; Lopes, C.; Moreira, P. Dietary intake of alpha-linolenic acid and low ratio of n-6:n-3 PUFA are associated with decreased exhaled NO and improved asthma control. Br. J. Nutr. 2011, 106, 441–450. [Google Scholar] [CrossRef] [Green Version]
- Kompauer, I.; Demmelmair, H.; Koletzko, B.; Bolte, G.; Linseisen, J.; Heinrich, J. Association of fatty acids in serum phospholipids with lung function and bronchial hyperresponsiveness in adults. Eur. J. Epidemiol. 2008, 23, 175–190. [Google Scholar] [CrossRef] [PubMed]
- Mickleborough, T.D.; Murray, R.L.; Ionescu, A.A.; Lindley, M.R. Fish oil supplementation reduces severity of exercise-induced bronchoconstriction in elite athletes. Am. J. Respir. Crit. Care Med. 2003, 168, 1181–1189. [Google Scholar] [CrossRef] [PubMed]
- Beck, K.L.; Thomson, J.S.; Swift, R.J.; von Hurst, P.R. Role of nutrition in performance enhancement and postexercise recovery. Open Access J. Sports Med. 2015, 6, 259–267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeromson, S.; Gallagher, I.J.; Galloway, S.D.; Hamilton, D.L. Omega-3 Fatty Acids and Skeletal Muscle Health. Mar. Drugs 2015, 13, 6977–7004. [Google Scholar] [CrossRef]
- Calder, P.C.; Yaqoob, P.; Harvey, D.J.; Watts, A.; Newsholme, E.A. Incorporation of fatty acids by concanavalin A-stimulated lymphocytes and the effect on fatty acid composition and membrane fluidity. Biochem. J. 1994, 300 ( Pt 2), 509–518. [Google Scholar] [CrossRef] [Green Version]
- Murphy, M.G. Dietary fatty acids and membrane protein function. J. Nutr. Biochem. 1990, 1, 68–79. [Google Scholar] [CrossRef]
- McGlory, C.; Galloway, S.D.; Hamilton, D.L.; McClintock, C.; Breen, L.; Dick, J.R.; Bell, J.G.; Tipton, K.D. Temporal changes in human skeletal muscle and blood lipid composition with fish oil supplementation. Prostaglandins Leukot. Essent. Fatty Acids 2014, 90, 199–206. [Google Scholar] [CrossRef]
- Laiglesia, L.M.; Lorente-Cebrian, S.; Prieto-Hontoria, P.L.; Fernandez-Galilea, M.; Ribeiro, S.M.; Sainz, N.; Martinez, J.A.; Moreno-Aliaga, M.J. Eicosapentaenoic acid promotes mitochondrial biogenesis and beige-like features in subcutaneous adipocytes from overweight subjects. J. Nutr. Biochem. 2016, 37, 76–82. [Google Scholar] [CrossRef]
- Macaluso, F.; Barone, R.; Catanese, P.; Carini, F.; Rizzuto, L.; Farina, F.; Di Felice, V. Do fat supplements increase physical performance? Nutrients 2013, 5, 509–524. [Google Scholar] [CrossRef] [Green Version]
- Tiryaki-Sönmez, G.S.B.; Vatansever-Ozen, S. Omega-3 fatty acids and exercise: A review of their combined effects on body composition and physical performance. Biomed. Human Kinetics 2011, 3, 23–29. [Google Scholar]
- Hessvik, N.P.; Bakke, S.S.; Fredriksson, K.; Boekschoten, M.V.; Fjorkenstad, A.; Koster, G.; Hesselink, M.K.; Kersten, S.; Kase, E.T.; Rustan, A.C.; et al. Metabolic switching of human myotubes is improved by n-3 fatty acids. J. Lipid Res. 2010, 51, 2090–2104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rokling-Andersen, M.H.; Rustan, A.C.; Wensaas, A.J.; Kaalhus, O.; Wergedahl, H.; Rost, T.H.; Jensen, J.; Graff, B.A.; Caesar, R.; Drevon, C.A. Marine n-3 fatty acids promote size reduction of visceral adipose depots, without altering body weight and composition, in male Wistar rats fed a high-fat diet. Br. J. Nutr. 2009, 102, 995–1006. [Google Scholar] [CrossRef] [Green Version]
- Logan, S.L.; Spriet, L.L. Omega-3 Fatty Acid Supplementation for 12 Weeks Increases Resting and Exercise Metabolic Rate in Healthy Community-Dwelling Older Females. PLoS One 2015, 10, e0144828. [Google Scholar] [CrossRef] [PubMed]
- Nedelec, M.; McCall, A.; Carling, C.; Legall, F.; Berthoin, S.; Dupont, G. Recovery in soccer: Part I - post-match fatigue and time course of recovery. Sports Med. 2012, 42, 997–1015. [Google Scholar] [CrossRef] [PubMed]
- Pereira Panza, V.S.; Diefenthaeler, F.; da Silva, E.L. Benefits of dietary phytochemical supplementation on eccentric exercise-induced muscle damage: Is including antioxidants enough? Nutrition 2015, 31, 1072–1082. [Google Scholar] [CrossRef]
- Evans, W.J. Muscle damage: Nutritional considerations. Int. J. Sport Nutr. 1991, 1, 214–224. [Google Scholar] [CrossRef]
- Magee, P.; Pearson, S.; Whittingham-Dowd, J.; Allen, J. PPARgamma as a molecular target of EPA anti-inflammatory activity during TNF-alpha-impaired skeletal muscle cell differentiation. J. Nutr. Biochem. 2012, 23, 1440–1448. [Google Scholar] [CrossRef]
- Biswas, S.K. Does the Interdependence between Oxidative Stress and Inflammation Explain the Antioxidant Paradox? Oxid. Med. Cell Longev. 2016, 2016, 5698931. [Google Scholar] [CrossRef] [Green Version]
- Gammone, M.A.; Riccioni, G.; Parrinello, G.; D’Orazio, N. Omega-3 Polyunsaturated Fatty Acids: Benefits and Endpoints in Sport. Nutrients 2018, 11, 46. [Google Scholar] [CrossRef] [Green Version]
- Carlsen, K.H.; Anderson, S.D.; Bjermer, L.; Bonini, S.; Brusasco, V.; Canonica, W.; Cummiskey, J.; Delgado, L.; Del Giacco, S.R.; Drobnic, F.; et al. Exercise-induced asthma, respiratory and allergic disorders in elite athletes: Epidemiology, mechanisms and diagnosis: Part I of the report from the Joint Task Force of the European Respiratory Society (ERS) and the European Academy of Allergy and Clinical Immunology (EAACI) in cooperation with GA2LEN. Allergy 2008, 63, 387–403. [Google Scholar] [CrossRef] [Green Version]
- Kumar, A.; Mastana, S.S.; Lindley, M.R. n-3 Fatty acids and asthma. Nutr Res Rev 2016, 29, 1–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wendell, S.G.; Baffi, C.; Holguin, F. Fatty acids, inflammation, and asthma. J. Allergy Clin. Immunol. 2014, 133, 1255–1264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oliver, J.M.; Anzalone, A.J.; Turner, S.M. Protection Before Impact: The Potential Neuroprotective Role of Nutritional Supplementation in Sports-Related Head Trauma. Sports Med. 2018, 48, 39–52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hasadsri, L.; Wang, B.H.; Lee, J.V.; Erdman, J.W.; Llano, D.A.; Barbey, A.K.; Wszalek, T.; Sharrock, M.F.; Wang, H.J. Omega-3 fatty acids as a putative treatment for traumatic brain injury. J. Neurotrauma 2013, 30, 897–906. [Google Scholar] [CrossRef] [Green Version]
- Bryhn, M. Prevention of Sports Injuries by Marine Omega-3 Fatty Acids. J. Am. Coll. Nutr. 2015, 34 Suppl. 1, 60–61. [Google Scholar] [CrossRef]
- Prince Nelson, S.L.; Ramakrishnan, V.; Nietert, P.J.; Kamen, D.L.; Ramos, P.S.; Wolf, B.J. An evaluation of common methods for dichotomization of continuous variables to discriminate disease status. Commun. Stat. Theory Methods 2017, 46, 10823–10834. [Google Scholar] [CrossRef]
| Population (n) Sex Age ± SD (Years) * | Duration (Weeks) | Dose EPA/DHA (g/d) | Exercise Intervention/Test | Effects of EPA/DHA Compared to Control | Reference |
|---|---|---|---|---|---|
| Athletes | |||||
| Cyclists (16) M 23.2 ± 1.2 and 27.1 ± 2.7 | 8 w | 0.8 g EPA 2.4 g DHA | Oxygen peak consumption (VO2 peak) Sustained submaximal exercise tests at 55% of peak workload | Heart rate (HR), including peak heart rate during incremental workloads to exhaustion, reduced Steady-state submaximal exercise HR, reduced Whole body O2 consumption, reduced HR pressure product, reduced VO2 peak, no difference Time to voluntary fatigue, no difference | [16] |
| Cyclists (23) M and F 24.1 ± 2.4 and 26.9 ± 2.8 | 6 w | 2.0 g EPA 0.4 g DHA | 10-km time trials | 10-km time trial performance, no difference Exercise-induced increases in plasma cytokines, myeloperoxidase, blood total leukocytes, serum C-reactive protein (CRP), and creatine kinase (CK); or the decrease in the salivary Immune (Ig)A:protein ratio, no difference | [17] |
| Rugby players (20) M 22 ± 0.7 | 5 w | 1.1 g EPA 1.1 g DHA | Pre-season training Muscle soreness countermovement jump (CMJ) performance psychological well-being | Muscle soreness down Explosive power up Fatigue down | [18] |
| Football players (25) M 21.7 ± 1.0 and 23.2 ± 1.1 | 5 w | 1.9 g EPA + DHA | Endurance performance, Recovery Resting blood pressure (BP) Fasting serum triglycerides (TG) HR during treadmill running at 10 km/h | TG, decreased Diastolic blood pressure, decreased HR during submaximal exercise, decreased Time to exhaustion and recovery, no difference | [19] |
| Soccer players (26) M and F 24.5 ± 5.0 | 4 w | 4.9 g EPA 1.4 g DHA | During training | Strength, unchanged Power, unchanged Speed, unchanged Anaerobic endurance capacity, increased | [20] |
| Cyclists (13) M 23.1 ± 5.4 | 3 w | 0.66 g EPA 0.44 g DHA | Nitric oxide (NO) Asymmetric dimethyloarginine (ADMA) Maximal oxygen uptake (VO2 max) Flow-mediated dilatation (FMD) Pulse wave velocity | Baseline NO, increased NO concentration (ΔNO), increased Positive correlation between baseline post-intervention NO concentration and VO2 max (r = 0.72; p < 0.01) Positive correlation between ΔNO and ΔVO2max Association between higher FMD and higher ΔVO2max, improved | [21] |
| Cyclists (24) M 19–42 | 3 w | 1.06 g EPA 0.75 g DHA | Endurance test (bicycle time trial of approx. 1 h) Red blood cells (RBC) characteristics and lipid peroxidation | RBC characteristics, no difference Exercise performance, no difference Rate of LDL oxidation, decreased The amount of dienes, no difference Effects of exercise, no difference | [22] |
| Cyclists (26) M 24 ± 7 and 23 ± 5 | 8 w | 0.56 g DHA 0.14 g EPA | O2 consumption and fatigue Isometric quadriceps strength | Power output of maximal 6 s cycle sprinting, unchanged Power output during 5 min time trail (TT), unchanged Maximal voluntary contraction, unchanged Relative O2 consumption during the cycling time trial, reduced | [23] |
| High-intensity intermittent training athletes (27) M 26 ± 4 | Acute | Group 1: 0.75 g EPA, 0.05 g DHA per 10 kg body weight Group 2: 0.15 g EPA, 0.1 g DHA per 10 kg body weight | Recovery strategy following 100 plyometric drop jumps | Squat jump performance, increased in group1 CMJ performance, unchanged Functional and perceptual indices, unchanged Perceived soreness, unchanged | [24] |
| Amateurs | |||||
| Healthy (63) F 67.5 ± 0.4 | 24 w | n-6/n-3 polyunsaturated fatty acids (PUFA) ratio below 2 | Resistance training | Isometric strength performance, no difference Dynamic peak power and time to reach peak power (i.e., shorter time) during knee extension, increased Peak force and rate of force development during squat jump, increased | [25] |
| Healthy (60) M and F 60–85 | 24 w | 1.86 g EPA 1.5 g DHA | Age-associated loss of muscle mass and function Thigh muscle volume, handgrip strength, one-repetition maximum (1-RM) lower- and upper-body strength, and average power during isokinetic leg exercises | Thigh muscle volume, increased Handgrip strength, increased 1-RM muscle strength, increased Average isokinetic power, increased | [26] |
| Healthy (45) F 64 ± 1.4 | 21 w 3 d | 0.4 g EPA 0.3 g DHA | Muscle strength and functional capacity | The peak torque and rate of torque development for all muscles, increased The activation level and electromechanical delay of the muscles, improved Chair-rising performance, improved | [27] |
| Healthy (50) M and F 70.6 ± 4.5 | 18 w | 2.1 g EPA 0.6 g DHA | Resistance exercise Training-induced increases in muscle mass and function | Maximal isometric torque increased after exercise training in females, but not in men. Maximal isokinetic torque at 30, 90, and 240° s−1, 4-m walk time, chair-rise time, muscle anatomic cross-sectional area, and muscle fat, no difference Muscle quality in females after exercise training increased no differences in males. No differences in glucose, insulin, or inflammatory markers | [28] |
| Healthy with decreased muscle mass (53) M and F 74.6 ± 8.0 | 12 w | 0.66 g EPA 0.44 g DHA | Muscle strength Physical performance | Muscle strength, no difference Physical performance, no difference | [29] |
| Sedentary, overweight (65) M and F 25–65 | 12 w | 1.56 g DHA 0.36 g EPA | Moderate physical activity (3 d/week for 45 min), at 75 % of age-predicted maximal HR. Resting HR and the HR in response to submaximal exercise HR variability | HR variability, improved HR at rest, reduced HR during submaximal exercise, reduced | [30] |
| Healthy (19) M 24 ± 0 and 21 ± 0 years | 8 w | 3.5 g EPA, 0.9 g DHA | Resistance exercise Skeletal muscle biopsies were obtained before and after supplementation for assessment of muscle lipid composition and protein kinase activities. | Muscle protein synthesis, no difference Protein kinase B (PKB) activity at rest, reduced PKB and AMP-activated protein kinase a2 (AMPKα2) activity, decreased | [31] |
| Healthy (20) M 23 ± 1 | 8 w | 0.91 g EPA0.4 g DHA | Maximal O2 uptake and oxygen uptake during submaximal exercise | Negative linear correlation in change between erythrocyte EPA and whole O2 uptake during submaximal exercise pre- and post-supplementation | [32] |
| Healthy (21) M 21.0 ± 0.8 | 8 w | 0.6 g EPA 0.26 g DHA | Eccentric strength exercise Motor nerve function Muscle damage Changes in maximal voluntary isometric contraction torque Range of motion Upper arm circumference, Delayed onset muscle soreness (DOMS). | M-wave latency, Maximal voluntary contraction (MVC) torque, higher Range of motion (ROM), greater DOMS, reduced | [33] |
| Healthy (24) M 19.5 ± 0.8 | 8 w | 0.6 g EPA 0.26 g DHA | Eccentric contraction-induced muscle damage Changes in the MVC torque ROM Upper arm circumference, muscle soreness CK, myoglobin, interleukin-6 (IL-6), and tumor necrosis factor a (TNF-α) levels | MVC 2–5 days after exercise, increased ROM at 1–5 days after exercise, increased Muscle soreness 3 days after exercise, reduced Increase in serum IL-6 levels, reduced | [34] |
| Healthy (26) M 18–40 | 8 w | 0.56 g DHA 0.14 g EPA | HR, HR variability and HR recovery during rest, intense exercise and recovery | The mean HR during supine resting conditions, no difference HR variability at rest, decreasing trend Peak HR, no difference HR during submaximal exercise, decreased Supine HR recovery (half-time) after cycling, faster | [35] |
| Healthy (16) M and F 71 ± 2 | 8 w | 1.9 g EPA 1.5 g DHA | Hyperinsulinemic-hyperaminoacidemic clamp | Muscle protein synthesis in older people, increased | [36] |
| Healthy (9) M and F 39.7 ± 1.7 | 8 w | 1.9 g EPA 1.5 g DHA | Hyperinsulinemic-hyperaminoacidemic clamp | Muscle protein synthesis in young people, increased | [37] |
| Overweight (50) F 20–45 | 8 w | 0,6 g EPA 0.3 g DHA | Aerobic exercise | VO2max, increased | [38] |
| Healthy (68) M and F 18.6 ± 1.2 and 18.9 ± 1.1 | 4 w 2 d | FO 2.7 g | Eccentric exercise Omega-3 Index CRP and CK Lactate DOMS, extension and torque Quality of life | Pain following eccentric exercise, reduced Extension or strength, no difference Blood lactate, lower Emotional stability, improved CRP levels at 24 h, reduced | [39] |
| Healthy (32) M 22.0 ± 2 | 3 w 5 d | 0.06 g EPA 0.04 g DHA | Muscle damaging exercise (downhill running) | Strength loss (MVC), reduced skeletal troponin (sTnI) and TNF-α at 2, 24, 48, 72 and 96 h., Mb at 24, 48, 72, 96 h., reduced CK-MM at all-time, reduced DOMS at 72 and 96 h, reduced Protective effect against joint ROM loss at 96 h Pain, reduced potentiated twitch force (∆Qtw,pot), reduced | [40] |
| Healthy (30) M 25 ± 4.6 | 3 w | 0.38 g EPA 0.51 g DHA | 17 h training/week Maximal voluntary isometric contractions Wingate test 250 kJ time trial | Vastus lateralis, increased Maximal voluntary isometric contractions, no difference Wingate percent power drop, reduced Time trial, no difference | [41] |
| Healthy (30) M 24.1 ± 3.6 and 24.4 ± 2.6 | 2 w | 0.38 g EPA 0.51 g DHA | Sprint interval training with pre- and post-training TT Resting twitches, quadriceps MVC force, and potentiated twitch force | Maximal voluntary, no difference (∆Qtw,pot, no difference | [42] |
| Healthy (8) M 24 ± 1 | 2 w | 1.1 g EPA, 0.7 g DHA | VO2max, 30 min cycling | Substrate oxidation, no change Energy expenditure, no change Energy efficiency, no change | [43] |
| Healthy (17) F 22.5 ± 1.8 and 24.7 ± 3.6 | 1 w | 6 g FO (5:1 EPA:DHA) | Post resistance exercise muscle soreness Soreness during functional movements and limb circumferences | Resistance exercise-induced static and functional soreness responses, reduced Static and functional muscle soreness, no difference Upper arm and thigh circumferences, no difference | [44] |
| Healthy (22) M 23.0 ± 3.6 | Acute | 50 g high-fat meal (4.7 g EPA) 50 g high-fat meal (4.7 g DHA) | Exercise stress testing Cardiac output, Blood pressure and systemic vascular resistance (SVR) | SVR was lower at 5 h and during exercise following the DHA but not EPA meal Resting cardiac output, no difference 8-iso-PGF2α, no difference Cardiac output during exercise, no difference | [45] |
| Population (n) Sex Age ± SD (Years) * | Duration (Weeks) | Dose EPA/DHA (g/d) | Exercise Intervention/Test | Findings | Reference |
|---|---|---|---|---|---|
| Athletes | |||||
| Football players (15) M 18.9 ± 0.5 | 8 w | 1.14 g DHA | Mitochondria dynamics and antioxidant status in peripheral blood mononuclear cells (PBMC) | PBMCs, Mn-superoxide dismutase protein levels, and their capability to produce reactive oxygen species, no difference Proteins related to mitochondrial dynamics, increased The content in mitofusins (Mtf)-1 and Mtf-2, optic atrophy protein-1 (Opa-1), and mitochondrial transcription factor A (Tfam), increased Cytochrome c oxidase (COX-IV) activity and uncoupling proteins (uncoupling protein) UCP-2 and UCP-3 protein levels, increased | [47] |
| Football players (15) M 20.4 ± 0.5 and 19.3 ± 0.4 | 8 w | 1.14 g DHA | Pro-oxidant and antioxidant status of peripheral blood mononuclear cells (PBMC)s during training and acute exercise | UCP3 levels after training, increased Superoxide dismutase protein levels after acute exercise, increased Production of reactive oxygen species (ROS) after acute exercise, reduced. | [48] |
| Football players (15) M 19.7 ± 0.4 | 8 w | 1.16 g DHA | Eicosanoids levels and PBMCs eicosanoids production | Training: Cyclooxygenase 2 (COX-2) protein levels, no difference COX-1 protein levels, increased Acute exercise: COX-2 levels, increased Lipopolysaccharide (LPS)-stimulated PBMCs prostaglandin E (PGE)1 and PGE2 production, decreased Expression of NFκβ, COX-2, 15-LOX2, 5-LOX, or IL-1β genes in PBMCs, no difference | [49] |
| Football players (15) M 20.4 ± 0.5 and 19.3 ± 0.4 | 8 w | 1.16 g DHA | Cytokine production, by LPS-stimulated PBMCs after exercise | Exercise-induced increase in IL6, IL8, vascular endothelial growth factor , INFγ, TNFα, IL1α, IL1β, MCP1, decreased EGF production rates by LPS-stimulated PBMCs, reduced | [50] |
| Football players (15) M 19.7 ± 0.4 | 8 w | 1.14 g DHA | Plasma oxidative balance and anti-inflammatory markers after training and acute exercise | Biomarkers for oxidative balance in plasma, no difference During training, plasma protein markers of oxidative damage, haemolysis degree, antioxidant enzyme activities, increased Lipid oxidative damage, no difference PGE2 in plasma after acute exercise, increased | [51] |
| Football players (15) M 20.4 ± 0.5 and 19.3 ± 0.4 | 8 w | 1.14 g DHA | After training and acute exercise Oxidative balance Oxidative damage markers Activity and protein level of antioxidant enzymes | Enzyme activities in erythrocytes, increased Catalytic activity of superoxide dismutase, increased Peroxidative damage induced by training or exercise, reduced | [52] |
| Judoists (20) M 22.8 ± 1.4 and 22.3 ± 1.4 | 6 w | 0.6 g EPA 0.4 g DHA | Oxidative stress at rest and after training | Triglycerides, reduced Resting MDA concentrations, increased NO and oxidative stress, i.e., malondialdehyde (MDA), maximum rate of oxidation (Rmax), conjugated dienes (CD)max, and NO), increased Retinol and α-tocopherol, no difference | [53] |
| Football players (30) M 23 ± 1 year | 6 w | 0.55 g DHA 0.55 g EPA | Eccentric exercise Physiological markers of recovery measured over three days following eccentric exercise | Muscle soreness, reduced compared to protein Blood concentrations of CK, reduced compared to CHO Muscle function, no difference CRP, no difference | [54] |
| Taekwondo athletes (10) M 45.6 ± 1.6 and 22.8 ± 3.8 | 5 w | 0.82 g DHA + 0.33 g α-tocopherol | Maximal exercise test | Oxidative and nitrative damage, no change Antioxidant and mitochondrial gene expression, no change | [55] |
| Taekwondo athletes (18) M 45.6 ± 1.6 and 22.8 ± 3.8 | 5 w | 0.82 g DHA + 0.33 g α-tocopherol | Acute exercise test | Pro-inflammatory gene expression in young increased | [56] |
| Paddlers M (18) 23.1 ± 1 and 23.6 ± 1.9 | 4 w | 1.2 g DHA [56] 2.4 g EPA | During intense exercise | Production of tumor necrosis factor (TNF)-α, decreased Interleukin (IL)-1β, decreased Production of IL-6, increased Production of interferon (IFN)-γ, decreased Production of IL-10, increased | [57] |
| Amateurs | |||||
| Healthy (27) M 33.4 ± 4.2 | 8 w | 1.8 g FO | Eccentric exercise Knee ROM, perceived pain, and thigh circumference of the right leg | Pain and ROM before, immediately, and 24 h after the exercise, no difference Perceived pain and ROM at 48 h post-exercise, improved | [58] |
| Healthy (24) M 19.5 ± 0.8 | 8 w | 0.6 g EPA 0.26 g DHA | Eccentric contraction-induced muscle damagemuscle soreness | 3 days after exercise, muscle soreness of the brachialis, reduced MVC, increased | [34] |
| Healthy (24) M 21.0 ± 0.9 and 20.7 ± 1.1 | 6 w | 1.3 g FO | 1 h of exercise with a constant work load corresponding to 60% of their individual VO2max) followed by a maximal rate Blood antioxidant status and lipid profile | Resting concentration of triglycerides, decreased Superoxide dismutase activity, improved Catalase activity in response to exercise after 1 h of recovery, increased | [59] |
| Healthy (16) M 24 ± 3.8 | 6 w | 1.3 g EPA 0.3 g DHA | Single bout of exercise, maximal exercise test and a 1-h bout of endurance exercise at 70% VO2 peak Plasma IL-6, EPA, DHA and cortisol; PBMC IL-2, IL-4 and interferone (IFN)-γ production; neutrophil phagocytosis/oxidative burst; and natural killer (NK) cell cytotoxic activity | At 3 h post-exercise PBMC, IL-2 and NK cell activity increased PBMC, IL-4 and IFN-γ productions, plasma IL-6 and cortisol concentrations, as well as neutrophil activity, no difference | [60] |
| Healthy (20) M 23 ± 2.3 | 6 w | 1.3 g EPA 0.3 g DHA | Exercise-induced markers of oxidative stress and muscle damage Eccentric strength exercise | CK, protein carbonyls, endogenous DNA damage, muscle soreness or MVC, unchanged Plasma thiobarbituric acid reactive substances, decreased H2O2 stimulated DNA damage immediately post-exercise, decreased | [61] |
| Healthy (37) M and F 25.8 ± 5.3 | 6 w | 0.24 g EPA 0.12 g DHA | Maximal incremental exercise test and cycling TT. Post-exercise immune function and performance Plasma IL-6 and thiobarbituric acid reactive substances (TBARS) concentrations and, erythrocyte fatty acid composition NK cell cytotoxic activity and PBMC IL-2, IL-4, IL-10, IL-17 IFNγ production | PBMC IL-2 and NK cell cytotoxic activity 3 h post-exercise, increased Plasma IL-6 and TBARS, PBMC IL-4, IL-10, IL-17 and IFNγ production, along with performance and physiological measures during exercise, no difference | [62] |
| Healthy (32) M 22.0 ± 2 | 3 w 5 d | 0.06 g EPA 0.04 g DHA | Muscle damaging exercise (downhill running) | sTnI and TNF-α at 2, 24, 48, 72 and 96 h., Mb at 24, 48, 72, 96 h., reduced CK-MM at all-time, reduced DOMS at 72 and 96 h, reduced MVC, reduced Protective effect against joint ROM loss at 96 h Pain, reduced ∆Qtw,pot, reduced | [40] |
| Healthy (27) F 33.3 ± 2.4 and 31.9 ± 3.1 | 1 w 2 d | 3 g DHA | Eccentric strength exercise | Increase in soreness was 23% less Number of participants who were able to achieve full active elbow extension 48 h after eccentric exercise was greater in the DHA group No differences for passive elbow extension or arm swelling | [63] |
| Healthy (11) M and F 18–60 | 1 w | 2 g EPA 1 g DHA | Eccentric strength exercise Inflammation Soreness ratings Arm circumference and volume Temperature | Soreness, decreased Arm circumference, no difference Arm volume, no difference Skin temperature, no difference | [46] |
| Healthy (17) F 22.5 ± 1.8 and 24.7 ± 3.6 | 1 w | 6 g FO (5:1 EPA:DHA) | Post resistance exercise muscle soreness Soreness during functional movements and limb circumferences | Muscle soreness, no difference | [44] |
| Healthy (45) M 29.3 ± 6.2 and 31.1 ± 4.9 | Acute | 1.8 g FO | Plasma levels of PGE2, IL-6, TNF-α, CK, LDH, and myoglobin (Mb) after eccentric exercise | TNF-α and PGE2 immediately, 24, and 48 h after exercise, reduced Elevation concentration of IL-6, CK, and Mb at 24 and 48 h after exercise, reduced Plasma concentration of LDH immediately, 24, and 48 h after the exercise program, reduced | [64] |
| Population (n) Sex Age ± SD (Years) * | Duration (Weeks) | Dose of EPA/DHA (g/d) | Exercise Intervention/Test | Effects of EPA/DHA | Reference |
|---|---|---|---|---|---|
| Athletes | |||||
| Football players (81) M No age reported | Over the course of a season | 2, 4, or 6 g of DHA | Neuroprotection Neurofilament light (NFL) | DHA likely attenuated serum NFL coincident with increases in serum NFL by likely small and moderate magnitude (effect size = 0.4–0.7) | [66] |
| Endurance athletes with asthma (16) M 30 ± 9 and 25 ± 4 | 3 w | 3.7 g EPA 2.5 g DHA 1.8 g EPA 1.3 g DHA | Eucapnic voluntary hyperpnoea (EVH) challenge | The peak fall in forced expiratory volume (FEV)1 was similarly reduced in both intervention groups compared to placebo (p < 0·001). Baseline fraction of exhaled NO was reduced by 24 % (p = 0·020) and 31 % (p = 0·018) after 6·2 and 3·1 g/d n-3 omega-3, respectively. Peak increases in 9α, 11β PGF2 after EVH were reduced by 65 % (p = 0·009) and 56 % (p = 0·041) after 6·2 and 3·1 g/d n-3 PUFA, respectively | [67] |
| Amateurs | |||||
| Asthma (20) M and F 22.6 ± 2.1 | 3 w | 0.07 g EPA 0.05 g DHA | EVH challenge | Maximum fall in post-EVH FEV1 significantly reduced (p < 0.05) Pre- and post- EVH, EBC ph cyst-LT and 8-isoprostane, and urinary 9a, 11b-PGF2 and CC16 concentrations were significantly reduced (p < 0.05)exhaled breath condensate pH (EBC pH) and asthma symptom scores were significantly improved (p < 0.05) Rescue medication use significantly reduced (p < 0.05) | [68] |
| Asthma (23) M and F 19–54 | 3 w | 4.0 g EPA 2.0 g DHA | Bronchial hyperresponsiveness to mannitol | No changes in sputum eosinophils No differences in Forced expiratory volume | [69] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thielecke, F.; Blannin, A. Omega-3 Fatty Acids for Sport Performance—Are They Equally Beneficial for Athletes and Amateurs? A Narrative Review. Nutrients 2020, 12, 3712. https://doi.org/10.3390/nu12123712
Thielecke F, Blannin A. Omega-3 Fatty Acids for Sport Performance—Are They Equally Beneficial for Athletes and Amateurs? A Narrative Review. Nutrients. 2020; 12(12):3712. https://doi.org/10.3390/nu12123712
Chicago/Turabian StyleThielecke, Frank, and Andrew Blannin. 2020. "Omega-3 Fatty Acids for Sport Performance—Are They Equally Beneficial for Athletes and Amateurs? A Narrative Review" Nutrients 12, no. 12: 3712. https://doi.org/10.3390/nu12123712




