Ultra-Marathon-Induced Increase in Serum Levels of Vitamin D Metabolites: A Double-Blind Randomized Controlled Trial
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Overview
2.2. Participants
2.3. Pilot Study
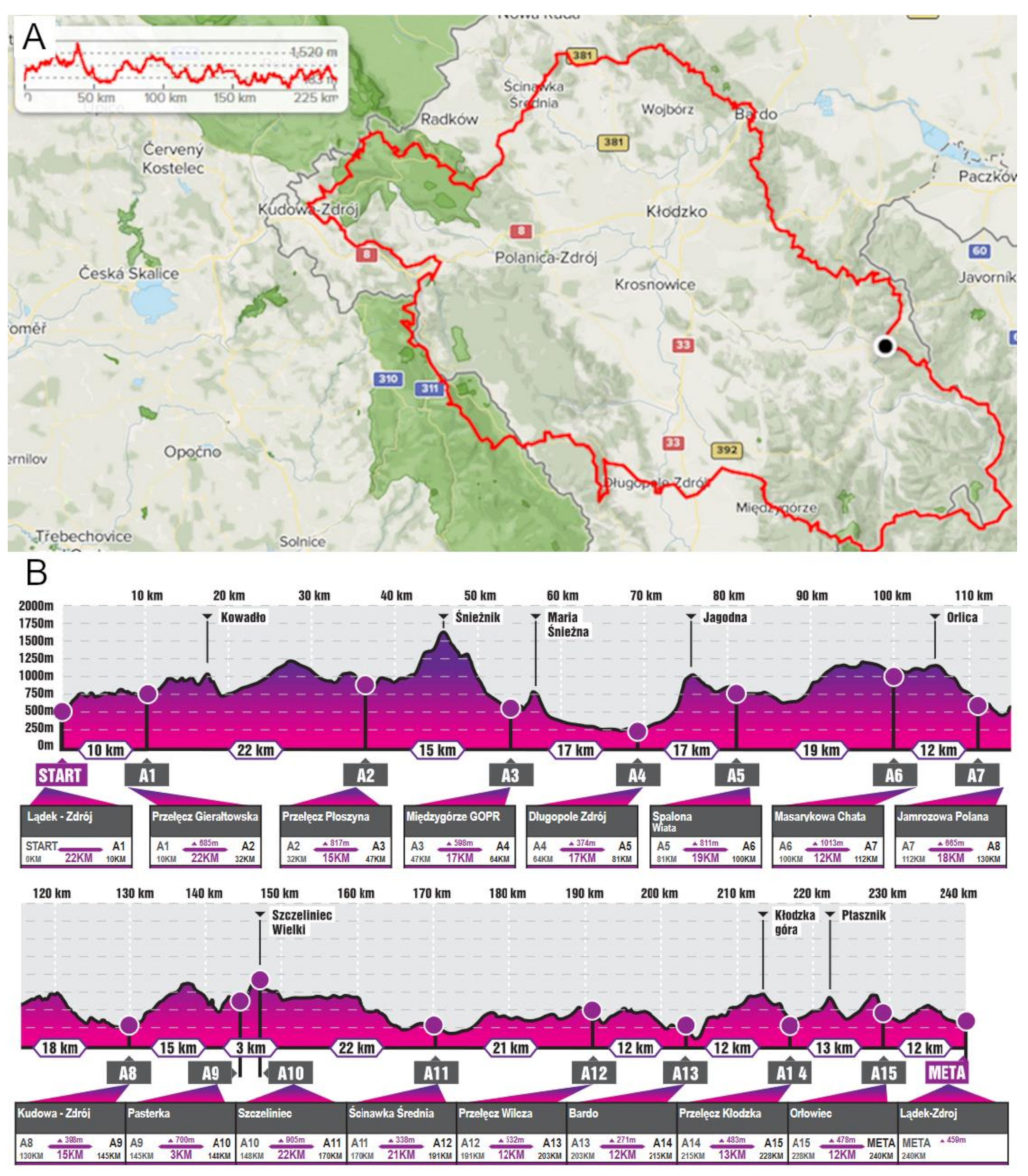
2.4. Ultra-Marathon Run
2.5. Vitamin D Supplementation
2.6. Sample Collection and Measurements of Vitamin D Metabolite Levels
2.7. Statistical Analysis
3. Results
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wang, Y.; Zhu, J.; DeLuca, H.F. Where is the vitamin D receptor? Arch. Biochem. Biophys. 2012, 523, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Wierzbicka, J.; Piotrowska, A.; Żmijewski, M.A. The renaissance of vitamin D. Acta Biochim. Pol. 2014, 61, 679–686. [Google Scholar] [CrossRef] [PubMed]
- Wierzbicka, E.; Szalecki, M.; Pludowski, P.; Jaworski, M.; Brzozowska, A. Vitamin D status, body composition and glycemic control in Polish adolescents with type 1 diabetes. Minerva Endocrinol. 2016, 41, 445–455. [Google Scholar] [PubMed]
- Strange, R.C.; Shipman, K.E.; Ramachandran, S. Metabolic syndrome: A review of the role of vitamin D in mediating susceptibility and outcome. World J. Diabetes 2015, 6, 896–911. [Google Scholar] [CrossRef] [PubMed]
- Prasad, P.; Kochhar, A. Interplay of vitamin D and metabolic syndrome: A review. Diabetes Metab. Syndr. 2016, 10, 105–112. [Google Scholar] [CrossRef]
- Pludowski, P.; Jaworski, M.; Niemirska, A.; Litwin, M.; Szalecki, M.; Karczmarewicz, E.; Michalkiewicz, J. Vitamin D status, body composition and hypertensive target organ damage in primary hypertension. J. Steroid Biochem. Mol. Biol. 2014, 144, 180–184. [Google Scholar] [CrossRef]
- De la Puente Yague, M.; Collado Yurrita, L.; Ciudad Cabanas, M.J.; Cuadrado Cenzual, M.A. Role of Vitamin D in Athletes and Their Performance: Current Concepts and New Trends. Nutrients 2020, 12, 579. [Google Scholar] [CrossRef] [Green Version]
- Ogan, D.; Pritchett, K. Vitamin D and the athlete: Risks, recommendations, and benefits. Nutrients 2013, 5, 1856–1868. [Google Scholar] [CrossRef] [Green Version]
- Janssen, H.C.; Emmelot-Vonk, M.H.; Verhaar, H.J.; van der Schouw, Y.T. Determinants of vitamin D status in healthy men and women aged 40–80 years. Maturitas 2013, 74, 79–83. [Google Scholar] [CrossRef]
- Theuri, G.; Kiplamai, F. Association between vitamin D levels and central adiposity in an eastern Africa outpatient clinical population. Dermatoendocrinol 2013, 5, 218–221. [Google Scholar] [CrossRef] [Green Version]
- Bescos Garcia, R.; Rodriguez Guisado, F.A. Low levels of vitamin D in professional basketball players after wintertime: Relationship with dietary intake of vitamin D and calcium. Nutr. Hosp. 2011, 26, 945–951. [Google Scholar] [CrossRef] [PubMed]
- Mentaverri, R.; Souberbielle, J.C.; Brami, G.; Daniel, C.; Fardellone, P. Pharmacokinetics of a New Pharmaceutical Form of Vitamin D3 100,000 IU in Soft Capsule. Nutrients 2019, 11, 703. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, X.; Cao, Z.B.; Taniguchi, H.; Tanisawa, K.; Higuchi, M. Effect of an Acute Bout of Endurance Exercise on Serum 25(OH)D Concentrations in Young Adults. J. Clin. Endocrinol. Metab. 2017, 102, 3937–3944. [Google Scholar] [CrossRef] [PubMed]
- Lutsey, P.L.; Eckfeldt, J.H.; Ogagarue, E.R.; Folsom, A.R.; Michos, E.D.; Gross, M. The 25-hydroxyvitamin D3 C-3 epimer: Distribution, correlates, and reclassification of 25-hydroxyvitamin D status in the population-based Atherosclerosis Risk in Communities Study (ARIC). Clin. Chim. Acta 2015, 442, 75–81. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, A.J.; Ritter, C.S.; Weiskopf, A.S.; Vouros, P.; Sasso, G.J.; Uskokovic, M.R.; Wang, G.; Reddy, G.S. Isolation and identification of 1alpha-hydroxy-3-epi-vitamin D3, a potent suppressor of parathyroid hormone secretion. J. Cell. Biochem. 2005, 96, 569–578. [Google Scholar] [CrossRef] [PubMed]
- Nemere, I.; Wilson, C.; Jensen, W.; Steinbeck, M.; Rohe, B.; Farach-Carson, M.C. Mechanism of 24,25-dihydroxyvitamin D3-mediated inhibition of rapid, 1,25-dihydroxyvitamin D3-induced responses: Role of reactive oxygen species. J. Cell. Biochem. 2006, 99, 1572–1581. [Google Scholar] [CrossRef]
- Seo, E.G.; Einhorn, T.A.; Norman, A.W. 24R,25-dihydroxyvitamin D3: An essential vitamin D3 metabolite for both normal bone integrity and healing of tibial fracture in chicks. Endocrinology 1997, 138, 3864–3872. [Google Scholar] [CrossRef]
- Seo, E.G.; Norman, A.W. Three-fold induction of renal 25-hydroxyvitamin D3-24-hydroxylase activity and increased serum 24,25-dihydroxyvitamin D3 levels are correlated with the healing process after chick tibial fracture. J. Bone Miner. Res. 1997, 12, 598–606. [Google Scholar] [CrossRef]
- Weinstock-Guttman, B.; Zivadinov, R.; Qu, J.; Cookfair, D.; Duan, X.; Bang, E.; Bergsland, N.; Hussein, S.; Cherneva, M.; Willis, L.; et al. Vitamin D metabolites are associated with clinical and MRI outcomes in multiple sclerosis patients. J. Neurol. Neurosurg. Psychiatry 2011, 82, 189–195. [Google Scholar] [CrossRef]
- Safdar, A.; Tarnopolsky, M.A. Exosomes as Mediators of the Systemic Adaptations to Endurance Exercise. Cold Spring Harb. Perspect. Med. 2018, 8. [Google Scholar] [CrossRef] [Green Version]
- Li, D.J.; Fu, H.; Zhao, T.; Ni, M.; Shen, F.M. Exercise-stimulated FGF23 promotes exercise performance via controlling the excess reactive oxygen species production and enhancing mitochondrial function in skeletal muscle. Metabolism 2016, 65, 747–756. [Google Scholar] [CrossRef] [PubMed]
- Shimada, T.; Hasegawa, H.; Yamazaki, Y.; Muto, T.; Hino, R.; Takeuchi, Y.; Fujita, T.; Nakahara, K.; Fukumoto, S.; Yamashita, T. FGF-23 is a potent regulator of vitamin D metabolism and phosphate homeostasis. J. Bone Miner. Res. 2004, 19, 429–435. [Google Scholar] [CrossRef] [Green Version]
- Kasprowicz, K.; Ratkowski, W.; Wolyniec, W.; Kaczmarczyk, M.; Witek, K.; Zmijewski, P.; Renke, M.; Jastrzebski, Z.; Rosemann, T.; Nikolaidis, P.T.; et al. The Effect of Vitamin D3 Supplementation on Hepcidin, Iron, and IL-6 Responses after a 100 km Ultra-Marathon. Int. J. Environ. Res. Public Health 2020, 17, 2962. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, M.D.; Ong, J.C.; Wang, G. Historical analysis of participation in 161 km ultramarathons in North America. Int. J. Hist. Sport 2010, 27, 1877–1891. [Google Scholar] [CrossRef] [PubMed]
- Knechtle, B.; Nikolaidis, P.T. Physiology and Pathophysiology in Ultra-Marathon Running. Front. Physiol. 2018, 9, 634. [Google Scholar] [CrossRef] [Green Version]
- Tiller, N.B.; Roberts, J.D.; Beasley, L.; Chapman, S.; Pinto, J.M.; Smith, L.; Wiffin, M.; Russell, M.; Sparks, S.A.; Duckworth, L.; et al. International Society of Sports Nutrition Position Stand: Nutritional considerations for single-stage ultra-marathon training and racing. J. Int. Soc. Sports Nutr. 2019, 16, 50. [Google Scholar] [CrossRef] [Green Version]
- Rola, R.; Kowalski, K.; Bienkowski, T.; Studzinska, S. Improved sample preparation method for fast LC-MS/MS analysis of vitamin D metabolites in serum. J. Pharm. Biomed. Anal. 2020, 190, 113529. [Google Scholar] [CrossRef]
- Van Beaumont, W. Evaluation of hemoconcentration from hematocrit measurements. J. Appl. Physiol. 1972, 32, 712–713. [Google Scholar] [CrossRef]
- Sherk, V.D.; Chrisman, C.; Smith, J.; Young, K.C.; Singh, H.; Bemben, M.G.; Bemben, D.A. Acute bone marker responses to whole-body vibration and resistance exercise in young women. J. Clin. Densitom. 2013, 16, 104–109. [Google Scholar] [CrossRef] [Green Version]
- Blum, M.; Dolnikowski, G.; Seyoum, E.; Harris, S.S.; Booth, S.L.; Peterson, J.; Saltzman, E.; Dawson-Hughes, B. Vitamin D(3) in fat tissue. Endocrine 2008, 33, 90–94. [Google Scholar] [CrossRef]
- Blum, M.; Dallal, G.E.; Dawson-Hughes, B. Body size and serum 25 hydroxy vitamin D response to oral supplements in healthy older adults. J. Am. Coll. Nutr. 2008, 27, 274–279. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Cao, Z.B.; Tanisawa, K.; Taniguchi, H.; Kubo, T.; Higuchi, M. Effects of chronic endurance exercise training on serum 25(OH)D concentrations in elderly Japanese men. Endocrine 2018, 59, 330–337. [Google Scholar] [CrossRef] [PubMed]
- Seuter, S.; Virtanen, J.K.; Nurmi, T.; Pihlajamäki, J.; Mursu, J.; Voutilainen, S.; Tuomainen, T.P.; Neme, A.; Carlberg, C. Molecular evaluation of vitamin D responsiveness of healthy young adults. J. Steroid Biochem. Mol. Biol. 2017, 174, 314–321. [Google Scholar] [CrossRef]
- Votruba, S.B.; Jensen, M.D. Regional fat deposition as a factor in FFA metabolism. Annu. Rev. Nutr. 2007, 27, 149–163. [Google Scholar] [CrossRef] [PubMed]
- Carrelli, A.; Bucovsky, M.; Horst, R.; Cremers, S.; Zhang, C.; Bessler, M.; Schrope, B.; Evanko, J.; Blanco, J.; Silverberg, S.J.; et al. Vitamin D Storage in Adipose Tissue of Obese and Normal Weight Women. J. Bone Miner. Res. 2017, 32, 237–242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Didriksen, A.; Burild, A.; Jakobsen, J.; Fuskevag, O.M.; Jorde, R. Vitamin D3 increases in abdominal subcutaneous fat tissue after supplementation with vitamin D3. Eur. J. Endocrinol. 2015, 172, 235–241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Owens, D.J.; Tang, J.C.; Bradley, W.J.; Sparks, A.S.; Fraser, W.D.; Morton, J.P.; Close, G.L. Efficacy of High-Dose Vitamin D Supplements for Elite Athletes. Med. Sci. Sports Exerc. 2017, 49, 349–356. [Google Scholar] [CrossRef]
- Pedersen, B.K. Physical activity and muscle-brain crosstalk. Nat. Rev. Endocrinol. 2019, 15, 383–392. [Google Scholar] [CrossRef]
- Chen, Y.; Kong, J.; Sun, T.; Li, G.; Szeto, F.L.; Liu, W.; Deb, D.K.; Wang, Y.; Zhao, Q.; Thadhani, R.; et al. 1,25-Dihydroxyvitamin D(3) suppresses inflammation-induced expression of plasminogen activator inhibitor-1 by blocking nuclear factor-kappaB activation. Arch. Biochem. Biophys. 2011, 507, 241–247. [Google Scholar] [CrossRef] [Green Version]
- Singh, R.J.; Taylor, R.L.; Reddy, G.S.; Grebe, S.K. C-3 epimers can account for a significant proportion of total circulating 25-hydroxyvitamin D in infants, complicating accurate measurement and interpretation of vitamin D status. J. Clin. Endocrinol. Metab. 2006, 91, 3055–3061. [Google Scholar] [CrossRef] [Green Version]
- Bailey, D.; Veljkovic, K.; Yazdanpanah, M.; Adeli, K. Analytical measurement and clinical relevance of vitamin D(3) C3-epimer. Clin. Biochem. 2013, 46, 190–196. [Google Scholar] [CrossRef] [PubMed]
- Rehan, V.K.; Torday, J.S.; Peleg, S.; Gennaro, L.; Vouros, P.; Padbury, J.; Rao, D.S.; Reddy, G.S. 1Alpha,25-dihydroxy-3-epi-vitamin D3, a natural metabolite of 1alpha,25-dihydroxy vitamin D3: Production and biological activity studies in pulmonary alveolar type II cells. Mol. Genet. Metab. 2002, 76, 46–56. [Google Scholar] [CrossRef]
- Granado-Lorencio, F.; Blanco-Navarro, I.; Perez-Sacristan, B.; Donoso-Navarro, E.; Silvestre-Mardomingo, R. Serum levels of 3-epi-25-OH-D3 during hypervitaminosis D in clinical practice. J. Clin. Endocrinol. Metab. 2012, 97, E2266–E2270. [Google Scholar] [CrossRef] [PubMed]
- Nikolaidis, P.T.; Veniamakis, E.; Rosemann, T.; Knechtle, B. Nutrition in Ultra-Endurance: State of the Art. Nutrients 2018, 10, 1995. [Google Scholar] [CrossRef] [Green Version]
- Knez, W.L.; Peake, J.M. The prevalence of vitamin supplementation in ultraendurance triathletes. Int. J. Sport Nutr. Exerc. Metab. 2010, 20, 507–514. [Google Scholar] [CrossRef] [PubMed]
- Costa, R.J.S.; Knechtle, B.; Tarnopolsky, M.; Hoffman, M.D. Nutrition for Ultramarathon Running: Trail, Track, and Road. Int. J. Sport Nutr. Exerc. Metab. 2019, 29, 130–140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taghiyar, M.; Ghiasvand, R.; Askari, G.; Feizi, A.; Hariri, M.; Mashhadi, N.S.; Darvishi, L. The effect of vitamins C and e supplementation on muscle damage, performance, and body composition in athlete women: A clinical trial. Int. J. Prev. Med. 2013, 4, S24–S30. [Google Scholar]


| Variable | UM-S (n = 13) | UM-C (n = 14) | Effect Size (η2) | ||
|---|---|---|---|---|---|
| Mean ± SD | (95% CI) | Mean ± SD | (95% CI) | ||
| Age (years) | 42.00 ± 8.44 | (36.00–47.00) | 40.00 ± 8.11 | (36.00–45.00) | 0.01 |
| Body mass | 74.29 ± 7.51 | (70.12–78.45) | 78.64 ± 10.66 | (72.20–85.09) | 0.05 |
| Body height (cm) | 174.80 ± 3.80 * | (172.54–177.45) | 181.30 ± 5.43 | (178.43–183.56) | 0.34 |
| Body mass index | 23.92 ± 2.42 | (21.10–25.65) | 24.18 ± 1.83 | (22.95–25.41) | 0.01 |
| Fat mass (%) | 12.58 ± 3.25 | 10.25–14.90 | 12.43 ± 4.65 | 9.31–15.56 | 0.02 |
| Variable | Effect | F | df | p | Effect Size (η2) | Post-Hoc Outcome |
|---|---|---|---|---|---|---|
| 25(OH)D3 | GR UM GR × UM | 6.59 67.00 7.43 | 1, 30 2, 60 2, 60 | 0.01 * 0.01 ** 0.01 ** | 0.19 0.70 0.20 | S > C I < II, III S-I < S-II, S-III C-I < C-II, C-III S-II > C-II S-III > C-III |
| 24,25(OH)2D3 | GR UM GR × UM | 2.91 49.90 3.72 | 1, 30 2, 60 2, 60 | 0.09 0.01 ** 0.02 * | 0.08 0.62 0.11 | I < II < III S-I < S-II, S-III C-I < C-II, C-III |
| 3-epi-25(OH)D3 | GR UM GR × UM | 7.84 58.32 7.66 | 1, 30 2, 60 2, 60 | 0.01 ** 0.01 ** 0.01 ** | 0.21 0.66 0.20 | S > C I < II < III S-I < S-II, S-III C-I < C-II, C-III S-II > C-II S-III > C-III |
| 25(OH)D2 | GR UM GR × UM | 0.26 34.06 1.05 | 1, 30 2, 60 2, 60 | 0.61 0.01 ** 0.35 | 0.01 0.53 0.03 | I < II < III |
| Ratio 25(OH)D3: 24,25(OH)2D3 | GR UM GR × UM | 6.79 70.82 8.06 | 1, 30 2, 60 2, 60 | 0.01 * 0.01 ** 0.01 ** | 0.18 0.70 0.21 | S > C I < II < III S-I < S-II, S-III C-I, C-II < C-III S-II > C-II S-III > C-III |
| Ratio 25(OH)D3: 3-epi-25(OH)D3 | GR UM GR × UM | 0.06 47.38 1.34 | 1, 30 2, 60 2, 60 | 0.81 0.04 * 0.26 | 0.01 0.10 0.04 | II < III |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mieszkowski, J.; Stankiewicz, B.; Kochanowicz, A.; Niespodziński, B.; Kowalik, T.; Żmijewski, M.A.; Kowalski, K.; Rola, R.; Bieńkowski, T.; Antosiewicz, J. Ultra-Marathon-Induced Increase in Serum Levels of Vitamin D Metabolites: A Double-Blind Randomized Controlled Trial. Nutrients 2020, 12, 3629. https://doi.org/10.3390/nu12123629
Mieszkowski J, Stankiewicz B, Kochanowicz A, Niespodziński B, Kowalik T, Żmijewski MA, Kowalski K, Rola R, Bieńkowski T, Antosiewicz J. Ultra-Marathon-Induced Increase in Serum Levels of Vitamin D Metabolites: A Double-Blind Randomized Controlled Trial. Nutrients. 2020; 12(12):3629. https://doi.org/10.3390/nu12123629
Chicago/Turabian StyleMieszkowski, Jan, Błażej Stankiewicz, Andrzej Kochanowicz, Bartłomiej Niespodziński, Tomasz Kowalik, Michał A. Żmijewski, Konrad Kowalski, Rafał Rola, Tomasz Bieńkowski, and Jędrzej Antosiewicz. 2020. "Ultra-Marathon-Induced Increase in Serum Levels of Vitamin D Metabolites: A Double-Blind Randomized Controlled Trial" Nutrients 12, no. 12: 3629. https://doi.org/10.3390/nu12123629





