Red and Processed Meat and Mortality in a Low Meat Intake Population
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Assessment of Exposures
2.3. Ascertainment of Outcomes
2.4. Assessment of Covariates
2.5. Statistical Analysis
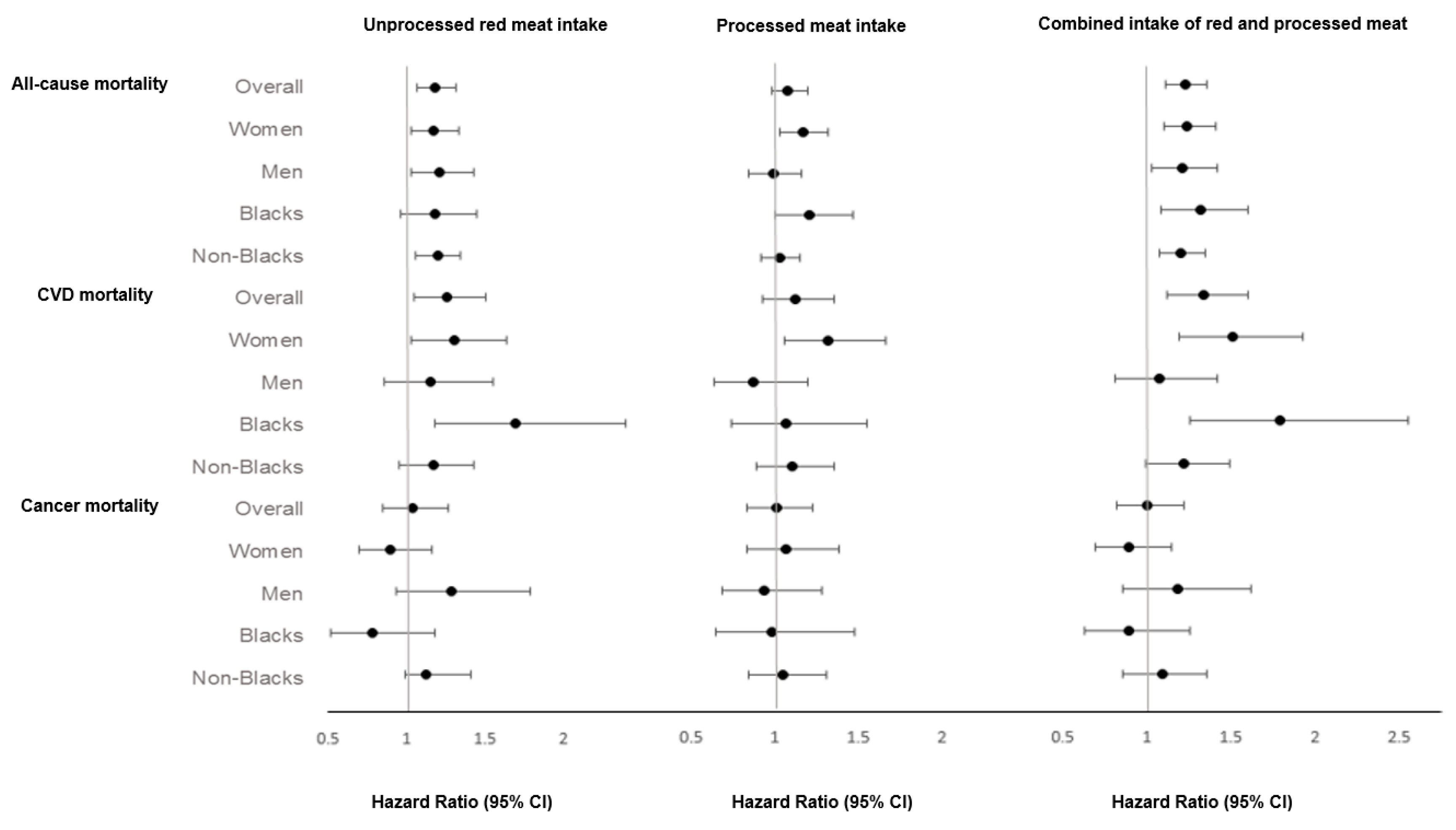
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bruinsma, J. World Agriculture: Towards 2015/2030: An FAO Perspective; Earthscan: London, UK, 2003. [Google Scholar]
- Daniel, C.R.; Cross, A.J.; Koebnick, C.; Sinha, R. Trends in meat consumption in the USA. Public Health Nutr. 2011, 14, 575–583. [Google Scholar] [CrossRef] [PubMed]
- Micha, R.; Wallace, S.K.; Mozaffarian, D. Red and processed meat consumption and risk of incident coronary heart disease, stroke, and diabetes mellitus: A systematic review and meta-analysis. Circulation 2010, 121, 2271–2283. [Google Scholar] [CrossRef] [PubMed]
- Pan, A.; Sun, Q.; Bernstein, A.M.; Schulze, M.B.; Manson, J.E.; Willett, W.C.; Hu, F.B. Red meat consumption and risk of type 2 diabetes: 3 cohorts of US adults and an updated meta-analysis. Am. J. Clin. Nutr. 2011, 94, 1088–1096. [Google Scholar] [CrossRef]
- Yuan, J.-M.; Talaei, M.; Wang, Y.-L.; Pan, A.; Koh, W.-P. Meat, Dietary Heme Iron, and Risk of Type 2 Diabetes Mellitus: The Singapore Chinese Health Study. Am. J. Epidemiol. 2017, 186, 824–833. [Google Scholar] [CrossRef]
- Cross, A.J.; Leitzmann, M.F.; Gail, M.H.; Hollenbeck, A.R.; Schatzkin, A.; Sinha, R. A prospective study of red and processed meat intake in relation to cancer risk. PLoS Med. 2007, 4, e325. [Google Scholar] [CrossRef] [PubMed]
- Pan, A.; Sun, Q.; Bernstein, A.M.; Schulze, M.B.; Manson, J.E.; Stampfer, M.J.; Willett, W.C.; Hu, F.B. Red meat consumption and mortality: Results from 2 prospective cohort studies. Arch. Intern. Med. 2012, 172, 555–563. [Google Scholar] [CrossRef]
- Sinha, R.; Cross, A.J.; Graubard, B.I.; Leitzmann, M.F.; Schatzkin, A. Meat intake and mortality: A prospective study of over half a million people. Arch. Intern. Med. 2009, 169, 562–571. [Google Scholar] [CrossRef] [PubMed]
- Rohrmann, S.; Overvad, K.; Bueno-de-Mesquita, H.B.; Jakobsen, M.U.; Egeberg, R.; Tjonneland, A.; Nailler, L.; Boutron-Ruault, M.C.; Clavel-Chapelon, F.; Krogh, V.; et al. Meat consumption and mortality—Results from the European Prospective Investigation into Cancer and Nutrition. BMC Med. 2013, 11, 63. [Google Scholar] [CrossRef]
- Orlich, M.J.; Jaceldo-Siegl, K.; Sabaté, J.; Fan, J.; Singh, P.N.; Fraser, G.E. Patterns of food consumption among vegetarians and non-vegetarians. Br. J. Nutr. 2014, 112, 1644–1653. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Butler, T.L.; Fraser, G.E.; Beeson, W.L.; Knutsen, S.F.; Herring, R.P.; Chan, J.; Sabate, J.; Montgomery, S.; Haddad, E.; Preston-Martin, S.; et al. Cohort profile: The Adventist Health Study-2 (AHS-2). Int. J. Epidemiol. 2008, 37, 260–265. [Google Scholar] [CrossRef]
- Jaceldo-Siegl, K.; Fan, J.; Sabate, J.; Knutsen, S.F.; Haddad, E.; Beeson, W.L.; Herring, R.P.; Butler, T.L.; Bennett, H.; Fraser, G.E. Race-specific validation of food intake obtained from a comprehensive FFQ: The Adventist Health Study-2. Public Health Nutr. 2011, 14, 1988–1997. [Google Scholar] [CrossRef]
- Willett, W. Nutritional Epidemiology; Oxford University Press: Oxford, UK, 2012. [Google Scholar]
- Fraser, G.; Yan, R. Guided multiple imputation of missing data: Using a subsample to strengthen the missing-at-random assumption. Epidemiology 2007, 18, 246–252. [Google Scholar] [CrossRef]
- Fraser, G.E.; Yan, R.; Butler, T.L.; Jaceldo-Siegl, K.; Beeson, W.L.; Chan, J. Missing data in a long food frequency questionnaire: Are imputed zeroes correct? Epidemiology 2009, 20, 289–294. [Google Scholar] [CrossRef] [PubMed]
- Rubin, D.B. Inference and missing data. Biometrika 1976, 63, 581–592. [Google Scholar] [CrossRef]
- Fraser, G.E.; Stram, D.O. Regression calibration in studies with correlated variables measured with error. Am. J. Epidemiol. 2001, 154, 836–844. [Google Scholar] [CrossRef] [PubMed]
- Fraser, G.E.; Stram, D.O. Regression calibration when foods (measured with error) are the variables of interest: Markedly non-Gaussian data with many zeroes. Am. J. Epidemiol. 2012, 175, 325–331. [Google Scholar] [CrossRef] [PubMed]
- Jaceldo-Siegl, K.; Knutsen, S.F.; Sabate, J.; Beeson, W.L.; Chan, J.; Herring, R.P.; Butler, T.L.; Haddad, E.; Bennett, H.; Montgomery, S.; et al. Validation of nutrient intake using an FFQ and repeated 24 h recalls in black and white subjects of the Adventist Health Study-2 (AHS-2). Public Health Nutr. 2010, 13, 812–819. [Google Scholar] [CrossRef] [PubMed]
- Willett, W.C.; Howe, G.R.; Kushi, L.H. Adjustment for total energy intake in epidemiologic studies. Am. J. Clin. Nutr. 1997, 65, 1220S–1228S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spiegelman, D.; Hertzmark, E.; Wand, H.C. Point and interval estimates of partial population attributable risks in cohort studies: Examples and software. Cancer Causes Control. 2007, 18, 571–579. [Google Scholar] [CrossRef]
- Field, A.; Miles, J.; Field, Z. Discovering Statistics Using R; Sage Publications: Thousand Oaks, CA, USA, 2012. [Google Scholar]
- Hmisc: Harrell Miscellaneous. R Package Version 4.1-1. 2018. Available online: https://cran.r-project.org/package=Hmisc (accessed on 12 September 2018).
- Regression Modeling Strategies. R Package Version 5.1-2. 2018. Available online: https://CRAN.R-project.org/package=rms (accessed on 6 November 2018).
- Boot: Bootstrap R (S-Plus) Functions. R package version 1.3-20. 2017. Available online: : https://CRAN.R-project.org/package=boot (accessed on 27 September 2018).
- Etemadi, A.; Sinha, R.; Ward, M.H.; Graubard, B.I.; Inoue-Choi, M.; Dawsey, S.M.; Abnet, C.C. Mortality from different causes associated with meat, heme iron, nitrates, and nitrites in the NIH-AARP Diet and Health Study: Population based cohort study. BMJ 2017, 357, j1957. [Google Scholar] [CrossRef]
- Bellavia, A.; Stilling, F.; Wolk, A. High red meat intake and all-cause cardiovascular and cancer mortality: Is the risk modified by fruit and vegetable intake? Am. J. Clin. Nutr. 2016, 104, 1137–1143. [Google Scholar] [CrossRef] [PubMed]
- Van den Brandt, P.A. Red meat, processed meat, and other dietary protein sources and risk of overall and cause-specific mortality in The Netherlands Cohort Study. Eur. J. Epidemiol. 2019, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Abete, I.; Romaguera, D.; Vieira, A.R.; de Munain, A.L.; Norat, T. Association between total, processed, red and white meat consumption and all-cause, CVD and IHD mortality: A meta-analysis of cohort studies. Br. J. Nutr. 2014, 112, 762–775. [Google Scholar] [CrossRef] [PubMed]
- Larsson, S.C.; Orsini, N. Red meat and processed meat consumption and all-cause mortality: A meta-analysis. Am. J. Epidemiol. 2013, 179, 282–289. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Lin, X.; Ouyang, Y.Y.; Liu, J.; Zhao, G.; Pan, A.; Hu, F.B. Red and processed meat consumption and mortality: Dose-response meta-analysis of prospective cohort studies. Public Health Nutr. 2016, 19, 893–905. [Google Scholar] [CrossRef]
- Lee, J.E.; McLerran, D.F.; Rolland, B.; Chen, Y.; Grant, E.J.; Vedanthan, R.; Inoue, M.; Tsugane, S.; Gao, Y.T.; Tsuji, I.; et al. Meat intake and cause-specific mortality: A pooled analysis of Asian prospective cohort studies. Am. J. Clin. Nutr. 2013, 98, 1032–1041. [Google Scholar] [CrossRef]
- Takata, Y.; Shu, X.O.; Gao, Y.T.; Li, H.; Zhang, X.; Gao, J.; Cai, H.; Yang, G.; Xiang, Y.B.; Zheng, W. Red meat and poultry intakes and risk of total and cause-specific mortality: Results from cohort studies of Chinese adults in Shanghai. PLoS ONE 2013, 8, e56963. [Google Scholar] [CrossRef]
- Aune, D.; Keum, N.; Giovannucci, E.; Fadnes, L.T.; Boffetta, P.; Greenwood, D.C.; Tonstad, S.; Vatten, L.J.; Riboli, E.; Norat, T. Whole grain consumption and risk of cardiovascular disease, cancer, and all cause and cause specific mortality: Systematic review and dose-response meta-analysis of prospective studies. BMJ 2016, 353, i2716. [Google Scholar] [CrossRef]
- Singh, P.N.; Fraser, G.E. Dietary risk factors for colon cancer in a low-risk population. Am. J. Epidemiol. 1998, 148, 761–774. [Google Scholar] [CrossRef]
- Zhu, B.; Sun, Y.; Qi, L.; Zhong, R.; Miao, X. Dietary legume consumption reduces risk of colorectal cancer: Evidence from a meta-analysis of cohort studies. Sci. Rep. 2015, 5, 8797. [Google Scholar] [CrossRef]
- Chan, D.S.; Lau, R.; Aune, D.; Vieira, R.; Greenwood, D.C.; Kampman, E.; Norat, T. Red and processed meat and colorectal cancer incidence: Meta-analysis of prospective studies. PLoS ONE 2011, 6, e20456. [Google Scholar] [CrossRef]
- Larsson, S.; Wolk, A. Red and processed meat consumption and risk of pancreatic cancer: Meta-analysis of prospective studies. Br. J. Cancer 2012, 106, 603. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Yang, X.; Zhang, C.; Zhu, C.; Tao, G.; Zhao, L.; Tang, S.; Shu, Z.; Cai, J.; Dai, S. Red and processed meat intake is associated with higher gastric cancer risk: A meta-analysis of epidemiological observational studies. PLoS ONE 2013, 8, e70955. [Google Scholar] [CrossRef]
- Fraser, G.E. Diet., life Expectancy, and Chronic Disease: Studies of Seventh-Day Adventists and Other Vegetarians; Oxford University Press: Oxford, UK, 2003. [Google Scholar]
- Orlich, M.J.; Singh, P.N.; Sabate, J.; Jaceldo-Siegl, K.; Fan, J.; Knutsen, S.; Beeson, W.L.; Fraser, G.E. Vegetarian dietary patterns and mortality in Adventist Health Study 2. JAMA Intern. Med. 2013, 173, 1230–1238. [Google Scholar] [CrossRef] [PubMed]
- Tantamango-Bartley, Y.; Jaceldo-Siegl, K.; Fan, J.; Fraser, G. Vegetarian diets and the incidence of cancer in a low-risk population. Cancer Epidemiol. Biomark. Prev. 2013, 22, 286–294. [Google Scholar] [CrossRef]
- American Heart Association. Saturated Fat. Available online: http://www.heart.org/en/healthy-living/healthy-eating/eat-smart/fats/saturated-fats (accessed on 14 October 2018).
- Mitra, S.; Goyal, T.; Mehta, J.L. Oxidized LDL, LOX-1 and atherosclerosis. Cardiovasc. Drugs Ther. 2011, 25, 419. [Google Scholar] [CrossRef] [PubMed]
- Madssen, E.; Laugsand, L.E.; Wiseth, R.; Morkedal, B.; Platou, C.; Vatten, L.; Janszky, I. Risk of acute myocardial infarction: Dyslipidemia more detrimental for men than women. Epidemiology 2013, 24, 637–642. [Google Scholar] [CrossRef]
- Montonen, J.; Boeing, H.; Fritsche, A.; Schleicher, E.; Joost, H.-G.; Schulze, M.B.; Steffen, A.; Pischon, T. Consumption of red meat and whole-grain bread in relation to biomarkers of obesity, inflammation, glucose metabolism and oxidative stress. Eur. J. Nutr. 2013, 52, 337–345. [Google Scholar] [CrossRef]
- Fang, X.; An, P.; Wang, H.; Wang, X.; Shen, X.; Li, X.; Min, J.; Liu, S.; Wang, F. Dietary intake of heme iron and risk of cardiovascular disease: A dose–response meta-analysis of prospective cohort studies. Nutr. Metab. Cardiovasc. Dis. 2015, 25, 24–35. [Google Scholar] [CrossRef]
- De Oliveira Otto, M.C.; Alonso, A.; Lee, D.-H.; Delclos, G.L.; Bertoni, A.G.; Jiang, R.; Lima, J.A.; Symanski, E.; Jacobs, D.R., Jr.; Nettleton, J.A. Dietary intakes of zinc and heme iron from red meat, but not from other sources, are associated with greater risk of metabolic syndrome and cardiovascular disease–3. J. Nutr. 2012, 142, 526–533. [Google Scholar] [CrossRef]
- Klipstein-Grobusch, K.; Grobbee, D.E.; den Breeijen, J.H.; Boeing, H.; Hofman, A.; Witteman, J.C. Dietary iron and risk of myocardial infarction in the Rotterdam Study. Am. J. Epidemiol. 1999, 149, 421–428. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Li, B.; Dong, X.; Zhang, X.-Q.; Zeng, Y.; Zhou, J.-L.; Tang, Y.-H.; Xu, J.-J. Is heme iron intake associated with risk of coronary heart disease? A meta-analysis of prospective studies. Eur. J. Nutr. 2014, 53, 395–400. [Google Scholar] [CrossRef]
- Koeth, R.A.; Wang, Z.; Levison, B.S.; Buffa, J.A.; Org, E.; Sheehy, B.T.; Britt, E.B.; Fu, X.; Wu, Y.; Li, L.; et al. Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat. Med. 2013, 19, 576–585. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Z.; Klipfell, E.; Bennett, B.J.; Koeth, R.; Levison, B.S.; Dugar, B.; Feldstein, A.E.; Britt, E.B.; Fu, X.; Chung, Y.M.; et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature 2011, 472, 57–63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scientific Advisory Committee on Nutrition. Salt and Health. Available online: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/338782/SACN_Salt_and_Health_report.pdf (accessed on 14 October 2018).
- He, F.J.; Li, J.; MacGregor, G.A. Effect of longer term modest salt reduction on blood pressure: Cochrane systematic review and meta-analysis of randomised trials. BMJ 2013, 346, f1325. [Google Scholar] [CrossRef] [PubMed]
- Smith-Spangler, C.M.; Juusola, J.L.; Enns, E.A.; Owens, D.K.; Garber, A.M. Population strategies to decrease sodium intake and the burden of cardiovascular disease: A cost-effectiveness analysis. Ann. Intern. Med. 2010, 152, 481–487. [Google Scholar] [CrossRef]
- Bibbins-Domingo, K.; Chertow, G.M.; Coxson, P.G.; Moran, A.; Lightwood, J.M.; Pletcher, M.J.; Goldman, L. Projected effect of dietary salt reductions on future cardiovascular disease. N. Engl. J. Med. 2010, 362, 590–599. [Google Scholar] [CrossRef]
- Grosso, G.; Micek, A.; Godos, J.; Pajak, A.; Sciacca, S.; Galvano, F.; Boffetta, P. Health risk factors associated with meat, fruit and vegetable consumption in cohort studies: A comprehensive meta-analysis. PLoS ONE 2017, 12, e0183787. [Google Scholar] [CrossRef]
- Martins, M.C.; Jaceldo-Siegl, K.; Orlich, M.; Fan, J.; Mashchak, A.; Fraser, G.E. A New Approach to Assess Lifetime Dietary Patterns Finds Lower Consumption of Animal Foods with Aging in a Longitudinal Analysis of a Health-Oriented Adventist Population. Nutrients 2017, 9, 1118. [Google Scholar] [CrossRef]
| Zero Intake | Quartiles of Intake g/day 1 | ||||
| Characteristic | 0 | Q1 | Q2 | Q3 | Q4 |
| Age (year), mean (SD) * | 57.3 (14.0) | 56.4 (13.8) | 55.7 (13.4) | 54.1 (12.7) | 52.7 (12.4) |
| Female, n (%) | 31,124 (66.8) | 4306 (66.0) | 4266 (66.9) | 3989 (62.7) | 3704 (58.2) |
| Blacks, n (%) | 11,985 (25.7) | 2089 (32.5) | 2153 (33.8) | 1805 (28.4) | 1631 (25.6) |
| Married, n (%) | 34,550 (74.1) | 4409 (68.0) | 4446 (69.7) | 4565 (71.8) | 4632 (72.7) |
| Graduate degree, n (%) | 9956 (21.4) | 987 (15.4) | 903 (14.2) | 817 (12.9) | 684 (10.7) |
| Current multivitamin users, n (%) | 22,462 (48.2) | 3238 (50.4) | 3086 (48.4) | 2905 (45.7) | 2790 (43.8) |
| Current smokers, n (%) | 121 (0.3) | 82 (1.3) | 116 (1.8) | 205 (3.2) | 291 (4.6) |
| Alcohol daily users, n (%) | 140 (0.3) | 53 (0.8) | 79 (1.2) | 122 (1.9) | 171 (2.7) |
| Exercise (≥150 min/week), n (%) 2 | 9812 (21.1) | 1109 (17.2) | 1118 (17.5) | 975 (15.3) | 896 (14.1) |
| Postmenopausal, n (%) 3 | 22,538 (72.4) | 3082 (71.6) | 3020 (70.8) | 2744 (68.8) | 2418 (65.3) |
| Current HRT users, n (%) 4 | 11,659 (37.5) | 1701 (39.5) | 1682 (39.4) | 1572 (39.4) | 1348 (36.4) |
| Diabetes, n (%) | 2698 (5.8) | 636 (9.9) | 692 (10.9) | 711 (11.2) | 698 (11.0) |
| Hypertension, n (%) | 8328 (17.9) | 1626 (25.3) | 1635 (25.6) | 1601 (25.2) | 1649 (25.9) |
| Hypercholesterolemia, n (%) | 7309 (15.7) | 1439 (22.4) | 1383 (21.7) | 1326 (20.9) | 1399 (22.0) |
| Current aspirin users, n (%) | 6264 (13.4) | 1233 (19.2) | 1308 (20.5) | 1236 (19.4) | 1312 (20.6) |
| BMI (kg/m2), mean (SD) * | 26.1 (5.3) | 28 (6.0) | 28.7 (6.0) | 29.2 (6.4) | 29.9 (6.7) |
| Total energy (kcal), mean (SD) * | 1901.3 (739.2) | 1934.3 (800.7) | 1853.7 (768.2) | 1844.7 (777.6) | 2071.2 (783.3) |
| Dietary variables (g/day), median, mean (SD) * | |||||
| Cruciferous vegetables | 22.9 32.6 (32.1) | 18.5 27.7 (29.7) | 18.6 26.6 (27.4) | 17.7 24.6 (24.2) | 15.4 23.1 (26.7) |
| Fruits | 306 356 (250.0) | 246 302.7 (241.9) | 231 281.9 (226.0) | 199.1 243.2 (199.0) | 155.9 200.3 (184.1) |
| Whole grain | 162.1 185.4 (123.1) | 122.1 149.4 (109.5) | 107.7 139 (110.5) | 92.9 120.5 (97.2) | 77.5 102.3 (87.5) |
| Legumes | 42.4 56 (48.0) | 32.3 45.1 (43.6) | 30.4 42 (41.9) | 27.1 36.7 (36.9) | 23.5 33.5 (34.6) |
| Nuts and seeds | 20.21 25.34 (21.7) | 14.06 19.80 (19.9) | 12.33 17.89 (18.4) | 11.55 16.33 (16.3) | 9.82 14.58 (15.5) |
| Total dairy | 46.8 115.7 (170.5) | 143.6 199.1 (202.7) | 163.7 215.1 (206.3) | 178 228.1 (205.9) | 184.1 232.9 (200.3) |
| Eggs | 3.3 7.7 (13.3) | 6.7 12.6 (17.4) | 7.1 13.7 (17.6) | 8.5 15.4 (18.5) | 15.2 18.9 (23.7) |
| Unprocessed poultry | 0 4.4 (13.9) | 5.9 14.5 (20.5) | 7.9 16.8 (21.1) | 12.2 21.3 (22.4) | 28.7 27.9 (23.3) |
| Processed meat | 0 0.3 (2.5) | 0.5 1.8 (5.6) | 0.9 2.6 (6.0) | 1.9 4 (6.9) | 3.3 7.4 (12.7) |
| Fish | 0 7.1 (17.3) | 9.0 14.9 (20.7) | 11.6 16 (19.5) | 12.1 16.4 (18.9) | 11.5 16 (18.8) |
| Unprocessed Red Meat Intake (g/day) 2 | ||||||||
| Zero Intake | Quartiles of Intake 3 | p-trend | 90th vs. 0 4 | 90th vs. 0 4 | ||||
| 0 | Q1 | Q2 | Q3 | Q4 | Uncalibrated | Calibrated | ||
| No. of participants | 46,613 | 6431 | 6377 | 6359 | 6369 | |||
| All-cause mortality | ||||||||
| No. of deaths (n = 7961) | 5376 | 727 | 673 | 593 | 592 | |||
| Model 1 | 1.00 | 1.16 (1.07–1.26) | 1.27 (1.17–1.38) | 1.39 (1.27–1.52) | 1.58 (1.45–1.72) | <0.0001 | 1.56 (1.46–1.67) | 2.37 (1.99–2.93) |
| Model 2 | 1.00 | 1.08 (0.99–1.18) | 1.16 (1.06–1.27) | 1.19 (1.08–1.32) | 1.26 (1.14–1.39) | <0.0001 | 1.25 (1.15–1.36) | 1.69 (1.40–2.16) |
| Model 3 | 1.00 | 1.06 (0.97–1.17) | 1.12 (1.02–1.24) | 1.14 (1.02–1.27) | 1.17 (1.05–1.32) | <0.001 | 1.18 (1.07–1.31) | 1.51 (1.22–1.98) |
| CVD mortality | ||||||||
| No. of deaths (n = 2598) | 1785 | 250 | 204 | 178 | 181 | |||
| Model 1 | 1.00 | 1.24 (1.08–1.43) | 1.27 (1.09–1.48) | 1.41 (1.20–1.65) | 1.55 (1.33–1.82) | <0.0001 | 1.58 (1.40–1.78) | 2.41 (1.86–3.24) |
| Model 2 | 1.00 | 1.20 (1.03–1.39) | 1.18 (1.01–1.39) | 1.27 (1.07–1.50) | 1.32 (1.10–1.57) | <0.001 | 1.36 (1.18–1.57) | 2.02 (1.44–3.04) |
| Model 3 | 1.00 | 1.15 (0.98–1.34) | 1.11 (0.93–1.32) | 1.17 (0.96–1.43) | 1.20 (0.97–1.47) | 0.051 | 1.26 (1.05–1.50) | 1.64 (1.09–2.57) |
| Cancer mortality | ||||||||
| No. of deaths (n = 1873) | 1228 | 175 | 160 | 159 | 151 | |||
| Model 1 | 1.00 | 1.13 (0.96–1.34) | 1.16 (0.98–1.37) | 1.38 (1.16–1.63) | 1.53 (1.29–1.82) | <0.0001 | 1.50 (1.31–1.72) | 2.17 (1.66–2.95) |
| Model 2 5 | 1.00 | 1.04 (0.88–1.23) | 1.04 (0.87–1.24) | 1.14 (0.95–1.37) | 1.19 (0.95–1.37) | 0.047 | 1.16 (0.99–1.37) | 1.41 (0.98–2.05) |
| Model 3 5 | 1.00 | 1.01 (0.85–1.21) | 1.00 (0.83–1.22) | 1.08 (0.88–1.33) | 1.07 (0.86–1.34) | 0.357 | 1.04 (0.85–1.27) | 1.18 (0.78–1.84) |
| Processed Meat Intake (g/day) 2 | ||||||||
| No. of participants | 48,127 | 6014 | 6044 | 6016 | 5948 | |||
| All-cause mortality | ||||||||
| No. of deaths (n = 7961) | 5544 | 657 | 598 | 552 | 610 | |||
| Model 1 | 1.00 | 1.04 (0.96–1.13) | 1.24 (1.14–1.35) | 1.27 (1.16–1.40) | 1.59 (1.46–1.74) | <0.0001 | 1.54 (1.43–1.66) | 1.81 (1.59–2.12) |
| Model 2 | 1.00 | 0.98 (0.90–1.08) | 1.10 (0.99–1.21) | 1.09 (0.99–1.21) | 1.27 (1.15–1.40) | <0.0001 | 1.20 (1.10–1.30) | 1.38 (1.18–1.68) |
| Model 3 | 1.00 | 0.95 (0.86–1.05) | 1.03 (0.94–1.14) | 1.02 (0.91–1.13) | 1.16 (1.04–1.29) | 0.018 | 1.08 (0.98–1.20) | 1.25 (0.95–1.94) |
| CVD mortality | ||||||||
| No. of deaths (n = 2598) | 1821 | 224 | 199 | 176 | 178 | |||
| Model 1 | 1.00 | 1.11 (0.95–1.28) | 1.32 (1.13–1.55) | 1.38 (1.15–1.67) | 1.53 (1.30–1.80) | <0.0001 | 1.54 (1.34–1.76) | 1.90 (1.56–2.37) |
| Model 2 | 1.00 | 1.05 (0.89–1.24) | 1.21 (1.02–1.44) | 1.24 (1.01–1.51) | 1.31 (1.09–1.57) | <0.001 | 1.28 (1.09–1.51) | 1.68 (1.28–2.32) |
| Model 3 | 1.00 | 1.01 (0.84–1.21) | 1.13 (0.93–1.37) | 1.14 (0.92–1.42) | 1.19 (0.97–1.47) | 0.054 | 1.12 (0.93–1.36) | 1.62 (0.97–3.71) |
| Cancer mortality | ||||||||
| No. of deaths (n = 1873) | 1294 | 142 | 148 | 128 | 161 | |||
| Model 1 | 1.00 | 0.92 (0.77–1.10) | 1.15 (0.95–1.39) | 1.12 (0.92–1.36) | 1.58 (1.32–1.88) | <0.0001 | 1.49 (1.28–1.73) | 1.61 (1.28–2.04) |
| Model 2 5 | 1.00 | 0.85 (0.71–1.02) | 1.00 (0.82–1.21) | 0.94 (0.77–1.15) | 1.19 (0.98–1.45) | 0.229 | 1.12 (0.94–1.33) | 1.09 (0.79–1.50) |
| Model 3 5 | 1.00 | 0.80 (0.66–0.96) | 0.93 (0.75–1.14) | 0.86 (0.69–1.06) | 1.06 (0.86–1.32) | 0.994 | 1.01 (0.83–1.23) | 0.74 (0.32–1.38) |
| Combined intake of red and processed meat (g/day) 2 | ||||||||
| No. of participants | 40,287 | 7966 | 7965 | 7966 | 7965 | |||
| All-cause mortality | ||||||||
| No. of deaths (n = 7961) | 4706 | 860 | 890 | 752 | 753 | |||
| Model 1 | 1.00 | 1.07 (0.99–1.15) | 1.20 (1.11–1.30) | 1.35 (1.24–1.46) | 1.60 (1.47–1.73) | <0.0001 | 1.55 (1.45–1.65) | 1.86 (1.68–2.09) |
| Model 2 | 1.00 | 1.03 (0.95–1.12) | 1.11 (1.02–1.21) | 1.18 (1.08–1.29) | 1.27 (1.16–1.40) | <0.0001 | 1.25 (1.16–1.36) | 1.44 (1.27–1.65) |
| Model 3 6 | 1.00 | 1.02 (0.93–1.12) | 1.09 (0.99–1.21) | 1.17 (1.04–1.30) | 1.25 (1.12–1.40) | <0.0001 | 1.23 (1.11–1.36) | 1.50 (1.26–1.83) |
| CVD mortality | ||||||||
| No. of deaths (n = 2598) | 1564 | 291 | 290 | 230 | 223 | |||
| Model 1 | 1.00 | 1.11 (0.96–1.27) | 1.27 (1.11–1.45) | 1.38 (1.20–1.58) | 1.56 (1.35–1.80) | <0.0001 | 1.57 (1.40–1.77) | 1.90 (1.59–2.26) |
| Model 2 | 1.00 | 1.09 (0.93–1.27) | 1.21 (1.04–1.40) | 1.25 (1.07–1.47) | 1.33 (1.12–1.57) | <0.0001 | 1.37 (1.19–1.58) | 1.66 (1.32–2.12) |
| Model 3 6 | 1.00 | 1.08 (0.90–1.28) | 1.18 (0.99–1.40) | 1.21 (1.00–1.47) | 1.29 (1.06–1.58) | 0.005 | 1.34 (1.12–1.60) | 1.73 (1.27–2.51) |
| Cancer mortality | ||||||||
| No. of deaths (n = 1873) | 1080 | 196 | 206 | 194 | 197 | |||
| Model 1 | 1.00 | 1.00 (0.85–1.18) | 1.12 (0.96–1.30) | 1.25 (1.05–1.48) | 1.57 (1.35–1.84) | <0.0001 | 1.48 (1.29–1.69) | 1.73 (1.44–2.09) |
| Model 2 5 | 1.00 | 0.94 (0.80–1.11) | 1.00 (0.85–1.18) | 1.05 (0.88–1.26) | 1.19 (1.00–1.43) | 0.103 | 1.14 (0.97–1.34) | 1.25 (0.97–1.60) |
| Model 3 5,6 | 1.00 | 0.88 (0.73–1.05) | 0.92 (0.77–1.10) | 0.97 (0.78–1.20) | 1.07 (0.87–1.32) | 0.604 | 1.00 (0.82–1.22) | 1.02 (0.70–1.42) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alshahrani, S.M.; Fraser, G.E.; Sabaté, J.; Knutsen, R.; Shavlik, D.; Mashchak, A.; Lloren, J.I.; Orlich, M.J. Red and Processed Meat and Mortality in a Low Meat Intake Population. Nutrients 2019, 11, 622. https://doi.org/10.3390/nu11030622
Alshahrani SM, Fraser GE, Sabaté J, Knutsen R, Shavlik D, Mashchak A, Lloren JI, Orlich MJ. Red and Processed Meat and Mortality in a Low Meat Intake Population. Nutrients. 2019; 11(3):622. https://doi.org/10.3390/nu11030622
Chicago/Turabian StyleAlshahrani, Saeed Mastour, Gary E. Fraser, Joan Sabaté, Raymond Knutsen, David Shavlik, Andrew Mashchak, Jan Irene Lloren, and Michael J. Orlich. 2019. "Red and Processed Meat and Mortality in a Low Meat Intake Population" Nutrients 11, no. 3: 622. https://doi.org/10.3390/nu11030622





