High-Fructose Diet-Induced Metabolic Disorders Were Counteracted by the Intake of Fruit and Leaves of Sweet Cherry in Wistar Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Experimental Diets
2.3. Biological Sample Collection
2.4. The Concentration of Crude Lipid and Fatty Acids Composition in Organs and Adipose Tissue
2.5. Biochemical Analysis
2.6. Gene Expression
2.7. Statistical Analysis
3. Results
3.1. Body Weight Gain and Weight of Organs
3.2. Crude Lipid Content and Concentration of Fatty Acids in Selected Organs and Adipose Tissue
3.3. Selected Biochemical Parameters
3.4. Biochemical Parameters Related to Oxidative Stress and Inflammation
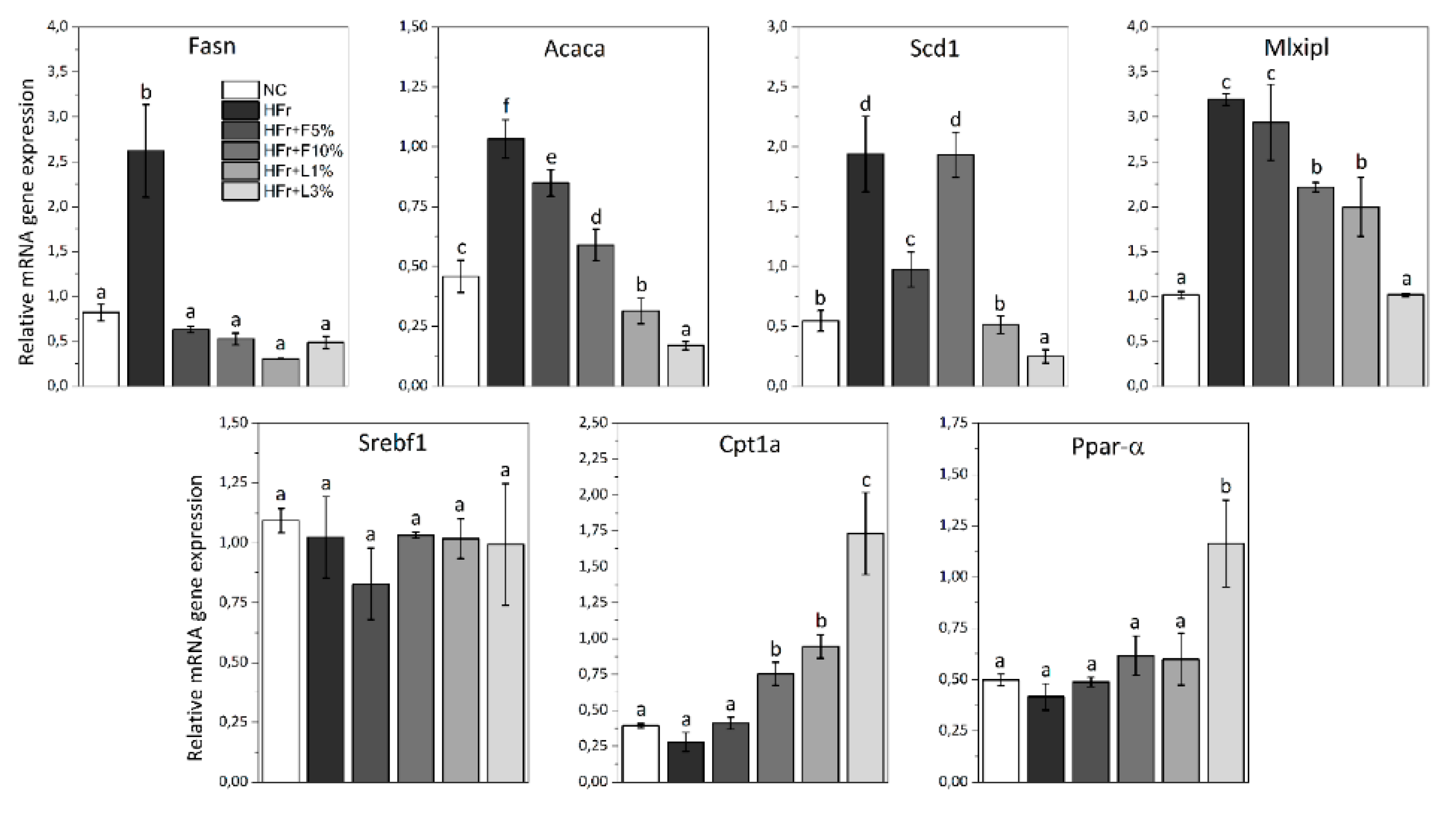
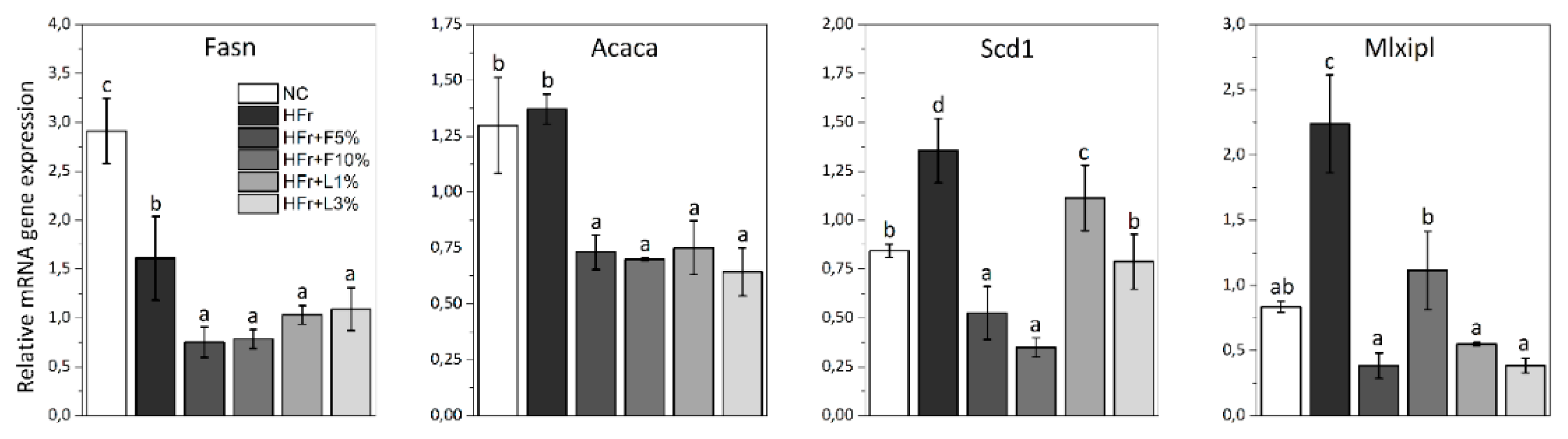
3.5. Gene Expression Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Ethical Standards
References
- WHO. Obesity and Overweight. Available online: https://www.who.int/en/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 20 August 2019).
- Tappy, L.; Le, K.A. Metabolic effects of fructose and the worldwide increase in obesity. Physiol. Rev. 2010, 90, 23–46. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.K.; Yetley, E.A. Intakes and food sources of fructose in the United States. Am. J. Clin. Nutr. 1993, 737–747. [Google Scholar] [CrossRef] [PubMed]
- Vos, M.B.; Kimmons, J.E.; Gillespie, C.; Welsh, J.; Blanck, H.M. Dietary Fructose Consumption Among US Children and Adults: The Third National Health and Nutrition Examination Survey. Medscape J. Med. 2008, 10, 160. [Google Scholar]
- Horst, K.W.; Serlie, M.J. Fructose Consumption, Lipogenesis, and Non-Alcoholic Fatty Liver Disease. Nutrients 2017, 9, 981. [Google Scholar] [CrossRef] [PubMed]
- Pektas, M.B.; Koca, H.B.; Sadi, G.; Akar, F. Dietary Fructose Activates Insulin Signaling and Inflammation in Adipose Tissue: Modulatory Role of Resveratrol. BioMed Res. Int. 2016, 2016, 8014252. [Google Scholar] [CrossRef] [PubMed]
- Zagrodzki, P.; Joniec, A.; Gawlik, M.; Gawlik, M.; Krośniak, M.; Fołta, M.; Bartoń, H.; Paśko, P.; Chłopicka, J.; Zachwieja, Z. High fructose model of oxidative stress and metabolic disturbances in rats. Part I. Antioxidant status of rats’ tissues. Bull. Vet. Inst. Pulawy 2007, 51, 407–412. [Google Scholar]
- Jegatheesan, P.; de Bandt, J.P. Fructose and NAFLD: The Multifaceted Aspects of Fructose Metabolism. Nutrients 2017, 9, 230. [Google Scholar] [CrossRef]
- Khanal, R.C.; Howard, L.R.; Wilkes, S.E.; Rogers, T.J.; Prior, R.L. Effect of Dietary Blueberry Pomace on Selected Metabolic Factors Associated with High Fructose Feeding in Growing Sprague–Dawley Rats. J. Med. Food 2012, 15, 802–810. [Google Scholar] [CrossRef]
- Varghese, S.M.; Thomas, J. The Effect of M. latifolia Leaf Extract on High-Fructose Corn Syrup (HFCS)-Induced Non-alcoholic Fatty Liver Disease in Rat Models. In Nonalcoholic Fatty Liver Disease; IntechOpen: London, UK, 2019. [Google Scholar]
- Ballistreri, G.; Continella, A.; Gentile, A.; Amenta, M.; Fabroni, S.; Rapisarda, P. Fruit quality and bioactive compounds relevant to human health of sweet cherry (Prunus avium L.) cultivars grown in Italy. Food Chem. 2013, 140, 630–638. [Google Scholar] [CrossRef]
- Delgado, J.; del Pilar Terrón, M.; Garrido, M.; Barriga, C.; Espino, J.; Paredes, S.D.; Rodríguez, A.B. Jerte Valley cherry-based product modulates serum inflammatory markers in rats and ringdoves. J. Appl. Biomed. 2012, 10, 41–50. [Google Scholar] [CrossRef] [Green Version]
- Der Werf, R.; Van Walter, C.; Bietiger, W.; Seyfritz, E.; Mura, C.; Peronet, C.; Legrandois, J.; Werner, D.; Ennahar, S.; Digel, F.; et al. Beneficial effects of cherry consumption as a dietary intervention for metabolic, hepatic and vascular complications in type 2 diabetic rats. Cardiovasc. Diabetol. 2018, 17, 104. [Google Scholar] [CrossRef] [PubMed]
- Ferretti, G.; Bacchetti, T.; Belleggia, A.; Neri, D. Cherry Antioxidants: From Farm to Table. Molecules 2010, 15, 6993–7005. [Google Scholar] [CrossRef] [PubMed]
- Dziadek, K.; Kopeć, A.; Czaplicki, S. The petioles and leaves of sweet cherry (Prunus avium L.) as a potential source of natural bioactive compounds. Eur. Food Res. Technol. 2018, 244, 1415–1426. [Google Scholar] [CrossRef]
- Dziadek, K.; Kopeć, A.; Tabaszewska, M. Potential of sweet cherry (Prunus avium L.) by-products: Bioactive compounds and antioxidant activity of leaves and petioles. Eur. Food Res. Technol. 2019, 245, 763–772. [Google Scholar] [CrossRef]
- Reeves, P.G. Symposium: Animal Diets for Nutritional and Toxicological Research Components of the AIN-93 Diets as Improvements in the AIN-76A Diet. J. Nutr. 1997, 127, 824S. [Google Scholar] [CrossRef]
- Dziadek, K.; Kopeć, A.; Piątkowska, E. Intake of fruit and leaves of sweet cherry beneficially affects lipid metabolism, oxidative stress and inflammation in Wistar rats fed with high fat-cholesterol diet. J. Funct. Foods 2019, 57, 31–39. [Google Scholar] [CrossRef]
- Kopeć, A.; Piątkowska, E.; Koronowicz, A.; Leszczyńska, T. Effect of long term administration of resveratrol on lipid concentration in selected organs and liver’s histology in rats fed high fructose diet. J. Funct. Food 2013, 5, 299–305. [Google Scholar] [CrossRef]
- Kopeć, A.; Piątkowska, E. Effect of resveratrol on selected biochemical parameters in rats fed high fructose diet. Acta Sci. Pol. Technol. Aliment. 2013, 12, 395–402. [Google Scholar]
- Friedewald, W.T.; Levy, R.I.; Fredrickson, D.S. Estimation of the Concentration of Low-Density Lipoprotein Cholesterol in Plasma, without Use of the Preparative Ultracentrifug. Clin. Chem. 1972, 18, 499–502. [Google Scholar]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for Lipid Peroxides in Animal Tissues Thiobarbituric Acid Reaction. Anal. Biochem. 1979, 358, 351–358. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real- Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Crescenzo, R.; Cigliano, L.; Mazzoli, A.; Cancelliere, R.; Carotenuto, R.; Tussellino, M.; Liverini, G.; Iossa, S. Early Effects of a Low Fat, Fructose-Rich Diet on Liver Metabolism, Insulin Signaling, and Oxidative Stress in Young and Adult Rats. Front. Physiol. 2018, 9, 411. [Google Scholar] [CrossRef] [PubMed]
- De Castro, U.G.M.; dos Santos, R.A.S.; Silva, M.E.; de Lima, W.G.; Campagnole-santos, M.J.; Alzamora, A.C. Age-dependent effect of high-fructose and high-fat diets on lipid metabolism and lipid accumulation in liver and kidney of rats. Lipids Health Dis. 2013, 12, 136. [Google Scholar] [CrossRef] [PubMed]
- Chong, M.F.; Fielding, B.A.; Frayn, K.N. Mechanisms for the acute effect of fructose on postprandial lipemia. Am. J. Clin. Nutr. 2007, 85, 1511–1520. [Google Scholar] [CrossRef] [PubMed]
- Dekker, M.J.; Su, Q.; Baker, C.; Rutledge, A.C.; Adeli, K. Fructose: A highly lipogenic nutrient implicated in insulin resistance, hepatic steatosis, and the metabolic syndrome. Am. J. Physiol. Metab. 2010, 299, E685–E694. [Google Scholar] [CrossRef]
- Hannou, S.A.; Haslam, D.E.; Mckeown, N.M.; Herman, M.A. Fructose metabolism and metabolic disease. J. Clin. Investig. 2018, 128, 545–555. [Google Scholar] [CrossRef]
- Samaan, R. Dietary Fiber for the Prevention of Cardiovascular Disease; Elsevier Inc.: Amsterdam, The Netherlands, 2017; ISBN 9780128051306. [Google Scholar]
- Liu, H.; Chang, C.; Yang, T.; Chiang, M. Long-term feeding of red algae (Gelidium amansii) ameliorates glucose and lipid metabolism in a high fructose diet-impaired glucose tolerance rat model. J. Food Drug Anal. 2017, 25, 543–549. [Google Scholar] [CrossRef]
- Chai, Y.; Lim, B.; Lee, J.; Kim, M.; Park, M.; Rhee, S. Effects of Manufactured Soluble Dietary Fiber from Quercus mongolica on Hepatic HMG-CoA Reductase and Lipoprotein Lipase Activities in Epididymal Adipose Tissue of Rats Fed High Cholesterol Diets. J. Med. Food 2003, 6, 329–336. [Google Scholar] [CrossRef]
- Kishimoto, Y.; Tani, M.; Kondo, K. Pleiotropic preventive effects of dietary polyphenols in cardiovascular diseases. Eur. J. Clin. Nutr. 2013, 67, 532–535. [Google Scholar] [CrossRef] [Green Version]
- Ong, K.W.; Hsu, A.; Tan, B.K.H. Anti-diabetic and anti-lipidemic effects of chlorogenic acid are mediated by ampk activation. Biochem. Pharmacol. 2013, 85, 1341–1351. [Google Scholar] [CrossRef]
- Matulewicz, N.; Karczewska-Kupczewska, M. Insulin resistance and chronic inflammation. Postepy Hig. Med. Dosw. 2016, 70, 1245–1257. [Google Scholar]
- Yeh, Y.; Lee, Y.; Hsieh, H.; Hwang, D. Dietary Caffeic Acid, Ferulic Acid and Coumaric Acid Supplements on Cholesterol Metabolism and Antioxidant Activity in Rats. J. Food Drug Anal. 2009, 17, 123–132. [Google Scholar]
- Ibitoye, O.B.; Ajiboye, T.O. Dietary phenolic acids reverse insulin resistance, hyperglycaemia, dyslipidaemia, inflammation and oxidative stress in high-fructose diet-induced metabolic syndrome rats. Arch. Physiol. Biochem. 2017, 124, 410–417. [Google Scholar] [CrossRef] [PubMed]
- Gibert-Ramos, A.; Crescenti, A.; Salvadó, M.J. Consumption of Cherry out of Season Changes White Adipose Tissue Gene Expression and Morphology to a Phenotype Prone to Fat Accumulation. Nutrients 2018, 10, 1102. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.; Hsu, M.; Huang, H.; Chung, D.; Chang, Y.; Wang, C. Mulberry Anthocyanins Inhibit Oleic Acid Induced Lipid Accumulation by Reduction of Lipogenesis and Promotion of Hepatic Lipid Clearance. J. Agric. Food Chem. 2013, 61, 6069–6076. [Google Scholar] [CrossRef]
- Tang, X.; Shen, T.; Jiang, X.; Xia, M.; Sun, X.; Guo, H.; Ling, W. Purified Anthocyanins from Bilberry and Black Currant Attenuate Hepatic Mitochondrial Dysfunction and Steatohepatitis in Mice with Methionine and Choline Deficiency. J. Agric. Food Chem. 2015, 63, 552–561. [Google Scholar] [CrossRef]
- Liao, C.; Ou, T.; Huang, H.; Wang, C. The inhibition of oleic acid induced hepatic lipogenesis and the promotion of lipolysis by caffeic acid via up-regulation of AMP-activated kinase. J. Sci. Food Agric. 2014, 94, 1154–1162. [Google Scholar] [CrossRef]
- Wan, C.; Wong, C.N.; Pin, W.; Wong, M.H.; Kwok, C.; Chan, R.Y.; Yu, P.H.; Chan, S. Chlorogenic Acid Exhibits Cholesterol Lowering and Fatty Liver Attenuating Properties by Up-regulating the Gene Expression of PPAR—A in Hypercholesterolemic Rats Induced with a High-Cholesterol Diet. Phyther. Res. 2012, 27, 545–551. [Google Scholar] [CrossRef]
- Ilavenil, S.; Kim, D.H.; Srigopalram, S.; Arasu, M.V.; Lee, K.D.; Lee, J.C.; Lee, J.S.; Renganathan, S.; Choi, K.C. Potential Application of p-Coumaric Acid on Differentiation of C2C12 Skeletal Muscle and 3T3-L1 Preadipocytes—An in Vitro and in Silico Approach. Molecules 2016, 21, 997. [Google Scholar] [CrossRef]
- Naowaboot, J.; Piyabhan, P.; Munkong, N.; Parklak, W.; Pannangpetch, P. Ferulic acid improves lipid and glucose homeostasis in high-fat diet-induced obese mice. Clin. Exp. Pharmacol. Physiol. 2016, 43, 242–250. [Google Scholar] [CrossRef]
- Sampath, H.; Ntambi, J.M. Regulation of gene expression by polyunsaturated fatty acids. Heart Metab. 2006, 32, 32–35. [Google Scholar]
- Vinitha, E.; Singh, H.J.C.; Kakalij, R.M.; Kshirsagar, R.P.; Kumar, B.H.; Diwan, P.V. Neuroprotective effect of Prunus avium on streptozotocin induced neurotoxicity in mice. Biomed. Prev. Nutr. 2014, 4, 519–525. [Google Scholar] [CrossRef]
- Wu, T.; Tang, Q.; Yu, Z.; Gao, Z.; Hu, H.; Chen, W.; Zheng, X.; Yu, T. Inhibitory effects of sweet cherry anthocyanins on the obesity development in C57BL/6 mice. Int. J. Food Sci. Nutr. 2014, 65, 351–359. [Google Scholar] [CrossRef] [PubMed]
- Šarić, A.; Sobočanec, S.; Balog, T.; Kušić, B.; Šverko, V.; Dragović-Uzelac, V.; Levaj, B.; Čosić, Z.; Šafranko, Ž.M.; Marotti, T. Improved Antioxidant and Anti-inflammatory Potential in Mice Consuming Sour Cherry Juice (Prunus Cerasus cv. Maraska). Plant Foods Hum. Nutr. 2009, 64, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Estruel-Amades, S.; Massot-Cladera, M.; Garcia-Cerdà, P.; Pérez-Cano, F.J.; Franch, À.; Castell, M.; Camps-Bossacoma, M. Protective Effect of Hesperidin on the Oxidative Stress Induced by an Exhausting Exercise in Intensively Trained Rats. Nutrients 2019, 11, 783. [Google Scholar] [CrossRef] [PubMed]
- Morillas-Ruiz, J.M.; Villegas Garcia, J.A.; Lopez, F.J.; Vidal-Guevara, M.L.; Zafrilla, P. Effects of polyphenolic antioxidants on exercise-induced oxidative stress. Clin. Nutr. 2006, 25, 444–453. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zhao, M.; Pu, Z.; Xu, G.; Li, X. Relationship between oxidative stress and inflammation in hyperuricemia. Analysis based on asymptomatic young patients with primary hyperuricemia. Medicine 2018, 97, e13108. [Google Scholar] [CrossRef]
- Kelley, D.S.; Adkins, Y.; Reddy, A.; Woodhouse, L.R.; Mackey, B.E.; Erickson, K.L. Sweet Bing Cherries Lower Circulating Concentrations of Markers for Chronic Inflammatory Diseases in Healthy Humans. J. Nutr. 2013, 143, 340–344. [Google Scholar] [CrossRef]
- Noratto, G.D.; Lage, N.N.; Chew, B.P.; Mertens-Talcott, S.U.; Talcott, S.T.; Pedrosa, M.L. Non-anthocyanin phenolics in cherry (Prunus avium L.) modulate IL-6, liver lipids and expression of PPARδ and LXRs in obese diabetic (db/db) mice. Food Chem. 2018, 266, 405–414. [Google Scholar] [CrossRef]
- Lalloyer, F.; Wouters, K.; Baron, M.; Caron, S.; Vallez, E.; Vanhoutte, J.; Baugé, E.; Shiri-Sverdlov, R.; Hofker, M.; Staels, B.; et al. Peroxisome proliferator-activated receptor-α gene level differently affects lipid metabolism and inflammation in apolipoprotein E2 knock-in mice. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 1573–1579. [Google Scholar] [CrossRef]
| Ingredient | Group | |||||
|---|---|---|---|---|---|---|
| NC | HFr | HFr + F5% | HFr + F10% | HFr + L1% | HFr + L3% | |
| g/kg | ||||||
| Corn Starch | 532.486 | 0 | 0 | 0 | 0 | 0 |
| Sucrose | 100 | 0 | 0 | 0 | 0 | 0 |
| Fructose | 0 | 632.486 | 586.586 | 540.786 | 627.286 | 616.986 |
| Casein | 200 | 200 | 200 | 200 | 200 | 200 |
| Soybean Oil | 70 | 70 | 70 | 70 | 70 | 70 |
| Fiber | 50 | 50 | 45.9 | 41.7 | 45.2 | 35.5 |
| Mineral Mix | 35 | 35 | 35 | 35 | 35 | 35 |
| Vitamin Mix | 10 | 10 | 10 | 10 | 10 | 10 |
| Leaves of sweet cherry | 0 | 0 | 0 | 0 | 10 | 30 |
| Fruits of sweet cherry | 0 | 0 | 50 | 100 | 0 | 0 |
| Choline | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 |
| Tert-Butylhydroquinone | 0.014 | 0.014 | 0.014 | 0.014 | 0.014 | 0.014 |
| Parameter | Group | |||||
|---|---|---|---|---|---|---|
| NC | HFr | HFr + F5% | HFr + F10% | HFr + L1% | HFr + L3% | |
| Weight (g) | ||||||
| Body weight gain | 485.00 ± 47.10 a | 465.50 ± 65.63 a | 422.43 ± 38.26 a | 465.25 ± 61.08 a | 426.29 ± 66.31 a | 479.00 ± 47.82 a |
| Liver | 17.49 ± 2.20 a | 24.39 ± 3.27 b | 20.63 ± 3.64 ab | 20.11 ± 2.12 a | 19.62 ± 4.02 a | 20.97 ± 4.02 ab |
| Heart | 1.53 ± 0.13 ab | 1.53 ± 0.17 ab | 1.47 ± 0.11 a | 1.64 ± 0.10 b | 1.57 ± 0.17 ab | 1.60 ± 0.10 ab |
| Kidneys * | 3.11 ± 0.34 a | 3.61 ± 0.40 bc | 3.37 ± 0.27 ab | 3.52 ± 0.38 bc | 3.47 ± 0.28 ab | 3.88 ± 0.35 c |
| Crude lipid content (g/100 g) | ||||||
| Liver | 15.27 ± 5.10 ab | 19.50 ± 2.89 b | 17.08 ± 4.41 ab | 13.17 ± 3.04 a | 12.45 ± 2.91 a | 12.37 ± 5.28 a |
| Heart | 8.18 ± 1.09 a | 7.65 ± 0.64 a | 8.75 ± 1.43 a | 8.20 ± 1.07 a | 8.01 ± 0.86 a | 7.26 ± 0.93 a |
| Kidneys | 12.65 ± 2.07 a | 11.17 ± 0.81 a | 12.41 ± 0.31 a | 11.45 ± 0.92 a | 11.15 ± 2.40 a | 11.10 ± 1.60 a |
| Serum parameters | ||||||
| Glucose (mg/dL) ** | 107 ± 16 a | 101 ± 9 a | 105 ± 6 a | 99 ± 8 a | 97 ± 14 a | 98 ± 13 a |
| ALT (U/L) | 15.41 ± 3.05 a | 27.82 ± 6.15 d | 21.87 ± 4.26 c | 25.78 ± 5.11 d | 17.44 ± 2.42 ab | 19.86 ± 3.14 bc |
| AST (U/L) | 30.54 ± 4.78 bc | 33.34 ± 3.17 c | 25.40 ± 3.41 a | 31.65 ± 5.10 c | 27.49 ± 2.62 ab | 26.02 ± 5.52 a |
| TC (mmol/L) | 3.15 ± 0.27 a | 3.25 ± 0.34 a | 3.21 ± 0.31 a | 3.02 ± 0.22 a | 3.18 ± 0.26 a | 3.11 ± 0.39 a |
| HDL (mmol/L) | 1.47 ± 0.21 ab | 1.41 ± 0.15 a | 1.60 ± 0.25 bc | 1.48 ± 0.22 ab | 1.66 ± 0.24 c | 1.74 ± 0.22 c |
| LDL+VLDL (mmol/L) | 0.69 ± 0.13 b | 0.66 ± 0.20 b | 0.58 ± 0.15 ab | 0.53 ± 0.13 a | 0.68 ± 0.18 b | 0.56 ± 0.13 ab |
| TG (mmol/L) | 2.32 ± 0.49 abc | 2.69 ± 0.59 c | 2.40 ± 0.52 bc | 2.27 ± 0.47 ab | 1.97 ± 0.43 a | 2.34 ± 0.43 abc |
| Parameter | Group | |||||
|---|---|---|---|---|---|---|
| NC | HFr | HFr+F5% | HFr+F10% | HFr+L1% | HFr+L3% | |
| Antioxidant status | ||||||
| TAS (mmol Trolox) | 0.83 ± 0.08 b | 0.64 ± 0.07 a | 0.66 ± 0.12 a | 0.73 ± 0.05 ab | 0.76 ± 0.09 ab | 0.70 ± 0.10 a |
| SOD (U/mL) * | 200.34 ± 18.45 bc | 201.52 ± 19.36 bc | 205.44 ± 15.52 c | 201.16 ± 16.38 bc | 188.93 ± 17.16 b | 168.43 ± 16.90 a |
| GPx (U/mL) ** | 114.88 ± 14.37 a | 111.57 ± 9.24 a | 127.10 ± 13.17 b | 147.95 ± 11.63 c | 145.18 ± 14.44 c | 138.95 ± 15.04 c |
| GR (U/L) | 34.59 ± 5.33 a | 36.64 ± 6.01 ab | 42.86 ± 10.08 bc | 43.34 ± 7.94 c | 42.32 ± 9.41 bc | 38.65 ± 5.64 abc |
| CAT (U/L) | 15.87 ± 3.50 c | 19.59 ± 2.20 d | 15.64 ± 2.80 c | 13.02 ± 2.43 ab | 13.92 ± 2.74 bc | 11.35 ± 1.86 a |
| HO-1 (µmol/L) | 0.60 ± 0.29 a | 0.55 ± 0.13 a | 0.43 ± 0.13 a | 0.57 ± 0.20 a | 0.60 ± 0.10 a | 0.56 ± 0.18 a |
| TBARS (nmol/mL) | 4.22 ± 0.49 ab | 4.44 ± 0.78 b | 3.21 ± 0.82 a | 3.37 ± 0.72 ab | 3.44 ± 0.31 ab | 4.24 ± 0.31 ab |
| Inflammation | ||||||
| CRP (ng/mL) | 78.82 ± 14.26 a | 115.79 ± 14.83 b | 78.72 ± 14.80 a | 80.37 ± 15.23 a | 70.64 ± 10.21 a | 80.77 ± 12.04 a |
| IL-6 (ng/mL) | 14.83 ± 1.33 a | 14.80 ± 0.47 a | 14.81 ± 0.40 a | 14.51 ± 0.24 a | 14.94 ± 1.03 a | 14.94 ± 0.48 a |
| TNF-α (ng/mL) | 11.25 ± 0.57 a | 11.35 ± 0.33 a | 11.45 ± 0.73 a | 11.00 ± 0.30 a | 11.42 ± 1.00 a | 11.03 ± 0.53 a |
| IL-10 (ng/mL) | 8.63 ± 0.98 a | 9.43 ± 1.00 a | 9.88 ± 1.53 ab | 12.28 ± 2.49 c | 9.92 ± 1.26 ab | 11.91 ± 0.88 bc |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dziadek, K.; Kopeć, A.; Piątkowska, E.; Leszczyńska, T. High-Fructose Diet-Induced Metabolic Disorders Were Counteracted by the Intake of Fruit and Leaves of Sweet Cherry in Wistar Rats. Nutrients 2019, 11, 2638. https://doi.org/10.3390/nu11112638
Dziadek K, Kopeć A, Piątkowska E, Leszczyńska T. High-Fructose Diet-Induced Metabolic Disorders Were Counteracted by the Intake of Fruit and Leaves of Sweet Cherry in Wistar Rats. Nutrients. 2019; 11(11):2638. https://doi.org/10.3390/nu11112638
Chicago/Turabian StyleDziadek, Kinga, Aneta Kopeć, Ewa Piątkowska, and Teresa Leszczyńska. 2019. "High-Fructose Diet-Induced Metabolic Disorders Were Counteracted by the Intake of Fruit and Leaves of Sweet Cherry in Wistar Rats" Nutrients 11, no. 11: 2638. https://doi.org/10.3390/nu11112638